Abstract
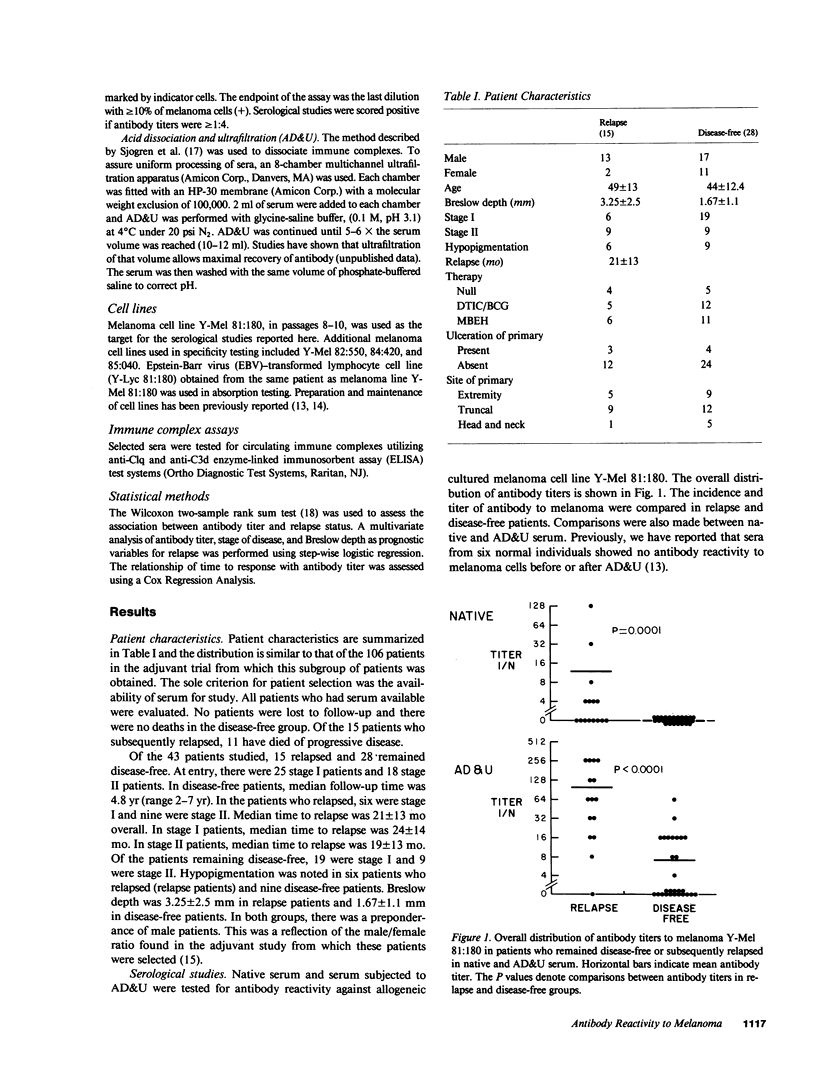
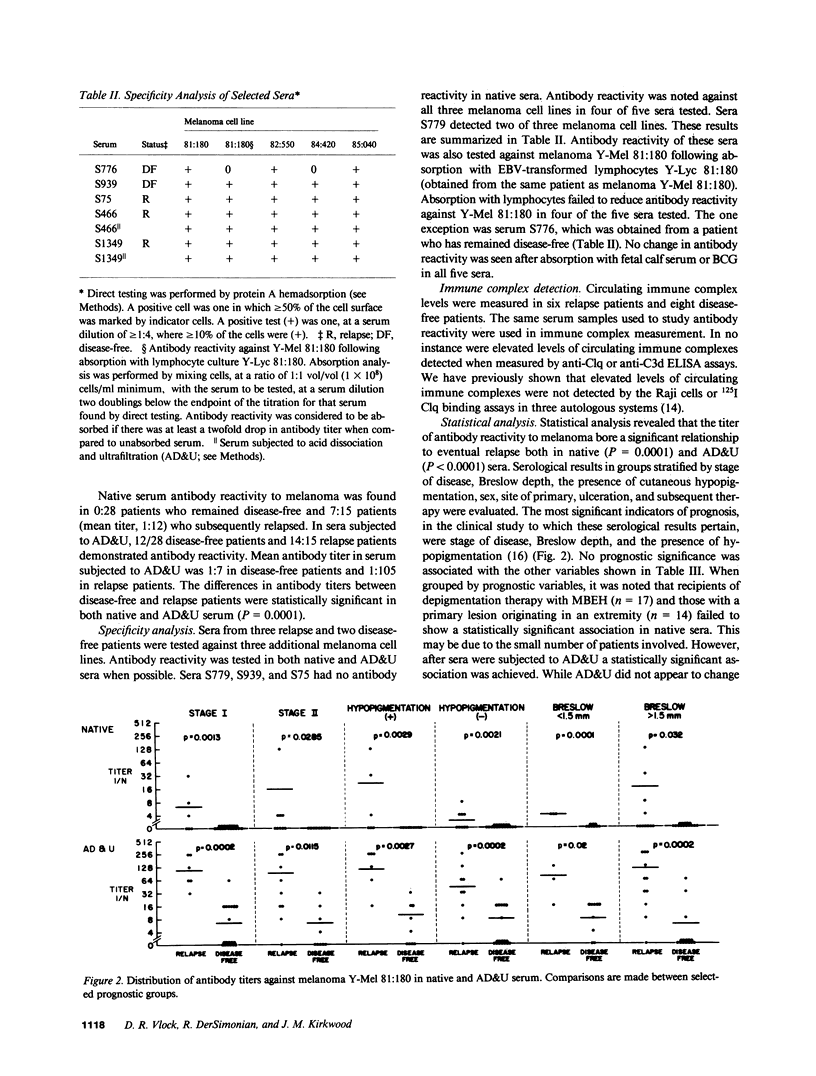
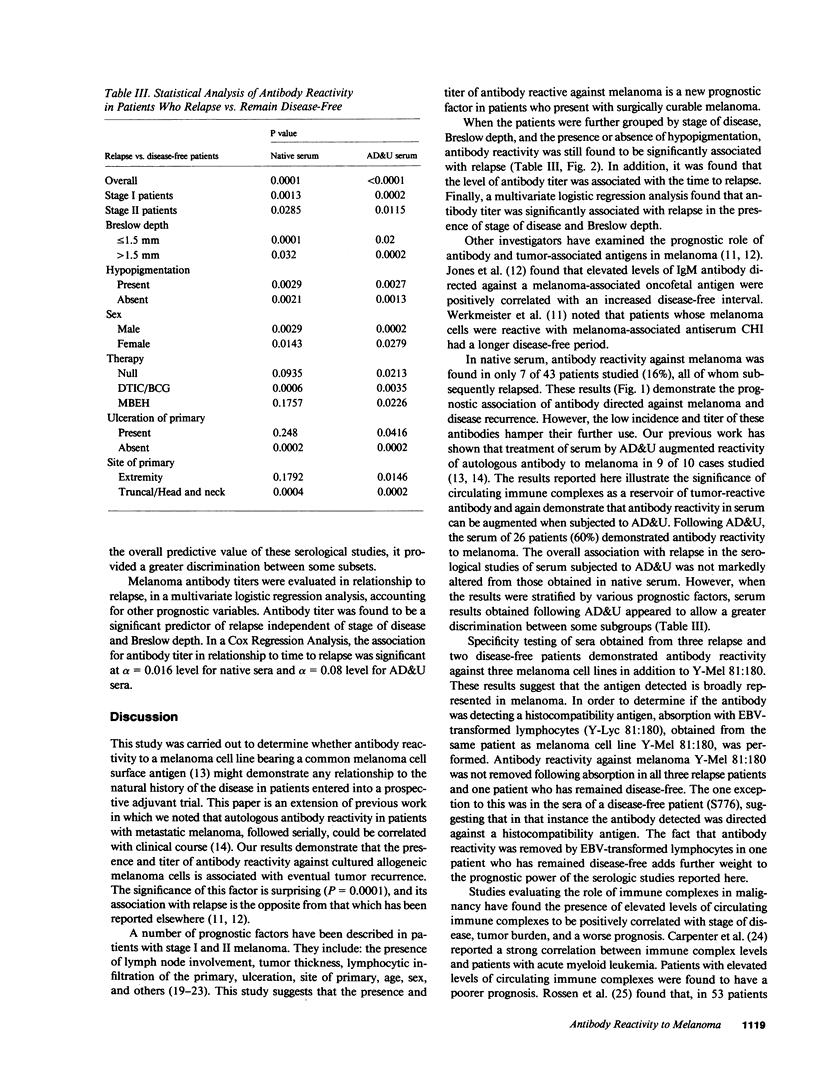
Antibody reactivity against cultured allogeneic melanoma Y-Mel 81:180 was studied in 43 patients who participated in an adjuvant trial for stage I and II melanoma. Serum samples were obtained at trial entry within 2 mo of definitive surgery. At the time of serum acquisition, all patients were free of disease by physical examination and routine radiologic and laboratory parameters. Serum antibody reactivity was tested for by protein A hemadsorption before and after acid dissociation and ultrafiltration of serum. We have previously shown that this technique for immune complex dissociation augments autologous antibody reactivity. Results of serum antibody reactivity were scored by an investigator blinded to the patient's clinical status. Of the 43 patients studied, 15 relapsed and 28 remained disease-free. At study entry, there were 25 stage I patients and 18 stage II patients. Breslow depth was 3.25 +/- 2.5 mm in relapse patients and 1.67 +/- 1.1 mm in disease-free patients. The presence and titer of antibody directed against melanoma in either native serum or serum dissociated from immune complexes was found to be associated with eventual relapse (P = 0.0001). When results were subgrouped by stage of disease, Breslow depth, and hypopigmentation, antibody reactivity was still correlated with eventual relapse. The incidence and titer of antibody reactivity against melanoma appears to be a new prognostic factor in predicting eventual disease recurrence.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin R. W., Price M. R., Robins R. A. Inhibition of hepatoma-immune lymph-node cell cytotoxicity by tumour-bearer serum, and solubilized hepatoma antigen. Int J Cancer. 1973 May;11(3):527–535. doi: 10.1002/ijc.2910110304. [DOI] [PubMed] [Google Scholar]
- Breslow A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970 Nov;172(5):902–908. doi: 10.1097/00000658-197011000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey T. E., Lloyd K. O., Takahashi T., Travassos L. R., Old L. J. AU cell-surface antigen of human malignant melanoma: solubilization and partial characterization. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2898–2902. doi: 10.1073/pnas.76.6.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey T. E., Lloyd K. O., Takahashi T., Travassos L. R., Old L. J. AU cell-surface antigen of human malignant melanoma: solubilization and partial characterization. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2898–2902. doi: 10.1073/pnas.76.6.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey T. E., Takahashi T., Resnick L. A., Oettgen H. F., Old L. J. Cell surface antigens of human malignant melanoma: mixed hemadsorption assays for humoral immunity to cultured autologous melanoma cells. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3278–3282. doi: 10.1073/pnas.73.9.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier N. A., Fiere D. M., Schuh D., Lange G. T., Lambert P. H. Circulating immune complexes and the prognosis of acute myeloid leukemia. N Engl J Med. 1982 Nov 4;307(19):1174–1180. doi: 10.1056/NEJM198211043071903. [DOI] [PubMed] [Google Scholar]
- Day C. L., Jr, Lew R. A., Mihm M. C., Jr, Harris M. N., Kopf A. W., Sober A. J., Fitzpatrick T. B. The natural break points for primary-tumor thickness in clinical Stage I melanoma. N Engl J Med. 1981 Nov 5;305(19):1155–1155. doi: 10.1056/NEJM198111053051916. [DOI] [PubMed] [Google Scholar]
- Galloway D. R., McCabe R. P., Pellegrino M. A., Ferrone S., Reisfeld R. A. Tumor-associated antigens in spent medium of human melanoma cells: immunochemical characterization with xenoantisera. J Immunol. 1981 Jan;126(1):62–66. [PubMed] [Google Scholar]
- Heaney-Kieras J., Bystryn J. C. Identification and purification of a Mr 75,000 cell surface human melanoma-associated antigen. Cancer Res. 1982 Jun;42(6):2310–2316. [PubMed] [Google Scholar]
- Hersey P., Edwards A., Murray E., McCarthy W. H., Milton G. W. Prognostic significance of leukocyte-dependent antibody activity in melanoma patients. J Natl Cancer Inst. 1983 Jul;71(1):45–53. [PubMed] [Google Scholar]
- Johnson O. K., Jr, Emrich L. J., Karakousis C. P., Rao U., Greco W. R. Comparison of prognostic factors for survival and recurrence in malignant melanoma of the skin, clinical Stage I. Cancer. 1985 Mar 1;55(5):1107–1117. doi: 10.1002/1097-0142(19850301)55:5<1107::aid-cncr2820550528>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Jones P. C., Sze L. L., Liu P. Y., Morton D. L., Irie R. F. Prolonged survival for melanoma patients with elevated IgM antibody to oncofetal antigen. J Natl Cancer Inst. 1981 Feb;66(2):249–254. [PubMed] [Google Scholar]
- KALISS N. Immunological enhancement of tumor homografts in mice: a review. Cancer Res. 1958 Oct;18(9):992–1003. [PubMed] [Google Scholar]
- Kirkwood J. M., Vlock D. R. Augmentation of autologous antibody to human melanoma following acid dissociation and ultrafiltration of serum. Cancer Res. 1984 Sep;44(9):4177–4182. [PubMed] [Google Scholar]
- Koprowski H., Steplewski Z., Herlyn D., Herlyn M. Study of antibodies against human melanoma produced by somatic cell hybrids. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3405–3409. doi: 10.1073/pnas.75.7.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M. G., Philips T. M., Rowden G. Beneficial and detrimental effects of humoral immunity in malignancy. Pathobiol Annu. 1978;8:217–239. [PubMed] [Google Scholar]
- Moses L. E., Emerson J. D., Hosseini H. Analyzing data from ordered categories. N Engl J Med. 1984 Aug 16;311(7):442–448. doi: 10.1056/NEJM198408163110705. [DOI] [PubMed] [Google Scholar]
- Murray E., McCarthy W. H., Hersey P. Blocking factors against leucocyte-dependent melanoma antibody in the sera of melanoma patients. Br J Cancer. 1977 Jul;36(1):7–14. doi: 10.1038/bjc.1977.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberbarnscheidt J., Kölsch E. Direct blockade of antigen-reactive B lymphocytes by immune complexes. An 'off' signal for precursors of IgM-producing cells provided by the linkage of antigen-and Fc-receptors. Immunology. 1978 Jul;35(1):151–157. [PMC free article] [PubMed] [Google Scholar]
- Old L. J. Cancer immunology: the search for specificity--G. H. A. Clowes Memorial lecture. Cancer Res. 1981 Feb;41(2):361–375. [PubMed] [Google Scholar]
- Pfreundschuh M., Shiku H., Takahashi T., Ueda R., Ransohoff J., Oettgen H. F., Old L. J. Serological analysis of cell surface antigens of malignant human brain tumors. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5122–5126. doi: 10.1073/pnas.75.10.5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossen R. D., Crane M. M., Morgan A. C., Giannini E. H., Giovanella B. C., Stehlin J. S., Twomey J. J., Hersh E. M. Circulating immune complexes and tumor cell cytotoxins as prognostic indicators in malignant melanoma: a prospective study of 53 patients. Cancer Res. 1983 Jan;43(1):422–429. [PubMed] [Google Scholar]
- Roth J. A. Tumor induced immunosuppression. Surg Gynecol Obstet. 1983 Feb;156(2):233–240. [PubMed] [Google Scholar]
- Ruell P., Murray E., McCarthy W. H., Hersey P. Evaluation of assays to detect immune complexes as an immunodiagnostic aid in patients with melanoma. Oncodev Biol Med. 1982;3(1):1–12. [PubMed] [Google Scholar]
- Shiku H., Takahashi T., Oettgen H. F. Cell surface antigens of human malignant melanoma. II. Serological typing with immune adherence assays and definition of two new surface antigens. J Exp Med. 1976 Oct 1;144(4):873–881. doi: 10.1084/jem.144.4.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiku H., Takahashi T., Resnick L. A., Oettgen H. F., Old L. J. Cell surface antigens of human malignant melanoma. III. Recognition of autoantibodies with unusual characteristics. J Exp Med. 1977 Mar 1;145(3):784–789. doi: 10.1084/jem.145.3.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren H. O., Hellström I., Bansal S. C., Hellström K. E. Suggestive evidence that the "blocking antibodies" of tumor-bearing individuals may be antigen--antibody complexes. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1372–1375. doi: 10.1073/pnas.68.6.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Andrews B. S., Urist M. M., Morton D. L., Dixon F. J. The nature of immune complexes in human cancer sera. J Immunol. 1977 Aug;119(2):657–663. [PubMed] [Google Scholar]
- Vlock D. R., Kirkwood J. M. Serial studies of autologous antibody reactivity to melanoma. Relationship to clinical course and circulating immune complexes. J Clin Invest. 1985 Aug;76(2):849–854. doi: 10.1172/JCI112042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury R. G., Brown J. P., Yeh M. Y., Hellström I., Hellström K. E. Identification of a cell surface protein, p97, in human melanomas and certain other neoplasms. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2183–2187. doi: 10.1073/pnas.77.4.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]



