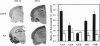Abstract
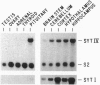
Subtractive library construction and differential screening were used to identify a cDNA for a cell type-specific immediate early gene induced in rat PC12 pheochromocytoma cells. Sequencing identified the protein product of this gene as rat synaptotagmin IV (SytIV). Synaptotagmins are synaptic vesicle proteins thought to play a role in depolarization-induced, calcium-mediated exocytosis and neurotransmitter release. SytIV mRNA accumulation is transiently induced in PC12 cells by potassium depolarization, calcium ionophore, ATP, and forskolin. In contrast, growth factors and phorbol 12-myristate 13-acetate induce little or no SytIV mRNA accumulation. Kainic acid-induced seizures in rats are followed by accumulation of SytIV message in the hippocampus and piriform cortex. The SytIV gene may provide a direct link between depolarization-induced neuronal gene expression and subsequent modulation of synaptic structure and function.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bommert K., Charlton M. P., DeBello W. M., Chin G. J., Betz H., Augustine G. J. Inhibition of neurotransmitter release by C2-domain peptides implicates synaptotagmin in exocytosis. Nature. 1993 May 13;363(6425):163–165. doi: 10.1038/363163a0. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Chapman E. R., Jahn R. Calcium-dependent interaction of the cytoplasmic region of synaptotagmin with membranes. Autonomous function of a single C2-homologous domain. J Biol Chem. 1994 Feb 25;269(8):5735–5741. [PubMed] [Google Scholar]
- Cho K. O., Skarnes W. C., Minsk B., Palmieri S., Jackson-Grusby L., Wagner J. A. Nerve growth factor regulates gene expression by several distinct mechanisms. Mol Cell Biol. 1989 Jan;9(1):135–143. doi: 10.1128/mcb.9.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Davletov B. A., Südhof T. C. A single C2 domain from synaptotagmin I is sufficient for high affinity Ca2+/phospholipid binding. J Biol Chem. 1993 Dec 15;268(35):26386–26390. [PubMed] [Google Scholar]
- DiAntonio A., Parfitt K. D., Schwarz T. L. Synaptic transmission persists in synaptotagmin mutants of Drosophila. Cell. 1993 Jul 2;73(7):1281–1290. doi: 10.1016/0092-8674(93)90356-u. [DOI] [PubMed] [Google Scholar]
- DiAntonio A., Schwarz T. L. The effect on synaptic physiology of synaptotagmin mutations in Drosophila. Neuron. 1994 Apr;12(4):909–920. doi: 10.1016/0896-6273(94)90342-5. [DOI] [PubMed] [Google Scholar]
- Egger C., Kirchmair R., Kapelari S., Fischer-Colbrie R., Hogue-Angeletti R., Winkler H. Bovine posterior pituitary: presence of p65 (synaptotagmin), PC1, PC2 and secretoneurin in large dense core vesicles. Neuroendocrinology. 1994 Feb;59(2):169–175. doi: 10.1159/000126655. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., Schwarz E., Komaromy M., Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984 Oct 15;179(1):125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- Geppert M., Archer B. T., 3rd, Südhof T. C. Synaptotagmin II. A novel differentially distributed form of synaptotagmin. J Biol Chem. 1991 Jul 25;266(21):13548–13552. [PubMed] [Google Scholar]
- Herfort M. R., Garber A. T. Simple and efficient subtractive hybridization screening. Biotechniques. 1991 Nov;11(5):598, 600, 602-4. [PubMed] [Google Scholar]
- Herschman H. R. Primary response genes induced by growth factors and tumor promoters. Annu Rev Biochem. 1991;60:281–319. doi: 10.1146/annurev.bi.60.070191.001433. [DOI] [PubMed] [Google Scholar]
- Hilbush B. S., Morgan J. I. A third synaptotagmin gene, Syt3, in the mouse. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8195–8199. doi: 10.1073/pnas.91.17.8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujubu D. A., Lim R. W., Varnum B. C., Herschman H. R. Induction of transiently expressed genes in PC-12 pheochromocytoma cells. Oncogene. 1987;1(3):257–262. [PubMed] [Google Scholar]
- Levi A., Eldridge J. D., Paterson B. M. Molecular cloning of a gene sequence regulated by nerve growth factor. Science. 1985 Jul 26;229(4711):393–395. doi: 10.1126/science.3839317. [DOI] [PubMed] [Google Scholar]
- Littleton J. T., Stern M., Schulze K., Perin M., Bellen H. J. Mutational analysis of Drosophila synaptotagmin demonstrates its essential role in Ca(2+)-activated neurotransmitter release. Cell. 1993 Sep 24;74(6):1125–1134. doi: 10.1016/0092-8674(93)90733-7. [DOI] [PubMed] [Google Scholar]
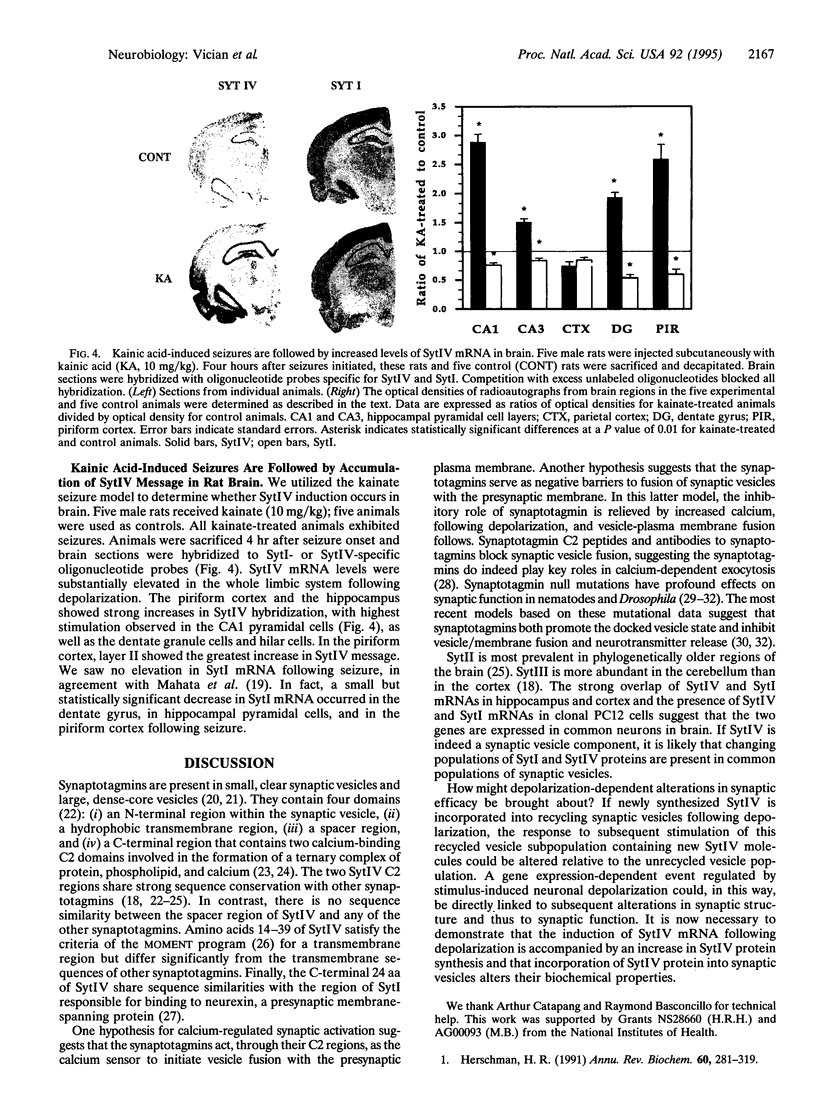
- Mahata S. K., Marksteiner J., Sperk G., Mahata M., Gruber B., Fischer-Colbrie R., Winkler H. Temporal lobe epilepsy of the rat: differential expression of mRNAs of chromogranin B, secretogranin II, synaptin/synaptophysin and p65 in subfield of the hippocampus. Brain Res Mol Brain Res. 1992 Nov;16(1-2):1–12. doi: 10.1016/0169-328x(92)90187-g. [DOI] [PubMed] [Google Scholar]
- Milbrandt J. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science. 1987 Nov 6;238(4828):797–799. doi: 10.1126/science.3672127. [DOI] [PubMed] [Google Scholar]
- Mizuta M., Inagaki N., Nemoto Y., Matsukura S., Takahashi M., Seino S. Synaptotagmin III is a novel isoform of rat synaptotagmin expressed in endocrine and neuronal cells. J Biol Chem. 1994 Apr 22;269(16):11675–11678. [PubMed] [Google Scholar]
- Morgan J. I., Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Nedivi E., Hevroni D., Naot D., Israeli D., Citri Y. Numerous candidate plasticity-related genes revealed by differential cDNA cloning. Nature. 1993 Jun 24;363(6431):718–722. doi: 10.1038/363718a0. [DOI] [PubMed] [Google Scholar]
- Nonet M. L., Grundahl K., Meyer B. J., Rand J. B. Synaptic function is impaired but not eliminated in C. elegans mutants lacking synaptotagmin. Cell. 1993 Jul 2;73(7):1291–1305. doi: 10.1016/0092-8674(93)90357-v. [DOI] [PubMed] [Google Scholar]
- Perin M. S., Brose N., Jahn R., Südhof T. C. Domain structure of synaptotagmin (p65) J Biol Chem. 1991 Jan 5;266(1):623–629. [PubMed] [Google Scholar]
- Perin M. S. The COOH terminus of synaptotagmin mediates interaction with the neurexins. J Biol Chem. 1994 Mar 18;269(11):8576–8581. [PubMed] [Google Scholar]
- Qian Z., Gilbert M. E., Colicos M. A., Kandel E. R., Kuhl D. Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature. 1993 Feb 4;361(6411):453–457. doi: 10.1038/361453a0. [DOI] [PubMed] [Google Scholar]
- Sive H. L., St John T. A simple subtractive hybridization technique employing photoactivatable biotin and phenol extraction. Nucleic Acids Res. 1988 Nov 25;16(22):10937–10937. doi: 10.1093/nar/16.22.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirone F., Shooter E. M. Early gene regulation by nerve growth factor in PC12 cells: induction of an interferon-related gene. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2088–2092. doi: 10.1073/pnas.86.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Solimena C., Takei K., Marek K. L., Midyett K., Südhof T. C., De Camilli P., Jahn R. Synaptotagmin: a membrane constituent of neuropeptide-containing large dense-core vesicles. J Neurosci. 1993 Sep;13(9):3895–3903. doi: 10.1523/JNEUROSCI.13-09-03895.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K., Andreasson K. I., Kaufmann W. E., Barnes C. A., Worley P. F. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron. 1993 Aug;11(2):371–386. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- Yamagata K., Sanders L. K., Kaufmann W. E., Yee W., Barnes C. A., Nathans D., Worley P. F. rheb, a growth factor- and synaptic activity-regulated gene, encodes a novel Ras-related protein. J Biol Chem. 1994 Jun 10;269(23):16333–16339. [PubMed] [Google Scholar]