Abstract
Mechanical thrombectomy holds promise for children with large cerebral arterial occlusions, although there are few reports in this population. We report a case of retrievable stent-assisted mechanical thrombectomy in a 5-year-old with basilar artery occlusion, despite late presentation and extensive initial diffusion-weighted imaging (DWI) restriction. This resulted in successful Thrombolysis in Cerebral Infarction 2B reperfusion and excellent clinical outcome. At 6-week follow-up he was completely back to baseline with no residual deficits (pediatric stroke outcome measure=0, modified Rankin scale=0). At 3-month follow-up the patient has not had any recurrent stroke or concern for stroke-like symptoms. We review the literature on mechanical thrombectomy and DWI changes in acute stroke in early to middle childhood (<12 years old).
Keywords: Pediatrics, Dissection, Stroke, Thrombectomy, MRI
Background
Mechanical thrombectomy (MT) holds promise for children with acute large artery occlusion, although few reports exist. We report a successful case of MT in a 5-year-old with basilar artery occlusion, despite late presentation and extensive initial diffusion-weighted imaging (DWI) restriction.
Case presentation
A previously healthy 5-year-old boy presented with mutism and lethargy following neck trauma from a fall. He presented first to a community hospital and was transferred to our center 8 h after onset. On arrival at our facility he was non-verbal, unable to follow commands, moving right greater than left upper extremity to stimulation and only withdrawing in lower extremities, with bilateral extensor plantar reflexes. Initial pediatric National Institutes of Health stroke scale (PedNIHSS)1 score was 22.
Investigations
Stroke protocol MRI demonstrated extensive restricted diffusion involving the bilateral pons, cerebellum, and right occipital lobe (figure 1). MR angiography and CT angiography confirmed proximal basilar occlusion (figure 2), with left vertebral artery irregularity concerning for dissection (figure 3A). The patient was taken as an emergency to the angiography suite 9 h after initial injury. No thrombolytics were given. He was loaded with 150 mg aspirin.
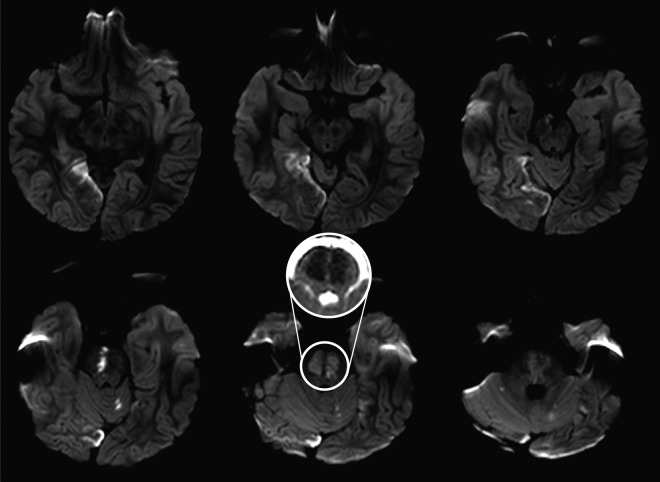
Figure 1.
Initial diffusion-weighted MRI. There are large areas of restricted diffusion involving the pons bilaterally, a large portion of the anterior and medial right occipital cortex shows restricted diffusion, and there are several scattered punctate foci of restricted diffusion within the cerebellum bilaterally. Inset: Apparent diffusion coefficient map showing restricted diffusion of bilateral pons.
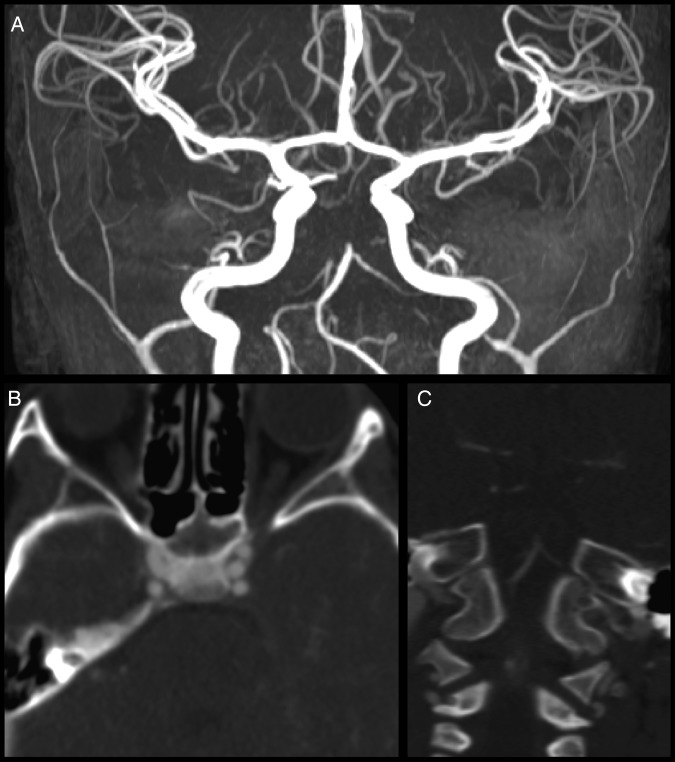
Figure 2.
(A) MR angiography shows an abrupt cut-off of the mid portion of the basilar artery, with lack of flow-related enhancement throughout the remainder of the expected course of the basilar artery. There is a large right posterior communicating artery which supplies the right P2 distribution. A very small left posterior communicating artery (Pcomm) is seen only over approximately a 7–8 mm segment. It appears that the right P1 segment is filling from the right Pcomm. (B) Axial CT angiography shows no filling of the basilar artery in the pontine cistern. (C) Coronal CT angiography shows complete occlusion of the basilar artery just distal to the origins of the anterior inferior cerebellar arteries.
Figure 3.
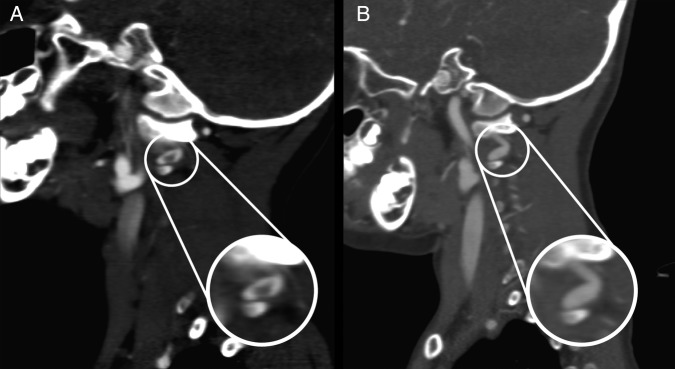
(A) Sagittal CT angiography (CTA) demonstrates possible dissection at the V3 segment of the vertebral artery. (B) Two-week follow-up sagittal CTA demonstrates an apparent smooth caliber change over a short segment of the left vertebral artery at C1–C2 at the area of previous thrombus on prior CTA. This possibly represents a pseudoaneurysm.
Treatment
Under general anesthesia, femoral access was gained with a micropuncture technique. An 070 Neuron (Penumbra, Alameda, California, USA) was used to perform baseline angiography of the left vertebral artery and confirmed the previously seen vertebral artery dissection and Thrombolysis In Cerebral Infarction (TICI) 0 complete basilar occlusion (Figure 4A). A Prowler Select Plus microcatheter (Codman, Raynham, Massachusetts, USA) was advanced to the right posterior cerebral artery (PCA) and a Solitaire 4 mm×20 mm stent retriever (Covidien, Plymouth, Minnesota, USA) was deployed from the proximal right P2 segment into the basilar artery (figure 4C). The Solitaire was held in place for 5 min, then withdrawn under vacuum aspiration into the 070 Neuron; two passes were required. After the second pass, angiography demonstrated TICI 2B reperfusion (figure 4B). Persistent thrombus was present in the left P2 segment; injection of the left internal carotid artery demonstrated good perfusion to this left PCA territory via collateral flow from the anterior circulation. Given acute angulation of P1 to P2 transition (causing the wire to preferentially select the posterior communicating artery rather than P2) and a desire to minimize contrast load, further intervention was not felt to be beneficial and the procedure was finished.
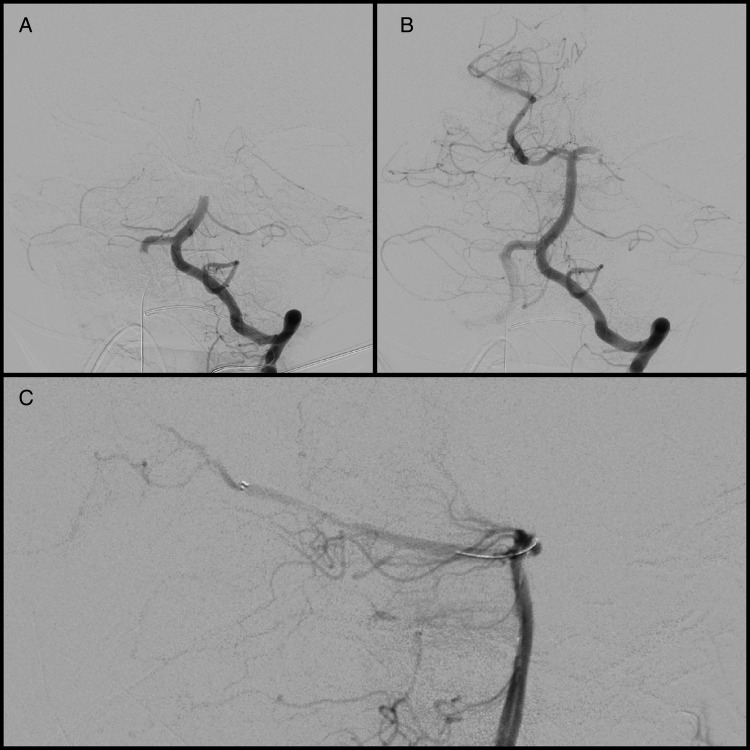
Figure 4.
(A) Initial angiography demonstrates Thrombolysis in Cerebral Infarction (TICI) 0 complete basilar occlusion. (B) Post-thrombectomy angiography demonstrates TICI 2B reperfusion of the basilar artery, bilateral superior cerebellar arteries, and proximal segment of the right posterior cerebral artery (PCA). There continued to be complete occlusion of the left PCA and distal branches of the right PCA. Anatomically, access to the right PCA was more straightforward and therefore the stent retriever was first deployed in the right PCA distal to the area of occlusion and aspiration was repeated followed by selection of the left PCA for further thrombectomy. Follow-up angiography showed excellent return of flow in the previously occluded distal right PCA segment; there was recanalization of the left P1 segment with continued distal thrombus. Multiple attempts were made for further thrombectomy of the distal left PCA but, given the burden of clot and anatomy, only the left posterior communicating artery could be accessed. (C) Unsubtracted lateral angiography demonstrates Solitaire stent retriever in place within the distal right PCA segment during attempt at removing distal PCA thrombus.
Outcome and follow-up
The patient remained intubated immediately post-thrombectomy. This was done because of poor baseline examination and a desire to prevent movement while the femoral arteriotomy healed. At postoperative evaluation he had briskly purposeful movements of all extremities. The following day he was extubated and was moving all extremities, left arm slightly less than others. His speech did not immediately return; however, over the ensuing 48 h he began to speak and his speech returned to near baseline by discharge (post-thrombectomy day 8).
Given the concern for extension of the left PCA thrombus and presumed vertebral dissection, enoxaparin (1.2 mg/kg twice daily) was started post-thrombectomy. Routine follow-up head CT demonstrated a small left frontal lobe hematoma (figure 5A) on post-thrombectomy day 2 without referable neurologic deficits. Contemporaneous MRI demonstrated some FLAIR changes in areas of admission DWI changes, although not nearly as extensive as the admission DWI changes might have indicated (figure 5B). Enoxaparin was briefly held and he was converted to an unfractioned heparin drip. After confirming stability of the hematoma over 48 h, enoxaparin was restarted for outpatient anticoagulation. Eight days post-thrombectomy the patient's functional improvement was so robust that he was discharged home with outpatient therapy. On the day of discharge he was alert, interactive, and with near baseline speech. His discharge PedNIHSS was 1 for mild pronator drift, with a modified Rankin scale (mRS) of 2. Follow-up CTA 2 weeks post-thrombectomy demonstrated a possible pseudoaneurysm at a site of likely prior dissection (figure 3B). After discussion at a multidisciplinary pediatric neurovascular board, enoxaparin was continued. At 6-week follow-up he was completely back to baseline, with no residual deficits (PedNIHSS=0, mRS=0). Three months have passed since the patient's initial stroke event and he has not had any recurrent stroke or concern for stroke-like symptoms. Enoxaparin has recently been discontinued and the patient is maintained on aspirin 81 mg daily.
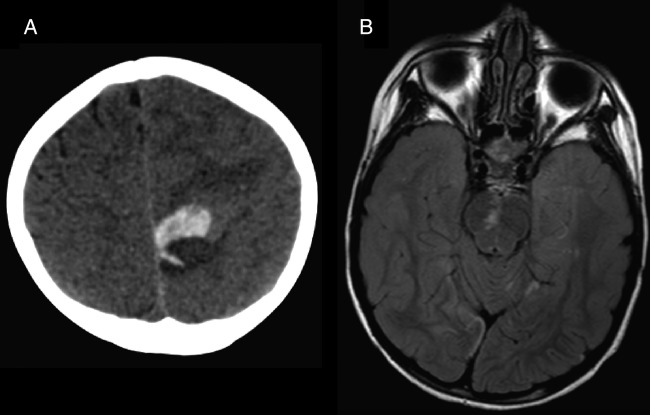
Figure 5.
(A) Head CT 2 days post-thrombectomy shows new acute high medial left frontal lobe hematoma with mild/moderate surrounding edema and local mass effect. This was thought to represent hemorrhagic transformation of distal emboli that spontaneously recanalized as it was not in an area previously at risk. (B) MRI FLAIR sequence obtained 3 days after mechanical thrombectomy shows some persistent changes, although there has been some reversal of diffusion-weighted imaging changes compared with the admission MRI, especially within the brainstem (compare figure 1, middle bottom row).
Discussion
Evidence for MT in patients with stroke in early to middle childhood (<12 years) is limited (table 1).2–12 Including this report, we could identify only 16 cases. Median NIHSS score was 18. Eight (50%) involved the posterior circulation and thrombolytics were administered in seven cases (44%). Five (31%) had arterial dissection. Ten (63%) had preoperative DWI available, each showing infarct. The median time to arterial puncture was 7 h. Seven cases (44%) employed the Solitaire device. All but two cases (88%) achieved excellent recovery. Although reporting bias must be considered,13 the available evidence suggests that MT is safe and effective in acute stroke in childhood.14
Table 1.
Outcomes of mechanical thrombectomy in acute childhood stroke
| Author | NIHSS | Location | Mechanism | DWI | Time to arterial puncture | Device | Thrombolytic | Post-MT antithrombotics | Clinical outcome |
|---|---|---|---|---|---|---|---|---|---|
| Gruber et al2 | NR | R MCA | CE | No preop MRI | 48 h | Guidewire | IA rTPA (0.11 mg/kg) | NR | Asymptomatic at 2 weeks |
| Cognard et al3 | NR | BA | VAD | BA territory | 36 h | Balloon angioplasty | IA UK (900 000 µ) | IV heparin | Asymptomatic at 3 months |
| Grigoriadis et al4 | NR | BA | VAD | BA territory | 44 h | Balloon angioplasty | IA UK (200 000 µ) | ASA, unspecific anticoagulant | Asymptomatic at 9 months |
| Tsivgoulis et al5 | 17 | R ICA | CE | No preop MRI | 3.4 h | Merci | IV (0.9 mg/kg), IA (1.1 µ) tPA | NR | NIHSS 2, mRS 1 at 3 months |
| Grunwald et al6 | 26 | L ICA | CE | L ICA territory | 3 h | Penumbra | None | ASA | NIHSS 0 at 30 days |
| Dubedout et al7 | 20 | BA | Unknown | L Pons | 6 h | CAPTURE | None | Heparin, ASA | NIHSS 0, mRS 0 at 30 days |
| Fink et al8 | 6 | BA | Unknown | R Pons, cerebellum | 4 h | Solitaire | IV tPA (0.6 mg/kg) | NR | Full recovery at 3 months |
| Tatum et al9 | 17 | R ICA | CE | R pons, MCA territory, BG | 4 h | Penumbra | IA tPA (14 mg) | NR | Ped-mRS 3 at 90 days |
| Tatum et al9 | 12 | R MCA | Post-embo | No preop MRI | 8 h | Penumbra | None | NR | Ped-mRS 1 at 90 days |
| Sainz de la Maza et al10 | 18 | R ICA | TCA | R MCA territory | 8 h | Solitaire | None | NR | NIHSS 1, mRS 1 at 3 months |
| Hu et al11 | 16 | L ICA | Unknown | L FTL | NR | Solitaire/ Penumbra | IV tPA (dose not reported) | NR | NIHSS 3 at 6 months |
| Hu et al11 | 17 | R ICA | CE | No preop MRI | NR | Solitaire | None | NR | NIHSS 2 at 3 months |
| Bodey et al12 | 27 | BA | VAD | Bilateral pons, R cerebellum | 36 h | Revive | None | IV heparin | Ped-mRS 3 at 6 months |
| Bodey et al12 | 29 | BA | Dehydration | No preop MRI | 6 h | Solitaire | None | IV heparin | mRS 2 at 6 months |
| Bodey et al12 | 28 | BA | VAD | No preop MRI | 4 h | Solitaire | None | IV heparin. warfarin, ASA | mRS 0 at 6 months |
ASA, aspirin; BA, basilar artery; CE, cardioembolic; DWI, diffusion-weighted imaging; ICA, internal carotid artery; IV, intravenous; MCA, middle cerebral artery; mRS, Modified Rankin Scale; MT, mechanical thrombectomy; NIHSS, National Institutes of Health Stroke Scale; NR, not reported; TCA, transient cerebral arteriopathy; tPA, tissue plasminogen activator; VAD, vertebral artery dissection.
Posterior circulation strokes with brainstem involvement can be devastating. Mourand et al15 developed a brainstem DWI scoring system in adults for predicting functional outcome after clot extraction in basilar artery occlusion. If applied to our patient, the probability of a follow-up mRS of ≥5 would be 80%. The excellent outcome of our patient despite conventional adult estimates prompts further assessment of the prognostic significance of DWI in this population. In children who have not undergone MT, it is known that larger DWI changes generally correlate with poor outcomes.16 However, there are no studies of initial DWI and outcomes after MT in pediatric stroke. From our review of the literature, noting several cases with extensive DWI changes, there appears to be a dramatic opportunity for recovery from an insult to the developing pediatric brain compared with adults, despite often ominous neuroimaging findings (table 1).
There is limited information on the appropriate time window for MT in childhood stroke. The SWIFT trial allowed treatment within 8 h for adults.17 The 9 h interval in our patient suggests that MT may be useful in children even outside conventional windows. Compatibility of pediatric vessels with adult endovascular instruments is understudied; however, the Solitaire 4 mm×20 mm device used here is rated for vessels 2–4 mm in diameter (Covidien Neurovascular Catalog 2014) and appears to accommodate the pediatric vasculature safely. The patient's P1 diameter was 3 mm bilaterally and the basilar apex was 3.5 mm. Some have suggested that, in the hands of operators experienced with pediatric vasculature, MT may even be safer than intra-arterial tissue plasminogen activator.10
There are no guidelines for post-thrombectomy antithrombotics in children. We found five reports of heparin, four reports of aspirin, one report of warfarin, and one unspecified anticoagulant post-thrombectomy (table 1). The decision to use anticoagulation in this child was based on the acute dissection. This case demonstrates that, even with delayed presentation, MT might still be successful in children. This child presented with an NIHSS score of 22 with extensive DWI changes, yet at 6-week follow-up was normal with no deficits and continues to do well even on long-term follow-up.
Learning points.
In selected pediatric stroke patients, neurointervention may offer chances at improved neurologic recovery, taking into account patient age, duration of symptoms, degree of injury, and experience of the neurointerventionalist.
Mechanical thrombectomy in children might be effective even outside conventional treatment windows.
Extensive DWI changes on MRI are associated with poor outcomes; however, their presence alone should not necessarily exclude pediatric stroke patients from consideration for mechanical thrombectomy.
Currently, there is no clear best treatment for pediatric acute stroke.
Footnotes
Competing interests: JM is a consultant for Lazarus Effect, Medina Medical, Pulsar Vascular, Reverse Medical, and Edge Therapeutics; an investor in Blockade Medical and Medina Medical; and is on the advisory board for Codman Neurovascular.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Ichord RN, Bastian R, Abraham L et al. Interrater reliability of the Pediatric National Institutes of Health Stroke Scale (PedNIHSS) in a multicenter study. Stroke 2011;42:613–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gruber A, Nasel C, Lang W et al. Intra-arterial thrombolysis for the treatment of perioperative childhood cardioembolic stroke. Neurology 2000;54:1684–6. [DOI] [PubMed] [Google Scholar]
- 3.Cognard C, Weill A, Lindgren S et al. Basilar artery occlusion in a child: ‘clot angioplasty’ followed by thrombolysis. Childs Nerv Syst 2000;16:496–500. [DOI] [PubMed] [Google Scholar]
- 4.Grigoriadis S, Gomori JM, Grigoriadis N et al. Clinically successful late recanalization of basilar artery occlusion in childhood: What are the odds?: Case report and review of the literature. J Neurol Sci 2007;260:256–60. [DOI] [PubMed] [Google Scholar]
- 5.Tsivgoulis G, Horton JA, Ness JM et al. Intravenous thrombolysis followed by intra-arterial thrombolysis and mechanical thrombectomy for the treatment of pediatric ischemic stroke. J Neurol Sci 2008;275:151–3. [DOI] [PubMed] [Google Scholar]
- 6.Grunwald IQ, Walter S, Shamdeen MG et al. New mechanical recanalization devices—the future in pediatric stroke treatment? J Invasive Cardiol 2010;22:63–6. [PubMed] [Google Scholar]
- 7.Dubedout S, Cognard C, Cances C et al. Successful clinical treatment of child stroke using mechanical embolectomy. Pediatr Neurol 2013;49:379–82. [DOI] [PubMed] [Google Scholar]
- 8.Fink J, Sonnenborg L, Larsen LL et al. Basilar artery thrombosis in a child treated with intravenous tissue plasminogen activator and endovascular mechanical thrombectomy. J Child Neurol 2013;28:1521–6. [DOI] [PubMed] [Google Scholar]
- 9.Tatum J, Farid H, Cooke D et al. Mechanical embolectomy for treatment of large vessel acute ischemic stroke in children. J Neurointerv Surg 2013;5:128–34. [DOI] [PubMed] [Google Scholar]
- 10.Sainz de la Maza S, De Felipe A, Matute MC et al. Acute ischemic stroke in a 12-year-old successfully treated with mechanical thrombectomy. J Child Neurol 2014;29:269–73. [DOI] [PubMed] [Google Scholar]
- 11.Hu YC, Chugh C, Jeevan D et al. Modern endovascular treatments of occlusive pediatric acute ischemic strokes: case series and review of the literature. Childs Nerv Syst 2014;30:937–43. [DOI] [PubMed] [Google Scholar]
- 12.Bodey C, Goddard T, Patankar T et al. Experience of mechanical thrombectomy for paediatric arterial ischaemic stroke. Eur J Paediatr Neurol. Published Online First: 29 July 2014. doi: 10.1016/j.ejpn.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 13.Amlie-Lefond C, deVeber G, Chan AK et al. Use of alteplase in childhood arterial ischaemic stroke: a multicentre, observational, cohort study. Lancet Neurol 2009;8:530–6. [DOI] [PubMed] [Google Scholar]
- 14.Ellis MJ, Amlie-Lefond C, Orbach DB. Endovascular therapy in children with acute ischemic stroke: review and recommendations. Neurology 2012;79:S158–64. [DOI] [PubMed] [Google Scholar]
- 15.Mourand I, Machi P, Nogué E et al. Diffusion-weighted imaging score of the brain stem: a predictor of outcome in acute basilar artery occlusion treated with the solitaire FR device. AJNR Am J Neuroradiol 2014;35:1117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zecavati N, Singh R, Farias-Moeller R et al. The utility of infarct volume measurement in pediatric ischemic stroke. J Child Neurol 2014;29:811–17. [DOI] [PubMed] [Google Scholar]
- 17.Saver JL, Jahan R, Levy EI et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet 2012;380:1241–9. [DOI] [PubMed] [Google Scholar]