Abstract
Rapid evolving cognitive impairment can be the onset of either a progressive or a treatable dementia. We describe a case of a man in whom the clinical and laboratory presentation suggested a diagnosis of Creutzfeldt-Jakob disease, which subsequently turned into a diagnosis of voltage-gated potassium channel complex antibodies limbic encephalitis. The natural history and an increased knowledge of limbic encephalitis and the related antibodies led us to the correct diagnosis.
Background
A clinical picture of ‘rapid evolving dementia’ prompts physicians to investigate potentially treatable neurological disorders.1 Diagnostic and therapeutic tools in this scenario are now under review.2 3 We describe the case of a 63-year-old man who experienced sudden onset of cognitive impairment and epileptic seizures. With laboratory support, a diagnosis of sporadic Creutzfeldt-Jakob disease (CJD) was established, but after 2 months a significant improvement was noted. The natural history ruled out a prion disease, so alternative diagnoses were investigated. In particular we searched for voltage-gated potassium channel (VGKC) complex antibodies limbic encephalitis, an autoimmune encephalitis associated with high concentrations of serum antibodies against components of VGKC complexes.4 Patients with disease typically present with acute/subacute onset of memory loss, confusion, seizures, sleep disturbances and psychiatric features, mimicking CJD.
Case presentation
A 63-year-old man presented with sudden onset of amnaesia with behavioural disturbances. He experienced episodes of agitation with verbal and physical aggressiveness, loss of spatial recognition and severe insomnia. Brain MRI showed mild brainstem and cerebellar atrophy, while blood flow single-photon emission CT disclosed a severe hypoperfusion of the bilateral frontal and parietal cortices consistent with a neurodegenerative disease.
We observed this patient for the first time about 45 days after clinical onset, when partial seizures started. His medical history was unremarkable. On admission, neurological examination revealed cognitive impairment with loss of recent memory and anosognosia. Sporadic myoclonic jerks and mild dysarthria were observed.
Investigations
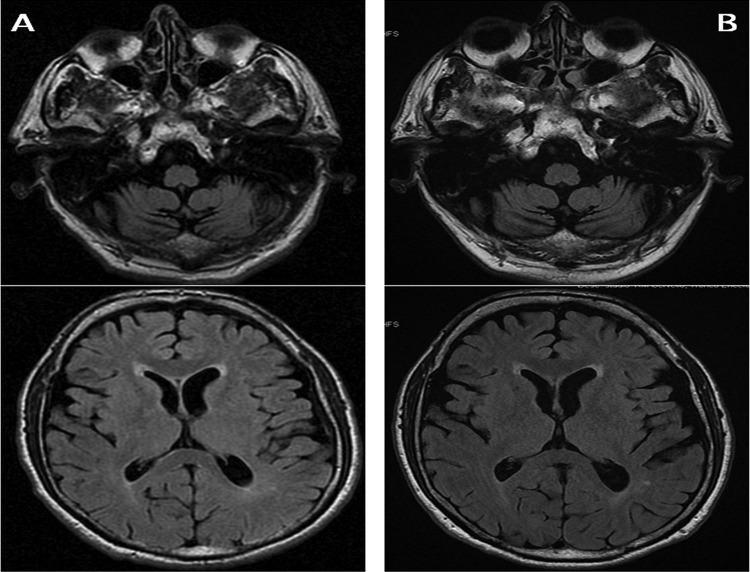
Extensive biochemical tests (including thyroid and liver function, autoimmune screening, syphilis, HIV) were unremarkable (only mild hyponatraemia 132 mEq/L). Neuropsychological examination revealed only memory difficulties as in amnaesic mild cognitive impairment. EEG showed no epileptic or periodic spikes and electroneurography and electromyography were normal. Autoimmune encephalitis was suspected and all available antibodies against neuronal cells (antibodies against Purkinje cells, neuronal nuclei, anti-Ri, anti-Hu, anti-Yo, antiamphiphysin, anti-CV2, anti-Ma) were tested with negative results. Lumbar puncture revealed elevated protein (57 mg/dL, normal value <50) with normal cell count and no viral agents. Analysis of neurodegenerative proteins disclosed an accumulation of protein 14-3-3 in the cerebrospinal fluid (CSF) with markedly elevated total τ protein >1300 pg/mL (normal value 171±127). Repeated brain MRI (figure 1A), including H+ spectroscopy, confirmed mild brainstem and cerebellar atrophy but no thalamic or metabolic abnormalities.
Figure 1.
Evidence of mild brainstem and cerebellar atrophy at disease onset (A), not typical of Creutzfeldt-Jakob disease, with no change after 5 years (B).
Differential diagnosis
According to the revised diagnostic criteria,5 a diagnosis of ‘probable CJD’ was established and no aetiopathogenetic therapy was prescribed (only antiepileptic drugs).
Outcome and follow-up
Surprisingly, 2 months later a significant improvement was noted with almost full recovery. Relatives reported only a tendency to irritability and hyperphagia (about 8 kg body weight gain) disappearing in a few months. The patient's natural history suggested an alternative diagnosis, but he did not attend follow-up visits and imaging because of his supposed complete recovery.
Five years later a second episode of confusion and memory loss occurred. The patient did not recognise well-known places, people and situations. This episode lasted for 72 h again with a full recovery. EEG and brain MRI were unremarkable (figure 1B). At this time a serological assay for limbic encephalitis, that is, N-methyl-d-aspartate and VGKC receptor antibodies, was performed, revealing a VGKC antibody titre of 358 pM. In addition, ‘real-time quaking-induced conversion (RT-QuIC)’, a new test designated to detect the abnormal form of prion protein,6 on a CSF sample stored up during the first clinical episode was performed, with negative result. A ‘correct diagnosis’ of anti-VGKC antibody limbic encephalitis was finally established but no therapy was started because of the absence of neurological symptoms. A total body CT scan failed to disclose occult malignancy and the patient is currently waiting for a positron emission tomography scan.
Discussion
Many variables should be considered in a diagnostic process. The clinical presentation of our patient with psychiatric and cognitive disturbance, epileptic seizures and mild cerebellar signs along with the evidence of CSF accumulation of 14-3-3 and τ proteins was consistent with CJD.5 By contrast, the natural history and an increased knowledge of limbic encephalitis, and the related antibodies led us to the correct diagnosis.4 7 Also, hyponatraemia could suggest an autoimmune basis for neurological symptoms.8
We emphasise two aspects, one related to diagnosis and the other to therapy. Diagnosis of prion disease is based on clinical and paraclinical tests. Clinical features include ‘short-term onset of cortical/subcortical signs’. Paraclinical findings could be obtained by EEG, MRI and CSF examination.5 EEG is positive in only some CJD subtypes or in the terminal stages of illness. Typical MRI lesions (hyperintensity on fluid-attenuated inversion recovery or diffusion-weighted imaging in at least two cortical regions) have an increasing role as a potential biomarker for CJD. The role of CSF proteins is now under review. Since its first clinical applications over 15 years ago, the diagnostic utility of CSF 14-3-3 has been questioned: its sensitivity is higher (85–90%) than its specificity (<80%),2 leading to the possibility of false-positive results. Combination with other neurodegenerative-related proteins (ie τ or S100b) increases the positive predictive value,2 9 but an alternative potentially treatable disease cannot be excluded,1 as in our case.
The therapy for VGKC complex encephalitis, like other central nervous system (CNS) neuronal surface antibody-associated syndromes, is not evidence based.10 In paraneoplastic syndromes, neoplasm recognition and removal is the best therapy. In non-paraneoplastic conditions or while tumour screening is underway, immunotherapy (corticosteroids, immunoglobulins or plasma exchange) should be tried, but responses can be absent or slow. For non-responders or when a relapse occurs, even second-line chronic immunotherapy (rituximab, cyclophosphamide) may be unsuccessful.
Moreover, the history of patients with a full recovery without treatment, as in our case, suggests that recovery may be spontaneous and therapy may not be necessary.
In conclusion, in ‘progressive dementia’, an association between serological antibodies for ‘limbic encephalitis’ and CSF neurodegenerative proteins has been described in both directions (ie, protein 14-3-3 in CSF of patients with VGKC complex autoimmune encephalitis and VGKC antibodies in sera of patients with CJD),8 11 but only the clinical course is highly specific in this scenario. The utility of therapy in autoimmune CNS syndromes should be confirmed by ad hoc scientific trials.
Learning points.
Cerebrospinal fluid (CSF) 14-3-3 has higher sensitivity than specificity, leading to the possibility of false-positive results.
Protein 14-3-3 may be found in CSF of patients with voltage-gated potassium channel (VGKC) complex autoimmune encephalitis.
VGKC antibodies may be found in sera of patients with Creutzfeldt-Jakob disease.
Hyponatraemia is a diagnostic clue favouring an autoimmune basis for neurological disease.
The utility of therapy in autoimmune encephalitis should be validated.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Chitravas N, Jung RS, Kofskey DM et al. Treatable neurological disorders misdiagnosed as Creutzfeldt-Jakob disease. Ann Neurol 2011;70: 437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chohan G, Pennington C, Mackenzie JM et al. The role of cerebrospinal fluid 14-3-3 and other proteins in the diagnosis of sporadic Creutzfeldt-Jakob disease in the UK: a 10 year review. JNNP 2010;81:1243–8. [DOI] [PubMed] [Google Scholar]
- 3.Stoeck K, Sanchez-Juan P, Gawinwcka J et al. Cerebrospinal fluid biomarker supported diagnosis of Creutzfeldt-Jakob disease and rapid dementias: a longitudinal multicentre study over 10 years. Brain 2012;135:3051–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vincent A, Bien CG, Irani SR et al. Autoantibodies associated with disease of the CNS: new developments and future challenges. Lancet Neurol 2011;10:759–72. [DOI] [PubMed] [Google Scholar]
- 5.Zerr I, Kallenberg K, Summers DM et al. Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain 2009;132:2659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGuire LI, Peden AH, Orrú CD et al. Real time quaking-induced conversion analysis of cerebrospinal fluid in sporadic Creutzfeldt-Jakob disease. Ann Neurol 2012;72:278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paterson RW, Zandi MS, Armstrong R et al. Clinical relevance of positive voltage-gated potassium channel (VGKC)-complex antibodies: experience from a tertiary referral centre. JNNP 2014;85:625–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geshwind MD, Tan KM, Lennon VA et al. Voltage-gated potassium channel autoimmunity mimicking Creutzfeldt-Jakob disease. Arch Neurol 2008;65:1341–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parchi P, Capellari S. Diagnostic value of cerebrospinal fluid markers. Nat Rev Neurol 2013;9:10–11. [DOI] [PubMed] [Google Scholar]
- 10.Zuliani L, Graus F, Giometto B et al. Central nervous system neuronal surface antibody associated syndromes: review and guidelines for recognition. JNNP 2012;83:638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujita K, Yuasa T, Watanabe O et al. Voltage-gated potassium channel complex antibodies in Creutzfeldt-Jakob disease. J Neurol 2012;259:2249–50. [DOI] [PubMed] [Google Scholar]