Abstract
Cancer-associated retinopathy (CAR) is one of the paraneoplastic neurological syndromes and characterised by retinal degeneration. Autoimmunity between cancer cells and retinal proteins is considered a major cause of CAR. The presence of serum autoantibodies to retinal antigens plays an important role in the diagnosis. A 60-year-old man reporting of visual disturbance and paresthaesia of extremities presented to our hospital. CT scan revealed a massive tumour in the left lower lobe of the lung. Small cell lung cancer was diagnosed histologically with bronchoscopy. Ophthalmological examination showed retinopathy but not optic neuritis. Anti-CV2/collapsin response mediator protein (CRMP)-5 and anti-Hu antibodies were detected by further serum examination. It has been reported that anti-CV2/CRMP5 antibodies are present in patients with neoplasms accompanied by retinopathy as well as optic neuritis. This is the first case of CAR with presence of anti-CV2/CRMP5 antibodies without neuritis.
Background
Paraneoplastic syndromes (PNS) are diseases or symptoms that arise due to the presence of cancer in the body. These phenomena are mediated by humoral factors induced by tumour cells or by an immune response against the tumour. Cancer-associated retinopathy (CAR) is a rare PNS characterised by retinal degeneration. Although antirecoverin antibodies are identified as some of the major autoantibodies detected in patients with CAR, the positive rate of antirecoverin antibodies is only 10%.1 Anti-CV2/CRMP5 antibodies binding to oligodendrocytes are known to be associated with paraneoplastic neurological syndromes.2–4 We report a rare case of small cell lung cancer (SCLC) accompanied by CAR associated with anti-CV2/CRMP5 antibodies.
Case presentation
A 60-year-old man, who was a 35 pack-year smoker and had a history of cerebral infarction at the age of 57, experienced photophobia, visual loss and paresthaesia of extremities in August 2012. He visited three different ophthalmologists, and each doctor detected uncertain retinal degeneration. At the same time, he visited a neurologist to treat paresthaesia. The neurologist prescribed pregabalin, which improved the paresthaesia. In December 2012, the patient also experienced hoarseness; however, he did not receive any treatment for this symptom. When dysphagia developed in January 2013, he visited an otolaryngologist. A chest CT revealed a tumour in the lower lobe of the left lung and mediastinal lymphadenopathies. He was referred to our hospital for further examination and treatment of the lung tumour in February 2013.
Investigations
A CT scan at our hospital showed a massive tumour in the left lower lobe of the lung with left pleural effusion and right pleural metastasis (figure 1). Blood tests revealed high serum levels of pro-gastrin-releasing peptide (Pro-GRP) and neuron-specific enolase (NSE), which were considered as tumour markers of SCLC. SCLC was histologically diagnosed by tissue obtained by bronchoscopy.
Figure 1.
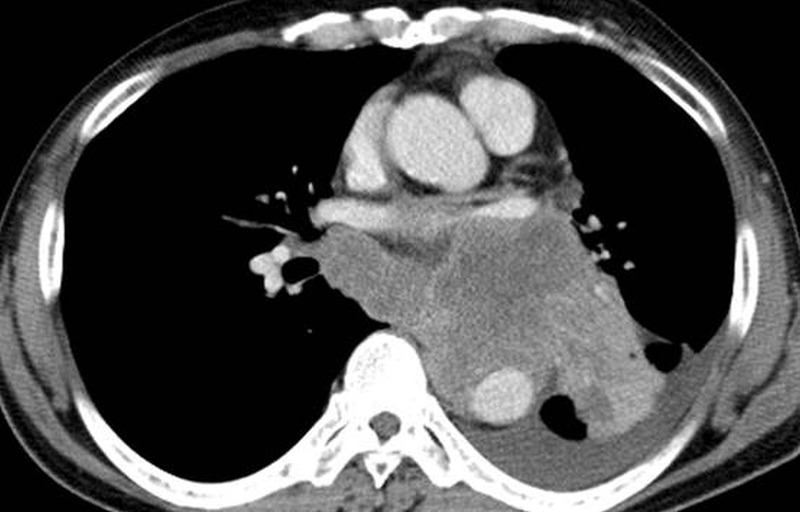
Chest CT scan on admission. Chest CT scan revealed a massive tumour invading into the mediastinum of the left lower lobe. Left pleural effusion was also present.
We suspected that the manifestations of his eyes were derived from PNS. Thus, we consulted an ophthalmologist about the ophthalmological findings, and he informed us that the patient's symptoms were due to bilateral retinal degeneration of unknown cause.
The patient's visual acuity was reduced (R 20/100, L 20/22), but funduscopy showed few abnormal findings. The Goldmann perimetry test showed central scotoma and paracentral scotoma in the right eye and ring scotoma in the left eye. Electroretinogram (ERG) revealed reduced a-wave, b-wave and oscillatory potentials (figure 2). The findings of ERG were compatible with retinal dystrophy. Visual evoked potential showed no prolongation of P100 latency and no decrease of the amplitude (figure 3). There were no other abnormal findings on the optic nerves by funduscopy and fluorescent fundus angiography.
Figure 2.
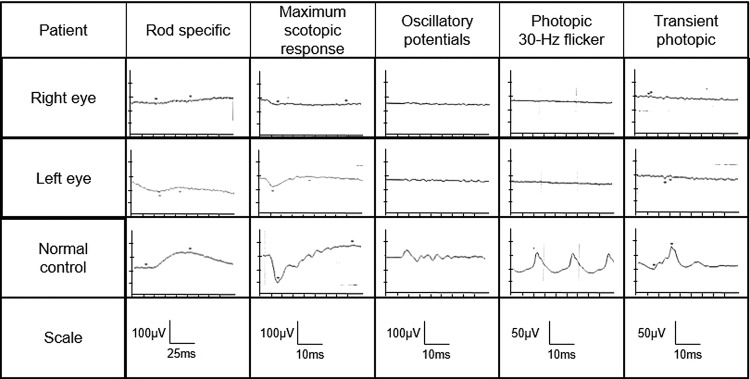
Electroretinogram (ERG) findings before admission. On full-field ERG, a-wave, b-wave and OPs (oscillatory potentials) decreased in right and left eyes, which indicated a reduction in retinal response in both eyes.
Figure 3.
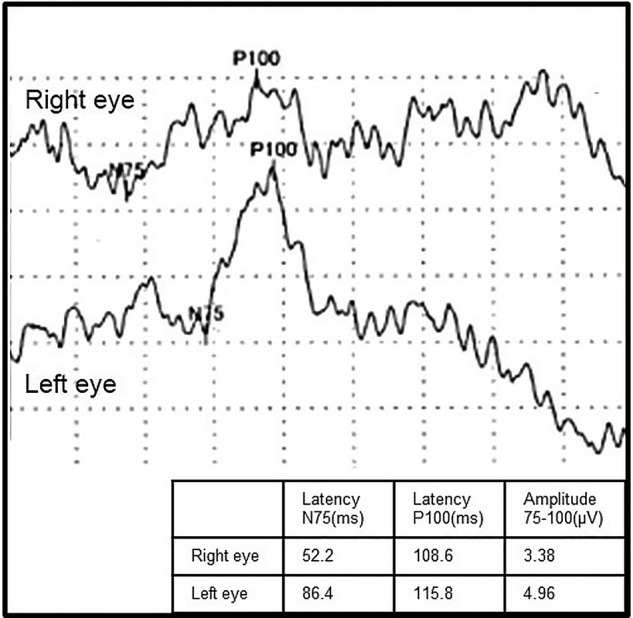
Visual evoked potential (VEP) findings before admission. VEP showed no prolongation of P100 latency and no decrease of the amplitude in both eyes.
We checked for autoantibodies, which are associated with PNS. A serum test for autoantibodies indicated a high titre of anti-CV2/CRMP5 antibodies and a low titre of anti-Hu antibodies. Antibodies against recoverin, which are strongly related to CAR, were negative (table 1). There are some reports that show a relation between CAR and anti-CV2/CRMP5 antibodies. On the basis of the patient's clinical signs and the result of serum autoantibodies, we diagnosed this case as CAR.
Table 1.
The serum autoantibodies
| Serum autoantibodies | Result 1 (pre-Tx.) | Result 2 (post-Tx.) |
|---|---|---|
| Recoverin | (−) | (−) |
| CV2/CRMP5 | (+++) | (+) |
| Hu | (+) | (−) |
| Amphiphysin | (−) | (−) |
| Ri | (−) | (−) |
| Yo | (−) | (−) |
| PNMA2 (Ma2/Ta) | (−) | (−) |
| SOX 1 | (−) | (−) |
| Titin | (−) | (−) |
PNMA2; paraneoplastic antigen Ma2; SOX1; sex-determining region Y-box 1; Tx; treatment.
Outcome and follow-up
After three cycles of chemotherapy with carboplatin plus irinotecan, the tumour size was reduced, and serum Pro-GRP and NSE levels decreased as well. The titre of anti-CV2/CRMP5 antibodies decreased and anti-Hu antibodies showed negative conversion (table 1). On the other hand, the visual disturbance had not improved during the entire course. Subsequently, the cancer progressed rapidly, and the patient died 7 months after diagnosis.
Discussion
The first case of CAR was reported by Sawyer et al5 in 1976. CAR is one of the paraneoplastic neurological syndromes and characterised by sudden, rapidly progressive loss of vision with night blindness associated with photosensitivity, ring scotoma, attenuated retinal arteriole, visual field defects and abnormal ERG.1 6 7 Cross reactivity between antigens expressed on cancer cells and retinal proteins is considered as an aetiology of CAR, and the presence of serum autoantibodies to retinal antigens is crucial for the diagnosis.8–10 Adamus1 reported a large cohort study of 209 cases with CAR, which showed that CAR may be associated with a variety of neoplasms. The major neoplasms associated with CAR are those of the lung (16%), breast (31%), colon (6%) and prostate (7%), as well as melanoma (16%), gynaecological neoplasm (9%) and haematological malignancy (15%). Retinopathy may develop either before or after diagnosis of cancer.
Some previous reports showed a variety of circulating serum autoantibodies to retinal proteins including recoverin,11 12 α-enolase,13 14 and transducin-α,15 but seronegative cases are also common.6 For years it has been believed that recoverin is the main CAR antigen16 and more involved in the development of CAR than other antigens.1 10 However, the positive rate of antirecoverin antibodies in patients with CAR is not high (10%).1
Collapsin response-mediator proteins (CRMPs) are a family of five cytosolic proteins highly expressed in the developing brain.17 18 CRMP5 exhibits in the cortex, hippocampus and cerebellum, and in the postmitotic neuronal precursors, suggesting that it plays a role in process extension.19 Adamus et al20 reported identifying CRMPs, including CRMP5, in the human retina.20 Anti-CV2/CRMP5 antibodies are reported to bind to the retina as well as to neurons, glia and optic nerve.21 Anti-CV2/CRMP5 antibodies are known to cause paraneoplastic neurological syndromes by inducing uveitis and optic neuritis, especially in SCLC patients.2–4 Cross et al21 reported that in 16 patients who had optic neuritis with presence of anti-CV2/CRMP5 antibodies, 5 also experienced retinitis. In their report, they demonstrated that abundant antigen existed in the retina and binding of anti-CV2/CRMP5 antibodies to the antigen caused CAR. Recently, Porto et al22 reported a case of subacute ataxia, sensory neuropathy and retinopathy with presence of anti-Hu and anti-CV2/CRMP5 antibodies. As in other previously reported cases, Porto's case was also accompanied by optic neuritis. Our current report is the first to show that serum anti-CV2/CRMP5 antibodies are associated with retinopathy in the absence of optic neuritis.
In our case, serum examination showed a low titre of anti-Hu antibodies. Cross et al21 showed that among 600 cases with presence of anti-Hu antibodies, none had visual loss. Therefore, we considered that anti-Hu antibodies were not associated with retinopathy in this case.
Thus far, there has been no established treatment of CAR, although various treatments have been attempted, including a combination of anti-cancer drugs, systemic immunosuppression with steroid, intravenous immunoglobulin infusion, rituximab (monoclonal antibodies against B cell CD20) and plasmapheresis.23–25 There are some papers reporting that steroid therapy improved visual function or prevented progression of the disease.10 26–28 However, there have not been any appropriate evidence-based studies with regard to steroid treatment.6 29 In our case, chemotherapy and steroid treatment had no effect on the visual symptoms. This negative outcome of treatments may be related to the inactivity of chemotherapy to SCLC.
One of the initial symptoms of this case was paresthaesia of the extremities. However, the patient was not actually diagnosed with a peripheral disorder because he did not undergo nerve conduction study before chemotherapy, and there was no abnormal finding in the study after treatment. However, it was presumed that the neurological symptom was related to presence of anti-CV2/CRMP5 or anti-Hu antibodies.
CAR is such a rare syndrome that it is difficult to diagnose.6 When we encounter patients with cancer who have unexplained visual loss, it is important to consider the possibility of CAR and to investigate the autoantibodies associated with CAR.
Learning points.
Cancer-associated retinopathy (CAR) is such a rare disease among paraneoplastic syndromes (PNS) that it is difficult to diagnose by optical examination alone.
Symptoms of PNS often appear before detection of cancer.
CAR is mediated by autoimmunity, therefore, detection of serum autoantibodies to retinal antigens facilitates the diagnosis of CAR.
CAR was induced not only by antibodies to recoverin, but also anti-CV2/CRMP5 antibodies.
Acknowledgments
The authors would like to thank Dr Ikuro Sato and Dr Shigemi Ito in the Department of Pathology, Miyagi Cancer Center, for the histological diagnosis. The authors would also like to express their gratitude to Dr Nobuyuki Sato in the Department of Neurology, Katta Public General Hospital, for help in diagnosing the neurological findings.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Adamus G. Autoantibody targets and their cancer relationship in the pathogenicity of paraneoplastic retinopathy. Autoimmun Rev 2009;8:410–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Honnorat J, Antoine JC, Derrington E et al. Antibodies to a subpopulation of glial cells and a 66 kDa developmental protein in patients with paraneoplastic neurological syndoromes. J Neurol Neurosurg Psychiatry 1996;61:270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de la Sayette V, Bertran F, Honnorat J et al. Paraneoplastic cerebellarsyndrome and optic neuritis with anti-CV2 antibodies: clinical response to excision of the primary tumor. Arch Neurol 1998;55:405–8. [DOI] [PubMed] [Google Scholar]
- 4.Honnorat J, Antoine JC. Paraneoplastic neurological syndromes. Orphanet J Rare Dis 2007;2:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawyer RA, Shelhorst JB, Zimmerman LE et al. Blindness caused by photoreceptor degeneration as a remote effect of cancer. Am J Ophthalmol 1976;81:606–13. [DOI] [PubMed] [Google Scholar]
- 6.Braithwait T, Vugler A, Tufail A. Autoimmune Retinopathy. Ophthalmologica 2012;228:131–42. [DOI] [PubMed] [Google Scholar]
- 7.Bataller L, Dalmau J. Neuro-ophathalmology and paraneoplastic syndromes. Curr Opin Neurol 2004;17:3–8. [DOI] [PubMed] [Google Scholar]
- 8.Chan JW. Paraneoplastic Retinopathies and Optic Neuropathies. Surv Ophthalmol 2003;48:12–38. [DOI] [PubMed] [Google Scholar]
- 9.Graus F, Delattre JY, Antoine JC et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry 2004;75:1135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohguro H, Yokoi Y, Ohguro I et al. Clinical and immunological aspects of cancer-associated retinopathy. Am J Ophthalmol 2004;137:1117–19. [DOI] [PubMed] [Google Scholar]
- 11.Dizhoor AM, Ray S, Kumar S et al. Recoverin: a calcium sensitive activator of retinal rod guanylate cyclase. Science 1991;251:915–18. [DOI] [PubMed] [Google Scholar]
- 12.Bazhin AV, Savchenko MS, Shifrina ON et al. Recoverin as a paraneoplastic antigen in lung cancer: the occurrence of anti-recoverin autoantibodies in sera and recoverin in tumors. Lung Cancer 2004;44:193–8. [DOI] [PubMed] [Google Scholar]
- 13.Adamus G, Aptsiauri N, Guy J et al. The occurrence of serum autoantibodies against enolase in cancer-associated retinopathy. Clin Immunol Immunopathol 1996;78:120–9. [DOI] [PubMed] [Google Scholar]
- 14.Ejima M, Misiuk-Hojlo M, Gorczyca WA et al. Antibodies to 46-kDa retinal antigen in a patient with breast carcinoma and cancer-associated retinopathy. Breast Cancer Res Treat 2008;110:269–71. [DOI] [PubMed] [Google Scholar]
- 15.Adamus G, Brown L, Weleber RG. Morecular biomarkers for autoimmune retinopathies: significance of anti-transducin-alpha autoantibodies. Exp Mol Pathol 2009;87:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polans AS, Buczylko J, Crabb J et al. A photoreceptor calcium binding protein is recognized by autoantibodies obtained from patients with cancer-associated retinopathy. J Cell Biol 1991;112:981–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang LH, Strittmatter SM. Brain CRMP forms heterotetramers similar to liver dihydropyrimidinase. J Neurochem 1997;69:2261–9. [DOI] [PubMed] [Google Scholar]
- 18.Inatome R, Tsujimura T, Hitomi T et al. Identification of CRAM, a novel unc-33 gene family protein that associates with CRMP3 and protein-tyrosine kinase(s) in the developing rat brain. J Biol Chem 2000;275:27291–302. [DOI] [PubMed] [Google Scholar]
- 19.Ricard D, Rogemond V, Charrier E et al. Isolation and expression pattern of human Unc-33-like phosphoprotein 6/collapsin response mediator protein 5 (Ulip6/CRMP5): coexistence with Ulip2/CRMP2 in Sema3a- sensitive oligodendrocytes. J Neurosci 2001;21:7203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adamus G, Bonnah R, Brown L et al. Detection of autoantibodies against heat shock proteins and collapsin response mediator proteins in autoimmune retinopathy. BMC Ophthalmol 2013;13:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cross SA, Salomao DR, Parisi JE et al. Paraneoplastic autoimmune optic neuritis with retinitis defined by CRMP-5-IgG. Ann Neurol 2003;54:38–50. [DOI] [PubMed] [Google Scholar]
- 22.Porto L, Miranda M, Gomes A et al. Paraneoplastic neurological syndrome as an initial indicator of small cell carcinoma of the lung. BMJ Case Rep 2013;2013;pii: bcr2012008432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dy I, Chintapatla R, Preeshagul I et al. Treatment of cancer-associated retinopathy with rituximab. J Natl Compr Canc Netw 2013;11:1320–4. [DOI] [PubMed] [Google Scholar]
- 24.Guy J, Aptsiauri N. Treatment of palaneoplastic visual loss with intravenous immunoglobulin: report of 3 cases. Arch Ophthalmol 1999;117:471–7. [DOI] [PubMed] [Google Scholar]
- 25.Murphy MA, Thirkill CE, Hart WM Jr. Paraneoplastic retinopathy: a novel autoantibody reaction associated with small-cell lung carcinoma. J Neuroophthalmol 1997;17:77–83. [PubMed] [Google Scholar]
- 26.Bidegain C, Rigalt J, Ribot E et al. [Small cell lung cancer and cancer-associated retinopathy]. Arch Bronconeumol 2005;41:99–101. [DOI] [PubMed] [Google Scholar]
- 27.Salgia R, Hedges TR, Rizk M et al. Cancer-associated retinopathy in a patient with non-small lung carcinoma. Lung Cancer 1998;22:149–52. [DOI] [PubMed] [Google Scholar]
- 28.Huynh N, Shildkrot Y, Lobo AM et al. Intravitreal triamcinolone for cancer-associated retinopathy refractory to systemic therapy. J Ophthalmic Inflamm Infect 2012;2:169–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adamus G, Ren G, Weleber RG. Autoantibodies against retinal proteins in paraneoplastic and autoimmune retinopathy. BMC Ophthalmol 2004;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]


