Abstract
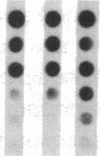
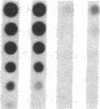
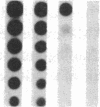
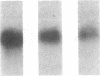
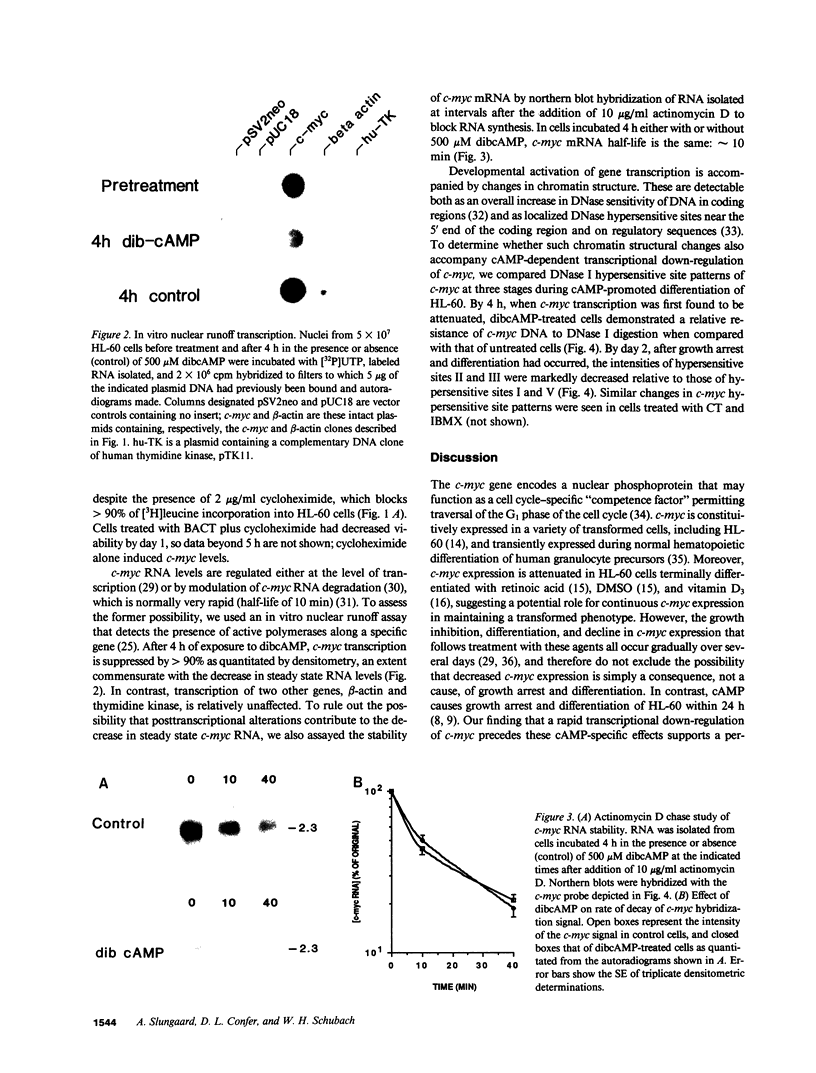
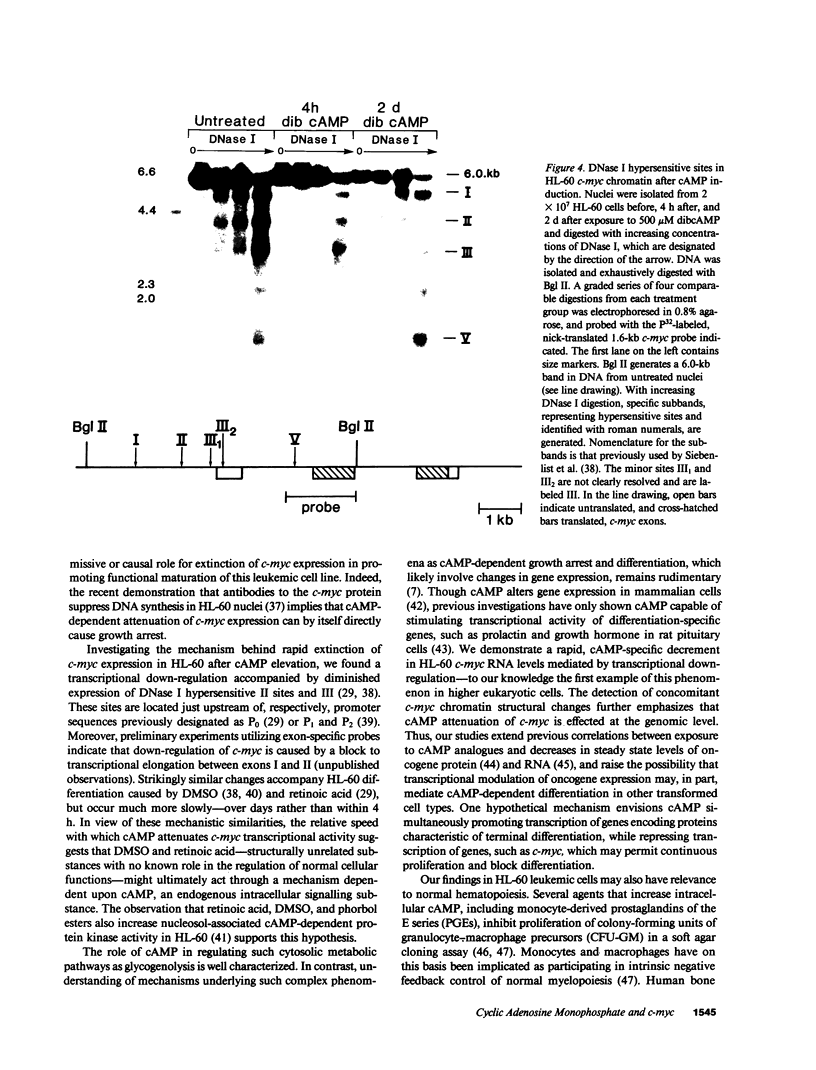
Pharmacologic elevation of cyclic AMP (cAMP) promotes growth arrest and differentiation in a variety of transformed mammalian cells, including the HL-60 human promyelocytic leukemia cell line. However, mechanisms underlying this phenomenon are poorly understood. Because cellular oncogenes play a pivotal role in regulating proliferation and differentiation, we examined whether cAMP-promoted differentiation of HL-60 was preceded by a decrease in the expression of c-myc, a cellular oncogene both amplified and constitutively expressed in HL-60. We find that cyclic AMP elevation in HL-60 caused by three different pharmacologic regimens is followed by an abrupt, greater than 90% decrease in steady state c-myc mRNA levels within 3 h, well before detectable changes in proliferation and differentiation. This decrease, which occurs despite protein synthetic blockade, is attributable to transcriptional down-regulation of c-myc and is accompanied by changes in chromatin structure near c-myc promoter sites. Our findings establish that cAMP, a ubiquitous intracellular regulatory messenger previously known only to enhance gene transcriptional activity in higher eukaryotic cells, can also suppress transcription of a cellular oncogene, thereby suggesting a potential mechanism for cAMP-promoted differentiation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley D. L., Groudine M. A block to elongation is largely responsible for decreased transcription of c-myc in differentiated HL60 cells. Nature. 1986 Jun 12;321(6071):702–706. doi: 10.1038/321702a0. [DOI] [PubMed] [Google Scholar]
- Bradshaw H. D., Jr, Deininger P. L. Human thymidine kinase gene: molecular cloning and nucleotide sequence of a cDNA expressible in mammalian cells. Mol Cell Biol. 1984 Nov;4(11):2316–2320. doi: 10.1128/mcb.4.11.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitman T. R., Selonick S. E., Collins S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980 May;77(5):2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplinski T. J., Niedel J. E. Cyclic nucleotide-induced maturation of human promyelocytic leukemia cells. J Clin Invest. 1982 Nov;70(5):953–964. doi: 10.1172/JCI110707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho-Chung Y. S., Clair T., Bodwin J. S., Berghoffer B. Growth arrest and morphological change of human breast cancer cells by dibutyryl cyclic AMP and L-arginine. Science. 1981 Oct 2;214(4516):77–79. doi: 10.1126/science.6269181. [DOI] [PubMed] [Google Scholar]
- Cho-Chung Y. S. On the mechanism of cyclic AMP-mediated growth arrest of solid tumors. Adv Cyclic Nucleotide Res. 1980;12:111–121. [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S., Groudine M. Amplification of endogenous myc-related DNA sequences in a human myeloid leukaemia cell line. Nature. 1982 Aug 12;298(5875):679–681. doi: 10.1038/298679a0. [DOI] [PubMed] [Google Scholar]
- Confer D. L., Eaton J. W. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science. 1982 Sep 3;217(4563):948–950. doi: 10.1126/science.6287574. [DOI] [PubMed] [Google Scholar]
- Confer D. L., Slungaard A. S., Graf E., Panter S. S., Eaton J. W. Bordetella adenylate cyclase toxin: entry of bacterial adenylate cyclase into mammalian cells. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:183–187. [PubMed] [Google Scholar]
- Dani C., Blanchard J. M., Piechaczyk M., El Sabouty S., Marty L., Jeanteur P. Extreme instability of myc mRNA in normal and transformed human cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7046–7050. doi: 10.1073/pnas.81.22.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani C., Mechti N., Piechaczyk M., Lebleu B., Jeanteur P., Blanchard J. M. Increased rate of degradation of c-myc mRNA in interferon-treated Daudi cells. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4896–4899. doi: 10.1073/pnas.82.15.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson P. J., Littlewood T. D., Forster A., Rabbitts T. H. Chromatin structure of transcriptionally active and inactive human c-myc alleles. EMBO J. 1985 Nov;4(11):2885–2891. doi: 10.1002/j.1460-2075.1985.tb04018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias L., Stewart T. Subcellular distribution of cyclic adenosine 3':5'-monophosphate-dependent protein kinase during the chemically induced differentiation of HL-60 cells. Cancer Res. 1984 Jul;44(7):3075–3080. [PubMed] [Google Scholar]
- Filmus J., Buick R. N. Relationship of c-myc expression to differentiation and proliferation of HL-60 cells. Cancer Res. 1985 Feb;45(2):822–825. [PubMed] [Google Scholar]
- Friedman D. L. Role of cyclic nucleotides in cell growth and differentiation. Physiol Rev. 1976 Oct;56(4):652–708. doi: 10.1152/physrev.1976.56.4.652. [DOI] [PubMed] [Google Scholar]
- Gallagher R., Collins S., Trujillo J., McCredie K., Ahearn M., Tsai S., Metzgar R., Aulakh G., Ting R., Ruscetti F. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979 Sep;54(3):713–733. [PubMed] [Google Scholar]
- Gowda S. D., Koler R. D., Bagby G. C., Jr Regulation of C-myc expression during growth and differentiation of normal and leukemic human myeloid progenitor cells. J Clin Invest. 1986 Jan;77(1):271–278. doi: 10.1172/JCI112287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso L. E., Pitot H. C. Transcriptional regulation of c-myc during chemically induced differentiation of HL-60 cultures. Cancer Res. 1985 Feb;45(2):847–850. [PubMed] [Google Scholar]
- Groudine M., Weintraub H. Propagation of globin DNAase I-hypersensitive sites in absence of factors required for induction: a possible mechanism for determination. Cell. 1982 Aug;30(1):131–139. doi: 10.1016/0092-8674(82)90019-8. [DOI] [PubMed] [Google Scholar]
- Hagiwara M., Pincus S. M., Chen I. L., Beckman B. S., Fisher J. W. Effects of dibutyryl adenosine 3',5'-cyclic monophosphate on erythropoietin production in human renal carcinoma cell cultures. Blood. 1985 Sep;66(3):714–717. [PubMed] [Google Scholar]
- Huang F. L., Cho-Chung Y. S. Hormone-regulated expression of cellular rasH oncogene in mammary carcinomas in rats. Biochem Biophys Res Commun. 1984 Aug 30;123(1):141–147. doi: 10.1016/0006-291x(84)90391-7. [DOI] [PubMed] [Google Scholar]
- Johnson G. S., Friedman R. M., Pastan I. Restoration of several morphological characteristics of normal fibroblasts in sarcoma cells treated with adenosine-3':5'-cyclic monphosphate and its derivatives. Proc Natl Acad Sci U S A. 1971 Feb;68(2):425–429. doi: 10.1073/pnas.68.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek L., Hyland J. K., Watt R., Rosenberg M., Baserga R. Microinjected c-myc as a competence factor. Science. 1985 Jun 14;228(4705):1313–1315. doi: 10.1126/science.4001943. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeffler H. P., Golde D. W. Humoral modulation of human acute myelogenous leukemia cell growth in vitro. Cancer Res. 1980 Jun;40(6):1858–1862. [PubMed] [Google Scholar]
- Kurland J. I., Bockman R. S., Broxmeyer H. E., Moore M. A. Limitation of excessive myelopoiesis by the intrinsic modulation of macrophage-derived prostaglandin E. Science. 1978 Feb 3;199(4328):552–555. doi: 10.1126/science.304600. [DOI] [PubMed] [Google Scholar]
- Linial M., Gunderson N., Groudine M. Enhanced transcription of c-myc in bursal lymphoma cells requires continuous protein synthesis. Science. 1985 Dec 6;230(4730):1126–1132. doi: 10.1126/science.2999973. [DOI] [PubMed] [Google Scholar]
- Matsui T., Nakao Y., Kobayashi N., Kishihara M., Ishizuka S., Watanabe S., Fujita T. Phenotypic differentiation-linked growth inhibition in human leukemia cells by active vitamin D3 analogues. Int J Cancer. 1984 Feb 15;33(2):193–202. doi: 10.1002/ijc.2910330207. [DOI] [PubMed] [Google Scholar]
- McCachren S. S., Jr, Nichols J., Kaufman R. E., Niedel J. E. Dibutyryl cyclic adenosine monophosphate reduces expression of c-myc during HL-60 differentiation. Blood. 1986 Aug;68(2):412–416. [PubMed] [Google Scholar]
- McGinnis W., Shermoen A. W., Heemskerk J., Beckendorf S. K. DNA sequence changes in an upstream DNase I-hypersensitive region are correlated with reduced gene expression. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1063–1067. doi: 10.1073/pnas.80.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch G. H., Rosenfeld M. G. Eukaryotic transcriptional regulation and chromatin-associated protein phosphorylation by cyclic AMP. Science. 1982 Dec 24;218(4579):1315–1317. doi: 10.1126/science.6293056. [DOI] [PubMed] [Google Scholar]
- Nagamine Y., Reich E. Gene expression and cAMP. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4606–4610. doi: 10.1073/pnas.82.14.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponte P., Gunning P., Blau H., Kedes L. Human actin genes are single copy for alpha-skeletal and alpha-cardiac actin but multicopy for beta- and gamma-cytoskeletal genes: 3' untranslated regions are isotype specific but are conserved in evolution. Mol Cell Biol. 1983 Oct;3(10):1783–1791. doi: 10.1128/mcb.3.10.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K. N., Hsie A. W. Morphologic differentiation of mouse neuroblastoma cells induced in vitro by dibutyryl adenosine 3':5'-cyclic monophosphate. Nat New Biol. 1971 Sep 29;233(39):141–142. doi: 10.1038/newbio233141a0. [DOI] [PubMed] [Google Scholar]
- Reitsma P. H., Rothberg P. G., Astrin S. M., Trial J., Bar-Shavit Z., Hall A., Teitelbaum S. L., Kahn A. J. Regulation of myc gene expression in HL-60 leukaemia cells by a vitamin D metabolite. Nature. 1983 Dec 1;306(5942):492–494. doi: 10.1038/306492a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schubach W., Groudine M. Alteration of c-myc chromatin structure by avian leukosis virus integration. Nature. 1984 Feb 23;307(5953):702–708. doi: 10.1038/307702a0. [DOI] [PubMed] [Google Scholar]
- Sheppard J. R. Restoration of contact-inhibited growth to transformed cells by dibutyryl adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1316–1320. doi: 10.1073/pnas.68.6.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Hennighausen L., Battey J., Leder P. Chromatin structure and protein binding in the putative regulatory region of the c-myc gene in Burkitt lymphoma. Cell. 1984 Jun;37(2):381–391. doi: 10.1016/0092-8674(84)90368-4. [DOI] [PubMed] [Google Scholar]
- Slungaard A., Confer D. L., Jacob H. S., Eaton J. W. Antineoplastic effects of Bordetella pertussis adenylate cyclase. Trans Assoc Am Physicians. 1983;96:401–405. [PubMed] [Google Scholar]
- Studzinski G. P., Brelvi Z. S., Feldman S. C., Watt R. A. Participation of c-myc protein in DNA synthesis of human cells. Science. 1986 Oct 24;234(4775):467–470. doi: 10.1126/science.3532322. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Vaulont S., Munnich A., Decaux J. F., Kahn A. Transcriptional and post-transcriptional regulation of L-type pyruvate kinase gene expression in rat liver. J Biol Chem. 1986 Jun 15;261(17):7621–7625. [PubMed] [Google Scholar]
- Westin E. H., Wong-Staal F., Gelmann E. P., Dalla-Favera R., Papas T. S., Lautenberger J. A., Eva A., Reddy E. P., Tronick S. R., Aaronson S. A. Expression of cellular homologues of retroviral onc genes in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2490–2494. doi: 10.1073/pnas.79.8.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]