Abstract
Cladophialophora bantiana, a dematiaceous fungus from the family Phaeohyphomycetes, is highly neurotropic and primarily reported as a rare cause of brain abscess. Pulmonary infection and disease outside the central nervous system is extremely rare, particularly in immunocompetent patients. We report an unusual case of disseminated cladosporiosis in a young man with a new diagnosis of prediabetes but no other identifiable risk factors for disease. Fungal cultures were positive for C. bantiana from brain abscess aspiration, vertebral bone cultures and subcarinal lymph node biopsy. Although the patient demonstrated initial good response to surgical debridement of brain abscesses plus antifungal therapy, he eventually expired from septic shock secondary to C. bantiana pneumonia and recurrent brain abscesses 2 years after initial diagnosis.
Background
Phaeohyphomycosis refers to infections caused by dematiaceous or pigmented fungi. These fungi have a worldwide distribution and can be found in soil and rotten organic material. The finding of dematiaceous fungi in cultures is often initially considered a contaminant, and this can result in delay in identification of a life-threatening disease with extremely high mortality.1 Additionally, infection by dematiaceous fungi is often thought to be opportunistic, but Cladophialophora bantiana has been shown to behave as a pathogen in immunosuppressed and immunocompetent individuals.1–3 Reported risk factors include underlying malignancy (lymphoma with neutropenia), organ transplant with immunosuppressant therapy, HIV infection and intravenous drug use, glucocorticoid or other immunosuppressant drug therapies and diabetes.1–4 Infection related to prediabetes is not reported. C. bantiana is the most common cause of central nervous system (CNS) infections caused by dematiaceous fungi, generally presenting as brain abscess, and in fewer cases, as meningitis or myelitis.5–8 This case outlines an extremely rare presentation of disseminated disease due to C. bantiana, with infection beyond the CNS, including pulmonary and bone involvement. Unlike prior reports, this individual reported no predisposing illnesses or immunocompromised state, and was taking no medications that have been linked to C. bantiana infection, though he was diagnosed with prediabetes on initial hospital admission.
Case presentation
A young Sudanese man was admitted with new onset of a generalised seizure, pleuritic chest pain and mild haemoptysis. He reported malaise for several months but specifically denied fever, chills, night sweats or weight changes. Additionally, he denied recent sick contacts and reported no recent travel outside of the USA. He denied history of medical illnesses, was on no medications, and denied recent trauma or tuberculosis exposure. His family history was non-contributory and social history revealed that he was single and living with two friends after emigrating from Sudan 5 years ago. He admitted to occasional alcohol and marijuana use, but denied intravenous or recreational drug use. He was a smoker with a 10–20 pack-year smoking history. On admission, he was lethargic but arousable and able to answer questions. He was tachycardic at 110 bpm, had a temperature of 101.5°F, with normal respirations (97% room air) and blood pressure. Cardiovascular, pulmonary and abdominal examinations were normal at admission. Neurological deficits were absent, but two large 4–5 cm ulcerated lesions were noted on the left posterior thigh.
Investigations
Blood chemistries found mild hyponatremia 129 mg/dL, elevated random blood glucose 157 mg/dL and normal kidney function. Subsequent fasting blood glucose levels ranged between 101 and 118 mg/dL with glycated haemoglobin of 6.1%, consistent with prediabetes. Anaemia with leukocytosis was present with haemoglobin 10.9 g/dL, mean corpuscular volume 77.7 fL, mean corpuscular haemoglobin concentration 33.3 g/dL, white cell count (WCC) 16.91 k/µL, neutrophils 13.5 k/µL and platelet count of 302 k/µL. Liver transaminases and function studies were normal except for a slightly elevated alkaline phosphatase at 172 U/L (normal range 40–140 U/L); remainder of liver tests revealed an aspartate transaminase 33 U/L, alanine transaminase 29 U/L, albumin 3.2 g/dL, total protein 7.7 g/dL and total bilirubin 0.5 mg/dL. HIV testing was negative and CD4 count was normal.
A chest X-ray demonstrated an ill-defined opacity at the left lung base and subsequent CT of the chest with contrast showed a T8 compression fracture, scattered pulmonary nodules and left lower lobar consolidation (figures 1 and 2). An MRI of the thoracic spine was consistent with osteomyelitis and loss of height of the T8 vertebral body (figure 3).
Figure 1.
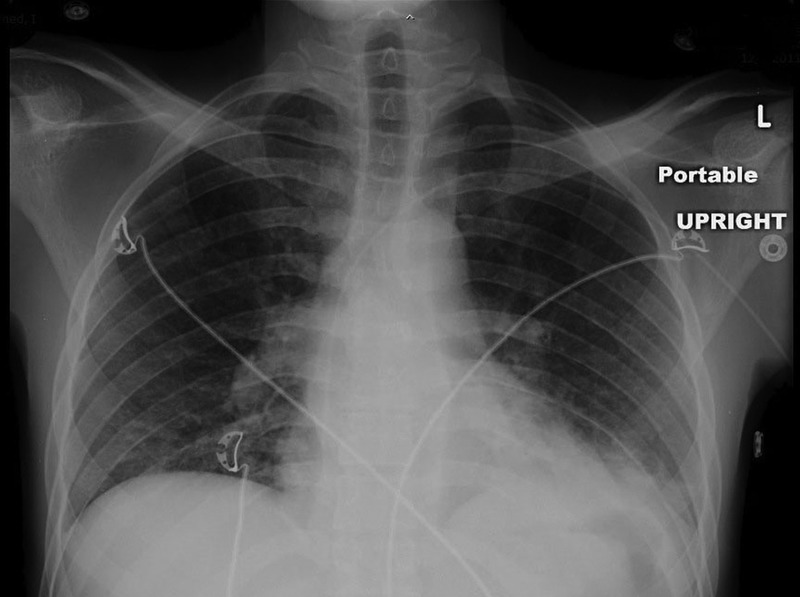
Chest X-ray revealing left lower lobe lung infiltrates; cardiomediastinal silhouette and pulmonary vasculature appear normal.
Figure 2.
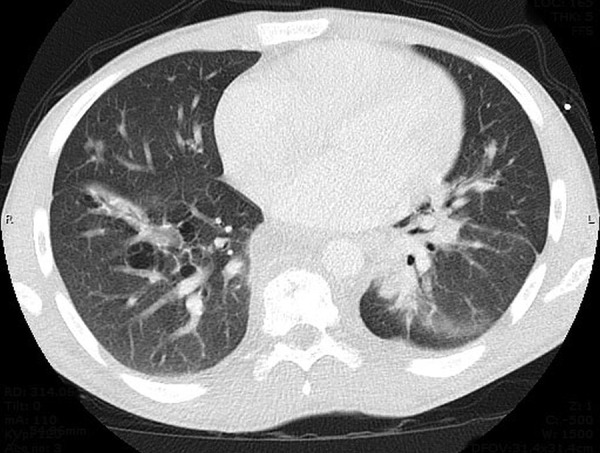
CT of the chest with contrast demonstrating dominant dense areas of consolidation involving left lower lobe with small patchy airspace disease involving right lower lobe.
Figure 3.
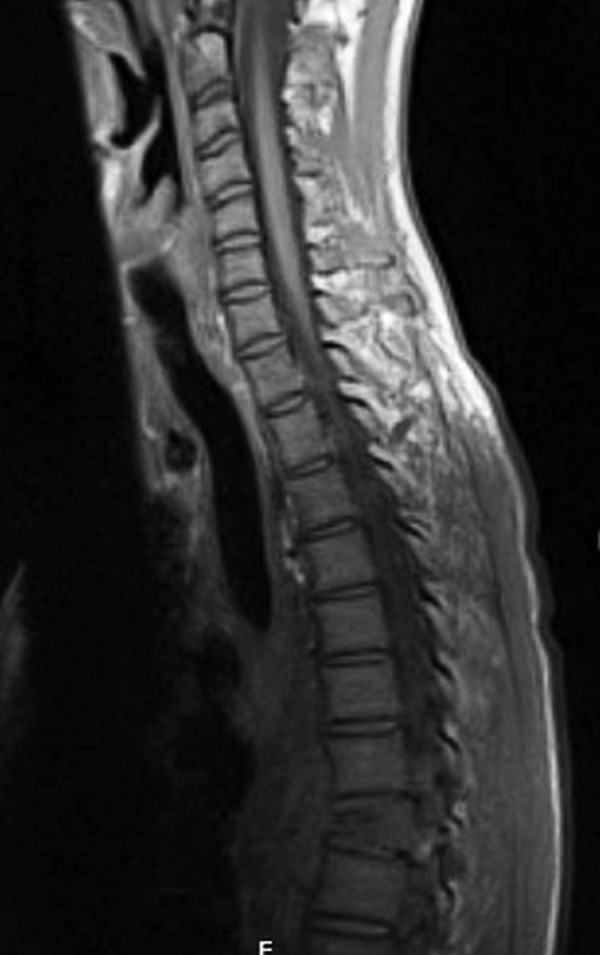
MRI of the spine with and without contrast revealing evidence of height collapse and compression fracture in the T8 vertebral body and appearance consistent with osteomyelitis.
Admission brain MRI showed bifocal hypodensities in the left frontal lobe indicative of old infarction versus infection and numerous ring-enhancing lesions in the brain parenchyma and leptomeninges (figures 4 and 5). The dominant lesion in the superior aspect of the left frontal lobe had surrounding vasogenic oedema and mild mass effect (figure 6).
Figure 4.
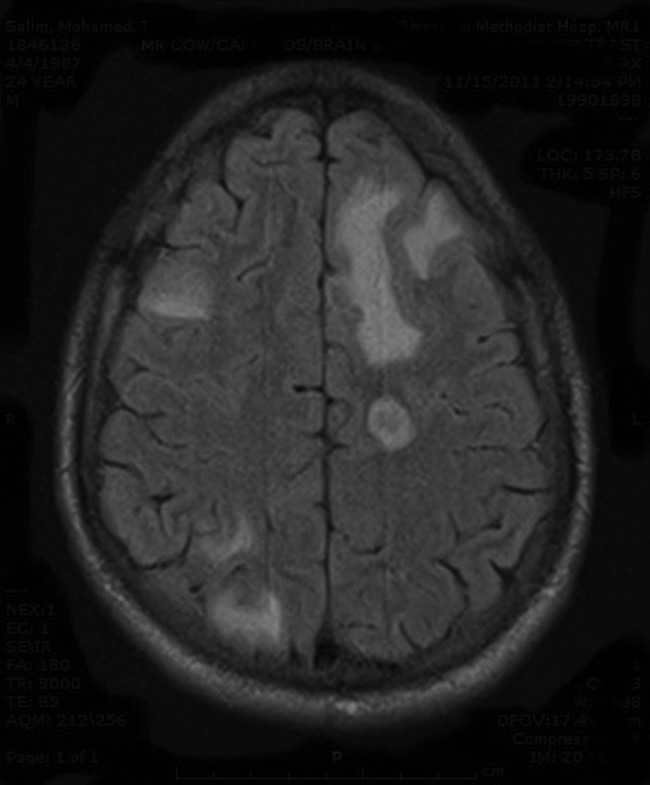
MRI of the head with and without contrast: multifocal areas of low attenuation in left frontal lobe.
Figure 5.
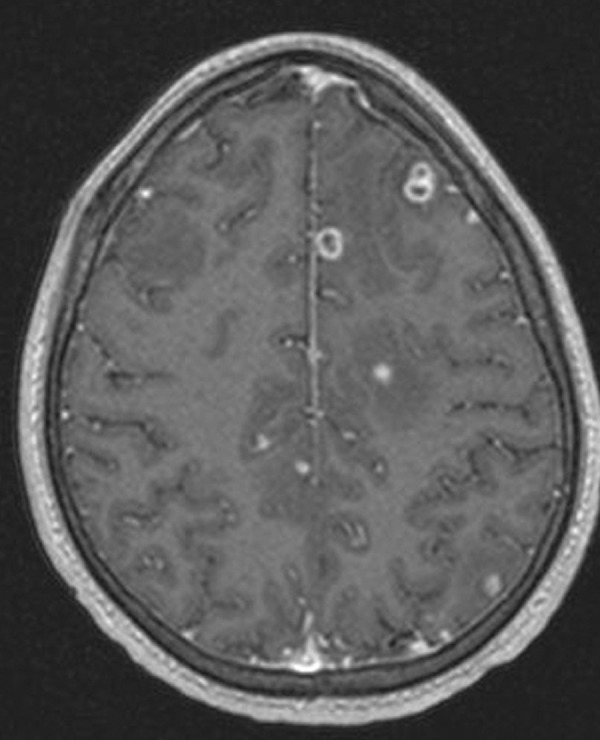
MRI of the head with and without contrast: numerous ring-enhancing lesions involving the brain parenchyma as well as the leptomeningeal surfaces. A cluster of these lesions are seen superiorly and inferiorly in the left frontal lobe.
Figure 6.
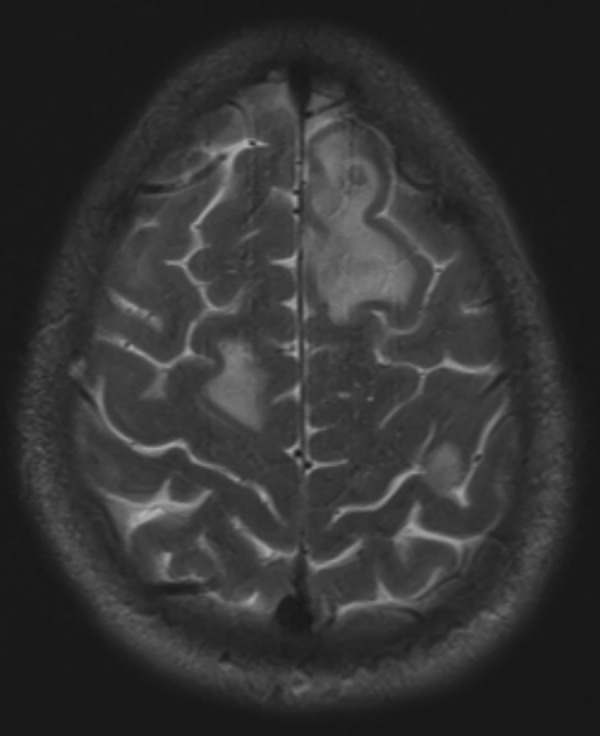
MRI of the head with and without contrast: dominant lesion in the superior aspect of the left frontal lobe had surrounding vasogenic oedema and mild mass effect.
Differential diagnosis
The patient was admitted with an initial diagnosis of pneumonia and new onset seizure after noting abnormal findings on chest X-ray and CT scan suspicious for infection. Acyclovir was started for possible herpes encephalitis and empiric moxifloxacin was initiated for pneumonia, awaiting cultures. Following the MRI and demonstration of numerous ring-enhancing lesions, other diagnoses were entertained. Differential diagnosis included malignancy, Mycobacterium tuberculosis, Cryptococcus neoformans, Candida, Aspergillus, toxoplasmosis and zygomycetes infection. Diagnostic testing included blood, cerebrospinal fluid, sputum, nares and urine cultures; all were negative. Additionally, cryptococcal antigen, purified protein derivative (PPD) and M. tuberculosis Quantiferon tests returned negative. Infectious disease consultants remained suspicious of active pulmonary M. tuberculosis with tuberculous brain abscess; thus, moxifloxacin and acyclovir were discontinued and four-drug antituberculous therapy was started. Pulmonary services were consulted for bronchoscopy and transbronchial needle aspiration; a subcarinal lymph node was sampled and pathology was consistent with necrotising granuloma, which was culture positive for Cladosporium spp. Subsequent biopsy of the brain hypodensities and spine grew C. bantiana; swabs of the skin ulcers were performed as well, but were negative for C. bantiana.
Treatment
Treatment included surgical as well as medical approach. The patient underwent spinal surgery with debridement of abscess, corpectomy and fusion fixation. Additionally he required debridement of multiple brain abscesses twice during initial hospitalisation. He was treated with combination of intravenous amphotericin for 7 weeks, flucytosine for 4 weeks, and was eventually discharged on long-term oral voriconazole as chemoprophylaxis against recurrent disease as recommended by infectious disease consultants. Repeat brain MRI at 4 months showed resolution of all brain abscesses (figure 7). Unfortunately, he then left for Sudan where he stayed for 5 months. While there, he was completely off antifungals and on return to the USA 5 months later, he was admitted with cough, chest pain and finger pain. Chest CT scan showed right lower lobe consolidation with cavitary lesions, thoracic and upper abdominal lymphadenopathy (figure 8). Bronchoscopy was again performed and was negative for acid-fast bacilli. Flucytosine and voriconazole were restarted, but at 2 months, abnormal liver transaminases and alkaline phosphatase (1750 U/L) were noted, thought related to azole treatment. He was admitted to an extended care facility to complete 6 weeks of therapy with intravenous amphotericin and flucytosine, and was then changed to oral posaconazole.
Figure 7.
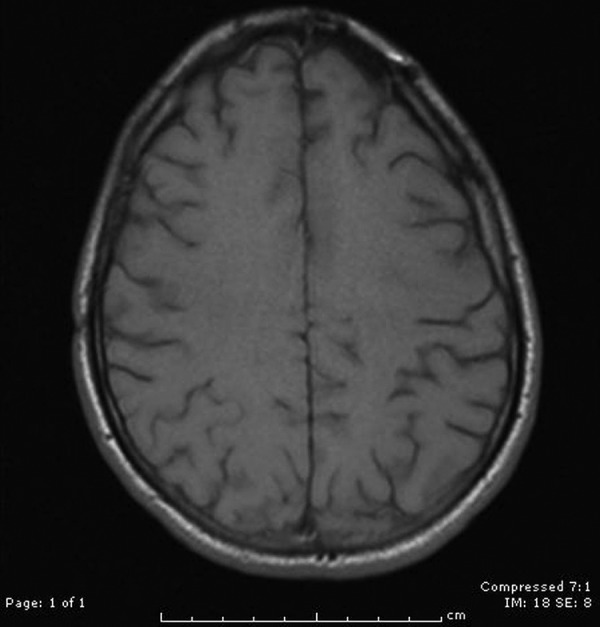
MRI of the head with and without contrast revealing resolution of the numerous ring-enhancing lesions, which were present during initial admission.
Figure 8.
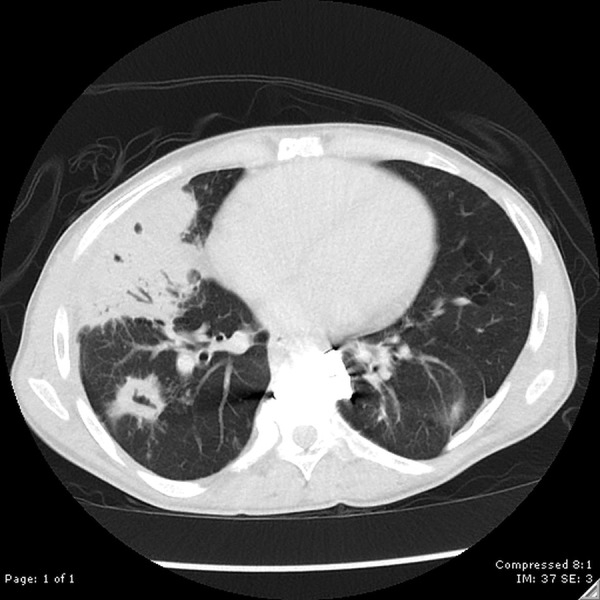
CT of the chest revealing dense right middle lobe consolidation with subtle internal necrosis as well as multiple cavitary nodular consolidations. Differential diagnosis includes an atypical infectious process, such as tuberculosis or fungal infection.
Outcome and follow-up
The patient remained compliant with treatment for the next 14 months and during that time, brain MRI showed no abscess. However, 2 years after initial diagnosis he presented with fever 102.9°F, headache and poor appetite. On examination, he was hypotensive with blood pressure 95/60 mm Hg and tachypnaeic at 22 breaths/min. Laboratory studies showed elevated WCC at 18 k/µL with a neutrophil predominance, but blood, urine and sputum cultures were negative. Repeat cryptococcal antigen, HIV, PPD and M. tuberculosis Quantiferon testing were also negative. Chest CT scan revealed consolidation in right and left lower lobes and new lesions were noted on MRI of the brain. There was a small 4 mm ring-enhancing lesion in the right frontal lobe and a new lesion in the body of the corpus callosum (figure 9). Despite aggressive care and treatment with amphotericin and flucytosine, the patient developed septic shock with respiratory failure and soon expired.
Figure 9.
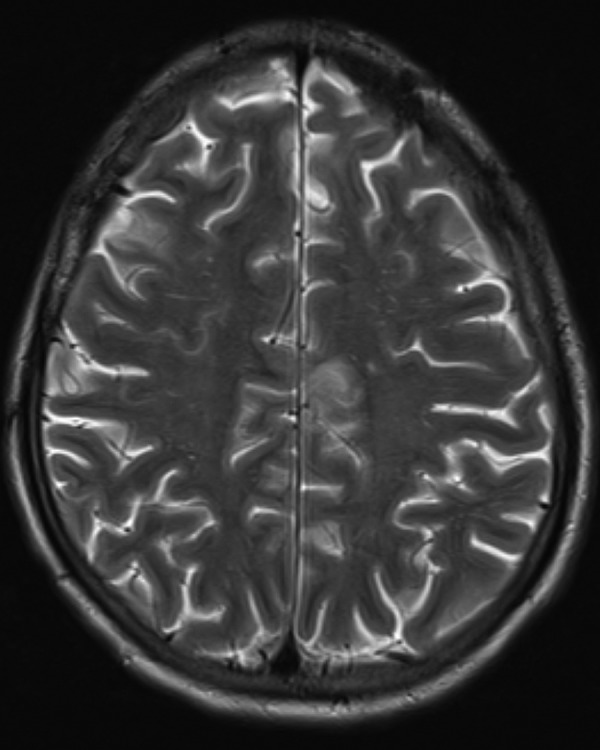
MRI of the head with and without contrast showing a small 4 mm ring-enhancing lesion in the right frontal lobe.
Discussion
C. bantiana infection is regarded as the most common cause of CNS infections caused by the dematiaceous pigmented fungi. In a review by Revankar et al,1 C. bantiana accounted for 48% of 101 cases of primary CNS phaeohyphomycosis. Slightly more than half of the patients with CNS-limited disease had no underlying disease or risk factor for infection, and diabetes was reported as a risk factor in only one patient.1 There appeared to be equal incidence between immunosuppressed and immunocompetent hosts, with male predominance.1 Risk factors include organ transplant, lymphoma, penetrating eye trauma, intravenous drug use, diabetes mellitus, HIV infection and immunosuppressant drug therapy (glucocorticoids and azathioprine).1–4
Diagnosis of C. bantiana infection is based on culture and demonstration of brown irregular hyphae under microscopic examination (figures 10 and 11). Suggested modes of infection include air-borne, haematogenous, lymphatic and possibly direct inoculation.
Figure 10.
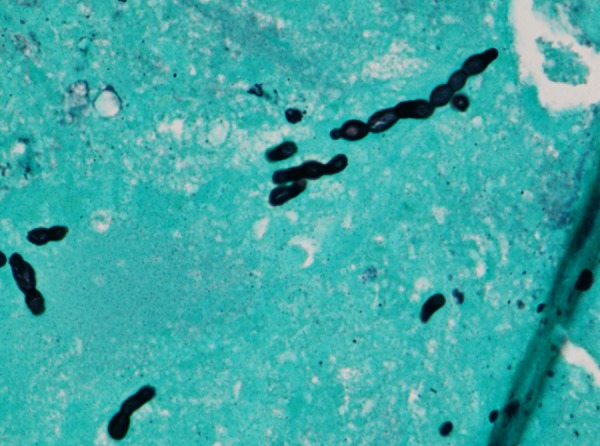
Cladophialophora bantiana organism (Silver stain; magnification ×600).
Figure 11.
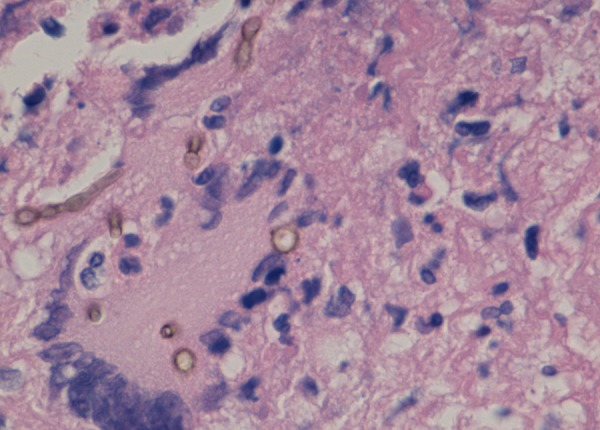
Cladophialophora bantiana organism demonstrated in brain abscess (magnification ×400).
Brain abscess is the most common clinical manifestation of C. bantiana infection, though meningitis and myelitis have been reported.4–7 Skin and subcutaneous tissue involvement are reported in the medical literature with evidence of C. bantiana localised infection caused by direct inoculation via trauma or penetrating wounds.9–13 Skin manifestations have been described in immunocompetent as well as immunosuppressed individuals. Although disseminated disease has been described in murine cases, disease outside the CNS is extremely rare in humans, with only few case reports. A literature search found five documented cases of disseminated infection, with four of these in immunosuppressed individuals. Two cases were reported in transplant recipients of solid organs, a third case occurred in a patient with idiopathic thrombocytopenic purpura on long-term corticosteroid therapy, the fourth case of pulmonary infiltrates and sputum isolation of C. bantiana was described in a patient with AIDS, and a fifth case of C. bantiana septic arthritis occurred in a male patient treated for sarcoidosis with corticosteroids.14–18 Prediabetes has not been described as a risk factor for disseminated C. bantiana infection.
Evidence-based treatment recommendations for limited as well as disseminated C. bantiana disease are lacking secondary to the extreme rarity of this type of infection. Most reports describe treatment of CNS-limited disease, but the exact role of surgery, best antifungal treatment and duration of therapy is not clearly established. In non-disseminated CNS infection the literature suggests that an aggressive approach combining surgical and antifungal treatment is warranted secondary to high mortality rates. Complete excision of brain abscesses appears to be associated with better outcomes than simple aspiration or partial excision.1 Historically, amphotericin B is the most commonly used antifungal agent, generally in combination with flucytosine, itraconazole or one of the newer azoles.1 4 Voriconazole, in particular, has good brain-barrier penetration, and has been increasingly used as a combination therapy with amphotericin B or as a solitary agent during remission.1 4 Despite treatment, mortality rates still approach 70%.1 Recent research for treatment of disseminated C. bantiana disease has occurred in murine models and combination therapy with posaconazole, micafungin and flucytosine for at least 30 days improves survival, though high mortality persists.19 In the few reported cases of disseminated disease in humans, multidrug therapy with amphotericin B, flucytosine and azole therapy have been used, similar to treatment of CNS-limited disease. Use of terbinafine, voriconazole and posaconazole have shown promise for treatment in disseminated disease from other species of dematiaceous fungi, and it has been suggested that long-term chemoprophylaxis may benefit patients at high risk.3
Learning points.
Phaeohyphyomycosis occurs secondary to dematiaceous or pigmented fungi, which comprise more than 100 species, and are found worldwide in rotten organic material and soil.
Although the majority of infections from phaeohyphomycotic organisms are considered opportunistic, Cladophialophora bantiana is a highly neurotropic pigmented fungus that is currently regarded as a pathogenic organism, accounting for the majority of central nervous system infections caused by pigmented fungi.
C. bantiana most often presents as brain abscess, and disseminated disease with pulmonary involvement is extremely rare, primarily reported in immunosuppressed patients.
A combined surgical and medical approach is considered best treatment, including abscess debridement and antifungal therapy with amphotericin and flucytosine, followed by long-term therapy with voriconazole or one of the newer azoles in attempt to chronically suppress fungal infection.
Despite therapy, mortality remains high, approaching 70%.
Footnotes
Contributors: AM was involved in case identification, literature search, writing of the case report, and is the guarantor. KJ contributed significantly to the discussion, editing and final proofing of the article.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Revankar SG, Sutton DA, Rinaldi MG. Primary central nervous system phaeohyphomycosis: a review of 101 cases. Clin Infect Dis 2004;38;206–16. [DOI] [PubMed] [Google Scholar]
- 2.Davis LE. Fungal infections of the central nervous system. Neurol Clin 1999;17:761–81. [DOI] [PubMed] [Google Scholar]
- 3.Revankar SG, Patterson JE, Sutton DA et al. . Disseminated phaeohyphomycosis: review of an emerging mycosis. Clin Infect Dis 2002;34:467–76. [DOI] [PubMed] [Google Scholar]
- 4.Levin TP, Baty DE, Fekete T et al. . Cladophialophora bantiana brain abscess in a solid-organ transplant recipient: case report and review of literature. J Clin Microbiol 42:4374–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shield GS, Castillo M. Myelitis caused by Cladophialophora bantiana. AJR Am J Roentgenol 2002;179:278–9. [DOI] [PubMed] [Google Scholar]
- 6.Deb S, Khan AK, Debasish B et al. . Intracranial necrotizing granuloma caused by Cladophialophora bantiana. Neurol India 2005;53:335–6. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Exophiala infection from contaminated injectable steroid preparation prepared by compound pharmacy—United States. JAMA 2003;289:291–3. [PubMed] [Google Scholar]
- 8.Naim UR, El Sheikh M, Abu Aisha H et al. . Cerebral phaeohyphomycosis. Bull Soc Pathol Exot Filiales 1987;80:320–8. [PubMed] [Google Scholar]
- 9.Pincus LB, Schwartz BS, Cunnungham G et al. . Cutaneous phaeohyphomycosis caused by Cladophialophora bantiana in a scar after treatment with intralesional corticosteroid injections. J Am Acad Dermatol 2009;61:537–8. [DOI] [PubMed] [Google Scholar]
- 10.Bonifaz A, De Hoog S, McGinnis MR et al. . Eumycetoma caused by Cladophialophora bantiana successfully treated with itraconazole. Med Mycol 2009;47:111–14. [DOI] [PubMed] [Google Scholar]
- 11.Applegren P, Farnebo F, Dotevall L et al. . Late-onset posttraumatic skin and soft-tissue infections caused by rapid-growing mycobacteria in tsunami survivors. Clin Infect Dis 2008;47:e11–16. [DOI] [PubMed] [Google Scholar]
- 12.Neoh CY, Tan SH, Perera P. Cutaneous phaeohyphomycosis due to Cladophialophora bantiana in an immunocompetent patient. Clin Exp Dermatol 2007;32:539–40. [DOI] [PubMed] [Google Scholar]
- 13.Petrini B, Farnebo F, Hedblad MA et al. . Concomitant late soft tissue infections by Cladophialophora bantiana and Mycobacterium abscessus following tsunami injuries. Med Mycol 2006;44:189–92. [DOI] [PubMed] [Google Scholar]
- 14.Keyser A, Schmid FX, Linde HJ et al. . Disseminated Cladophialophora bantiana infection in a heart transplant patient. J Heart Lung Transplant 2002;21:503–5. [DOI] [PubMed] [Google Scholar]
- 15.Brenner SA, Morgan J, Rickert PD et al. . Cladophialophora bantiana isolated from an AIDS patient with pulmonary infiltrates. J Med Vet Mycol 1996;34:427–9. [DOI] [PubMed] [Google Scholar]
- 16.Rattanaumpawan P, Tantimavanisch S, Tiengrim S et al. . Disseminated Cladophialophora bantiana infection in an idiopathic thrombocytopenic purpura patient; a case report. Mycoses 2011;54:544–8. [DOI] [PubMed] [Google Scholar]
- 17.Singh N, Chang FY, Gayowski T et al. . Infections due to dematiaceous fungi in organ transplant recipients: case report and review. Clin Infect Dis 1997;24:369–74. [DOI] [PubMed] [Google Scholar]
- 18.Lim A, Speers D, Inderjeeth C. Cladophialophora (Xylohypha) bantiana—an unusual cause of septic arthritis. Rheumatology (Oxford) 2013;52:958–9. [DOI] [PubMed] [Google Scholar]
- 19.Mariné M, Pastor FJ, Guarro J. Combined antifungal therapy in a murine model of disseminated infection by Cladophialophora bantiana. Med Mycol 2009;47:45–9. [DOI] [PubMed] [Google Scholar]


