Abstract
We present a case report of a 41-year-old woman of Malay ethnicity who presented with an 11-day history of fever and left-sided lymphadenopathy after consuming ‘Miracle Mineral Solution’ (sodium chlorite solution) for the first time. A diagnosis of Kikuchi-Fujimoto disease was established via lymph node biopsy after other differentials were excluded. The aetiology of Kikuchi-Fujimoto disease remains controversial, but viral, autoimmune and physicochemical causes have been suggested as possibilities. In this case, we hypothesise that oxidative injury from sodium chlorite initiated an inflammatory response, which triggered the onset of Kikuchi-Fujimoto disease.
Background
Kikuchi-Fujimoto disease (KFD) or histiocytic necrotising lymphadenitis is a rare condition that usually presents with self-limiting fever and tender lymphadenopathy. It was first described in Japan by Kikuchi and Fujimoto independently in 1972.1 The pathogenesis of KFD is still unclear, but autoimmune, viral, bacterial, parasitic and even physicochemical causes have been suggested. There have been only a handful of case reports of KFD occurring after a physical trigger, such as breast implants,2 pacemaker insertion,3 or gastric bypass surgery.4
‘Miracle Mineral Solution’ is a 28% sodium chlorite solution. It has been sold under the pseudonyms ‘Miracle Mineral Supplement’, ‘Master Mineral Solution’ and ‘Chlorine Dioxide Solution’.5 Its creator's instructions suggest mixing three drops of sodium chlorite solution with an acidic solution, such as lemon juice, which it reacts with to form chlorine dioxide.6
It is touted by its distributors and creator to cure malaria, AIDS, viral hepatitis and even cancer, without harming human cells. Its purported mechanism of action is via oxidation, in a manner similar to how chlorine dioxide is used for water disinfectant ex vivo. There is, however, no evidence published in any peer-reviewed journal to support the claims in an in vivo model. On the contrary, several government agencies in the USA,7 Canada8 and the UK,5 warn of side effects of nausea, severe vomiting, diarrhoea, dehydration and hypotension. Its sale is banned in those countries.
Case presentation
A 41-year-old woman of Malay ethnicity presented with an 11-day history of fever and left-sided lymphadenopathy. Her fever spiked to 40°C, was constant throughout the day and was only relieved by paracetamol. Her fever was associated with chills, rigors and a dry cough. She did not have any rash, neck stiffness, blurring of vision, sore throat, abdominal pain, diarrhoea, vomiting, dysuria or vaginal discharge.
She recently started work in a health science laboratory 1 month before presentation, where she was undergoing training for inspecting bovine heart valves. She wore personal protective equipment and did not come into contact with any live specimens. She had no recent travel, no sick contacts, no recent contact with live pets or birds, and no raw or unpasteurised food intake.
Her medical history included hepatitis B carrier and dengue fever.
One day prior to the onset of fever, she drank a glassful of ‘Miracle Mineral Solution’ diluted in water. It was prepared for her by a relative visiting from New Zealand, and she was uncertain how many drops of solution were used. She described the odour as smelling like household bleach.
Three days after the onset of fever, she saw a general practitioner, and was treated with oral antibiotics and paracetamol. The patient could not remember the name of the antibiotic. Six days after the onset, she reconsulted with her general practitioner because of persistent symptoms, and was prescribed a course of oral cefuroxime. Nine days after the onset, she visited the accident and emergency department where she was given another course of oral amoxicillin-clavulanate. The working diagnosis given by the general practitioner and emergency physician was of upper respiratory tract infection. She was admitted 11 days after onset of symptoms of fever for investigation.
On physical examination she was oriented and did not appear toxic or dehydrated. She had a temperature of 40°C, her blood pressure was 121/57 mm Hg, and she was tachycardic with a rate of 101 bpm and oxygen saturation of 97% on room air.
She had several palpable cervical lymph nodes along the jugulodigastric chain (level II) and posterior triangle of the neck (level V), the largest of which was 2×2 cm. Examination of the oral cavity showed no oral ulcers and no signs of pharyngeal or tonsil inflammation. Examination of heart, lungs and abdomen were unremarkable. She did not have any skin rash.
Investigations
Laboratory investigations showed raised acute phase inflammatory makers. C reactive protein was 89.2 mg/L, procalcitonin 0.51 μg/L and ferritin 494.3 μg/L. The patient's total white count was not elevated at 4.2×103/μL.
Serology was negative for antibodies to cytomegalovirus (IgM), Epstein-Barr virus (EBV) capsid antigen (IgM), HIV antibody, dengue (IgM, IgG), Brucella, Coxiella burnetti and Leptospira (IgM). Antinuclear antibody and anti-double-stranded DNA antibodies were negative. Peripheral blood film showed no malaria parasites.
Urine analysis was not suggestive of urinary infection. Blood and urine cultures yielded no growth.
CT showed left-sided level II, III, IV and V cervical lymphadenopathy with perinodal fat stranding detected (figure 1). The largest lymph node measured 1.8 by 1.5 cm in the left retromandibular area. There were no thoracic, abdominal or pelvic sources of infection or lymphadenopathy.
Figure 1.
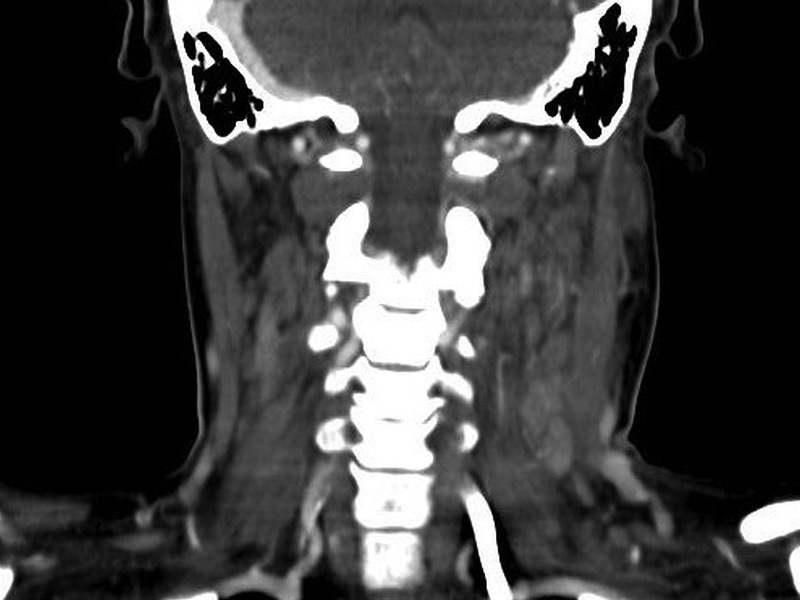
CT image of the cervical spine showing left-sided level II, III, IV and V cervical lymphadenopathy.
Fine needle aspiration of the lymph node was performed. Cytology showed necrotic tissue with no malignant cells. Culture of the fluid yielded no growth. Tuberculosis (TB) PCR of the lymph node aspirate showed no TB DNA.
An excisional lymph node biopsy was performed. Histology showed patchy areas of necrosis within the paracortex of the lymph node (figure 2). The necrotic areas contained abundant karyorrhectic debris surrounded by numerous histiocytes; some of the histiocytes possessed curved nuclei (crescentic histiocytes). The histiocytes were highlighted by CD68 and CD163 immunostains. CD8+ (figure 3) and CD3+ lymphocytes were predominant. There were a few CD4+ and CD20+ lymphocytes. Neutrophils were absent. There were no granulomas and no evidence of malignancy.
Figure 2.
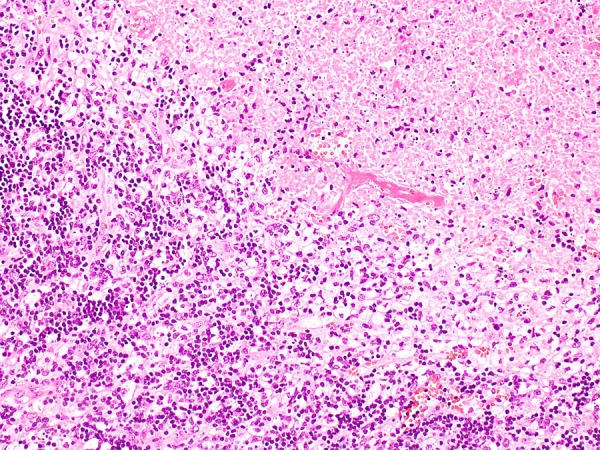
H&E stain showing necrosis with surrounding histiocytes (×10 magnification).
Figure 3.
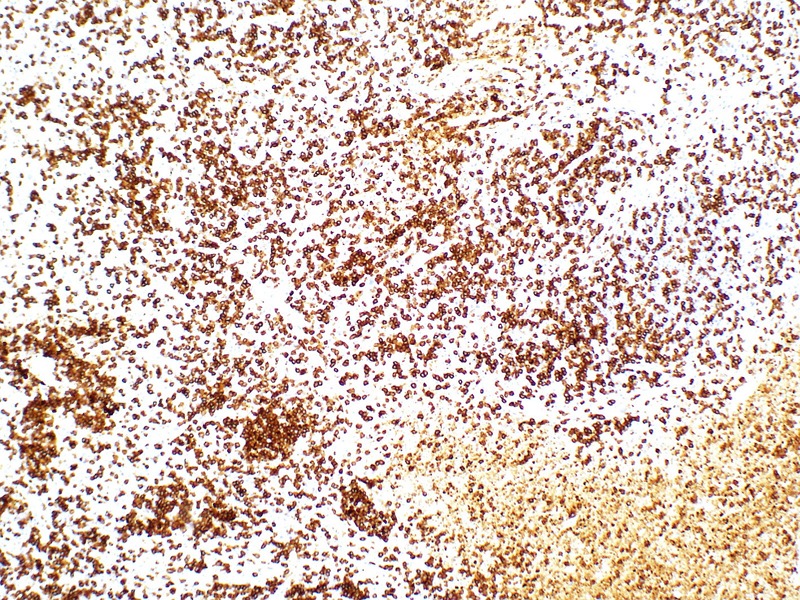
CD8 stain highlighting CD8+ T lymphocytes predominance (×20 magnification).
The Grocott's methenamine silver stain, periodic acid-schiff stain, Fite and Ziehl-Neelsen stains were negative for fungi and acid-fast bacilli.
The histological findings were in keeping with KFD.
Differential diagnosis
The patient was worked up extensively as a case of fever of unknown origin.
- Infective
- Viral—cytomegalovirus, EBV, dengue, influenza;
- Bacterial—pyogenic infections, TB, Brucella, Coxiella, Leptospira;
- Parasitic—malaria.
- Autoimmune
- Systemic lupus erythematosus (SLE).
- Neoplastic
- Lymphoma;
- Secondary lymph node metastasis.
Treatment
The patient was treated symptomatically. Her fever responded to oral paracetamol, but recurred approximately 5 h after every dose. Oral paracetamol 1 g every 6 h was prescribed for 72 h.
Outcome and follow-up
She defervesced 16 days after initial onset of fever. Outpatient follow-up in 2 weeks showed no recurrence of fever.
Discussion
Diagnosis of KFD was made on excisional lymph node biopsy. Histological features included coagulative necrosis with karyorrhectic debris, a large number of histiocytes, crescentic histiocytes and predominance of CD8+ lymphocytes over CD4+ lymphocytes.1
The cause of KFD remains unknown. Viral, autoimmune and even physicochemical causes have been suggested.
Viruses such as EBV, and human herpesvirus 6 and 8 have been shown to be associated with KFD based on serological studies,1 but none of these viruses have been shown to be causative, and the incidence of these viruses detected in patients with KFD are similar to control participants.9
Autoimmune conditions, in particular SLE, have been suggested as a possible cause due to the similarities in presentation with lupus lymphadenitis, higher prevalence in females, co-occurrence of SLE with KFD and histological features of necrotising lymphadenitis.10 The pathological features in a necrotic lymph node that favour SLE are the presence of haematoxylin bodies, a large number of plasma cells and sparse CD8+ cytotoxic T cells.1
In this case, the patient tested negative for viral serology and autoimmune markers. There are only two reported cases of human intoxication with sodium chlorite. One patient ingested an unmeasured amount of 28% sodium chlorite solution diluted with water.11 The other patient ingested 10 g diluted in 100 mL of water.12 That is approximately equivalent to 36 mL of 28% solution. The toxic effects of chlorite ions are due to oxidative damage. Early symptoms of toxicity include nausea, vomiting, abdominal pain, diarrhoea and dehydration likely due to irritation of the gastrointestinal mucosa. Later systemic toxic effects are due to oxidation of haemoglobin to methaemoglobin, resulting in respiratory distress, haemolysis and renal failure.12 The patient in this case did not have signs or systems of acute chlorite toxicity. It is unlikely that KFD resulted directly from systemic oxidative damage. Nonetheless, the patient was counselled on potential toxic effects of sodium chlorite solution and advised to discontinue the use of the ‘Miracle Mineral Solution’.
A possible hypothesis in this case is that chlorite ions caused mild local oxidative damage to oropharyngeal and gastrointestinal mucosa, which then initiated an inflammatory cascade,13 leading to an exuberant lymphocytic response.
KFD has been reported to occur after a leaking silicone breast implant,2 pacemaker insertion3 and gastric bypass surgery.4 In the first two cases, it was hypothesised that KFD resulted from an exaggerated or abnormal T lymphocyte response to the silicone or pacemaker material.
The Naranjo algorithm for likelihood of adverse drug reaction yields a score of 2 in this case, which indicates possible causality. However, owing to the rarity of reports about KFD occurring after a physicochemical insult, it is difficult to conclude whether this occurrence was coincidental or causal.
Physicians should be aware of a patient's use of alternative treatments when considering differentials in a diagnostic dilemma. Potential risks and benefits of alternative treatments should be discussed with patients, and patients advised accordingly.
KFD is a rare cause of fever and lymphadenopathy of unknown aetiology. There are few reports of it occurring after a physicochemical insult. More cases need to be reported to determine if it is a possible cause of KFD.
Learning points.
Kikuchi-Fujimoto disease (KFD) is a rare cause of fever and lymphadenopathy.
The aetiology of KFD is still unknown.
Physicians should be aware of any alternative treatments that their patients are on.
Patients should be advised and educated appropriately on the risks of unproven treatments.
There are few reports of KFD occurring after a physicochemical insult. More cases need to be reported to determine if it is a possible cause of KFD.
Acknowledgments
The authors would like to acknowledge Dr Lynne Goh Yin Lin and Dr Hema Salkade from the Changi General Hospital Department of Laboratory Medicine for providing the histological images.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bosch X, Guilabert A, Miquel R et al. Enigmatic Kikuchi-Fujimoto disease: a comprehensive review. Am J Clin Pathol 2004;122:141–52. [DOI] [PubMed] [Google Scholar]
- 2.Sever CE, Leith CP, Appenzeller J et al. Kikuchi's histiocytic necrotizing lymphadenitis associated with ruptured silicone breast implant. Arch Pathol Lab Med 1996;120:380–5. [PubMed] [Google Scholar]
- 3.Charalabopoulos K, Charalabopoulos A, Binolis J et al. Is implant pacemaker a physicochemical cause triggering Kikuchi-Fujimoto disease? In Vivo 2002;16:73–6. [PubMed] [Google Scholar]
- 4.Garcia-Arnes J, Bernal-Lopez MR, Gallego-Perales JL et al. Histiocytic necrotizing lymphadenitis (Kikuchi-Fujimoto disease) after laparoscopic Roux-en-Y gastric bypass for morbid obesity: a case report. J Med Case Rep 2012;6:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agency warning on chlorine solutions. Food Standards Agency News and Updates [Internet]. 2012. 2 April 2014. http://food.gov.uk/news-updates/news/2012/jul/cdswarning [Google Scholar]
- 6.Humble J. The Miracle Mineral Solution of the 21st Century. 4th Ed. 2009. Self-published. [02 April 2014] Available from: http://miraclemineral.org/free/Miracle_Mineral_Solution_of_the_21st_Century_by_Archbishop_Jim_Humble-part_1-Free-2006-jhbooks.org.pdf [Google Scholar]
- 7.FDA Warns Consumers of Serious Harm from Drinking Miracle Mineral Solution (MMS). FDA News Release [Internet]. 2010. [28 January 2014]. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2010/ucm220747.htm [Google Scholar]
- 8.Carruthers Czyewski P. Candian Adverse Reaction Newsletter [Internet]. 2012 Apr 22 (2):4 [28 January 2014]. http://www.hc-sc.gc.ca/dhp-mps/alt_formats/pdf/medeff/bulletin/carn-bcei_v22n2-eng.pdf [Google Scholar]
- 9.Rosado FG, Tang YW, Hasserjian RP et al. Kikuchi-Fujimoto lymphadenitis: role of parvovirus B-19, Epstein-Barr virus, human herpesvirus 6, and human herpesvirus 8. Hum Pathol 2013;44:255–9. [DOI] [PubMed] [Google Scholar]
- 10.Cramer J, Schmiedel S, Alegre NG et al. Necrotizing lymphadenitis: Kikuchi-Fujimoto disease alias lupus lymphadenitis? Lupus 2010;19:89–92. [DOI] [PubMed] [Google Scholar]
- 11.Romanovsky A, Djogovic D, Chin D. A case of sodium chlorite toxicity managed with concurrent renal replacement therapy and red cell exchange. J Med Toxicol 2013;9:67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin JL, Lim PS. Acute sodium chlorite poisoning associated with failure. Ren Fail 1993;15:645–8. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharyya A, Chattopadhyay R, Mitra S et al. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev 2014;94:329–54. [DOI] [PMC free article] [PubMed] [Google Scholar]


