Abstract
T cell immunoglobulin domain and mucin domain-containing molecule 3 (Tim-3) is a newly discovered immunomodulatory, which plays an important role in immunity regulation. Recent evidence suggests that Tim-3 is differentially regulated in a variety of tumors and has a potential as a therapeutic target. The aim of this study was to investigate the effect of Tim-3 on the development of prostate cancer (PCa). Tim-3 expressing on peripheral CD4+ T and CD8+ T cells was analyzed by flow cytometry. The relationships between Tim-3 expression and clinicopathological features were analyzed. Immunohistochemical expression of Tim-3 was examined in our large numbers of paraffin-fixed prostate tissues. Flow cytometry revealed that expression of Tim-3 was significantly increased on both CD4+ and CD8+ T cells in PCa patients than that in benign prostate hyperplasia (BPH) patients. Also, the level of Tim-3 on CD4+ T cells was positively correlated with CD8+ T cells in patients. Further analyses revealed that the levels of Tim-3 on CD4+ T cells and CD8+ T cells exhibited different expression patterns in terms of localization depending on pathological category of PCa and metastasis. Immunohistochemical analysis revealed that positive staining of Tim-3 in PCa but little or no staining of Tim-3 was observed in BPH epithelium. Tim-3 may affect the development and progression of PCa, which may provide knowledge for using Tim-3 as a novel therapy for effective PCa management.
Keywords: Prostate cancer, Tim-3, CD4+ T cells, CD8+ T cells
Prostate cancer (PCa) is a heterogeneous disease with an estimated 241,740 new cases and 28,170 deaths related to this disease in 2012, making PCa the second most frequently diagnosed cancer and the second-leading cause of cancer death in men [1]. There has been a trend towards increased incidence and morbidity of prostate cancer in Asia in the recent years [2]. With the development of serum prostate-specific antigen (PSA) screening, MRI imaging, and new prostate biopsies protocols in recent years, the accuracy of detection and localization of prostate tumors was improved obviously, but still 5 % of cases present with metastatic lesions at the time of diagnosis [3]. The most common site of metastasis for PCa is the bone, and frequently, metastasis is symptomatic [4].
The main options for localized PCa are active surveillance, radical prostatectomy, and radiotherapy (RT) with or without adjuvant androgen deprivation therapy (ADT) [5]. Radical prostatectomy is the standard treatment for organ-confined tumors; however, even after seemingly complete removal of tumor, 20 to 30 % of patients experience a recurrence, typically detected by a rise in PSA levels [6]. The incomplete understanding of molecular features of PCa might be one of the reasons for this unsatisfied situation, although recent gene expression studies have significantly improved our knowledge. Therefore, it is important to investigate the molecular mechanisms underlying the progression of PCa to provide effective strategies for the prevention and therapy of PCa.
T cell immunoglobulin and mucin-domain-containing molecule 3 (Tim-3), which could be identified as a specific cell surface marker of T-helper 1 (Th1) CD4+ T cells, is one of the Ig superfamily members and is preferentially expressed on fully differentiated Th1 lymphocytes but not on Th2 cells [7, 8]. Studies have shown that Tim-3 may not contribute to the T cell differentiation but might perform a critical function in the Th1 cell transportation [9, 10]. Interaction between Tim-3 and its ligand galectin-9 inhibits Th1 and Th17 responses and induces peripheral tolerance [11], suggesting an inhibitory role of Tim-3 in T cell responses. The soluble form of Tim-3 would reduce the antigen-specific T cell responses and downregulate the anti-tumor immunity in vivo by inhibiting the Th1 responses.
Recent studies have shown an important role of Tim-3 T cell exhaustion in a variety of tumors. Tim-3 may play important roles in the development of non-small-cell lung cancer (NSCLC) [12]. It has been shown that Tim-3-expressing CD4+ and CD8+ T cells are significantly increased in NSCLC patients. The expression levels of Tim-3 may be correlated with patients’ survival [12]. Tim-3 and PD-1 are co-expressed on CD8 tumor-infiltrating lymphocytes (TILs) in mice bearing transplanted tumors as well as on NY-ESO-1-specific CD8+ T cells in patients with advanced melanoma [13]. Blockade of the inhibitory Tim-3 pathway may prove useful in the treatment of a wide array of tumors, suggesting that Tim-3 pathway may act as one of the key factors in establishing T cell exhaustion [14]. However, there have been few studies reporting the expression of Tim-3 in PCa. This study was designed to explore the expression of Tim-3 in our large collection of clinical prostatic carcinoma samples and investigate its clinicopathological significance in PCa.
Materials and methods
Patients and tissue specimens
A total of 116 patients who had undergone radical prostatectomy and bilateral lymphadenectomy at the Department of Urology, Affiliated Hospital of Yan Bian University, between August 2001 and December 2010 and for whom archival tissues were included in this study aimed at detecting Tim-3 expression. No patient was managed preoperatively with either hormonal or radiation therapy, and no secondary cancers were observed. Ninety-two cases of benign prostate hyperplasia (BPH) were obtained from men undergoing suprapubic prostatectomy or transurethral plasmakinetic enucleation of prostate. The stages of cancer for all patients were determined by the American Joint Committee on Cancer (AJCC) 2002 system. The specimens were examined by two staff pathologists who were blinded to the clinical outcome and follow-up data. The evaluation of the specimen was performed according to the guidelines of the College of American Pathologists. Paraffin-embedded tumor tissues and peripheral blood samples from these patients were evaluated. This study was approved by the Ethics Committee of Affiliated Hospital of Yan Bian University. All patients provided informed consent.
Staining and flow cytometric analysis
Detection of Tim-3 was performed based on previously described methods. Peripheral blood sample was incubated for 30 min in 4 °C in a dark room with monoclonal antibodies or isotype-matched control. Pe-Cy5-conjugated anti-human CD3, FITC-conjugated anti-human CD4, Pe-Cy5-conjugated anti-human CD8, phycoerythrin (PE)-conjugated rat IgG2a isotype control (Bioscience, San Diego, CA, USA), and monoclonal PE-conjugated anti-human Tim-3 (R&D Systems, Minneapolis, MN 55413, USA) were used for flow cytometric analysis. Cells were analyzed using a Beckman Coulter flow cytometer (FC500, Fullerton, CA, USA), and the data were analyzed using the Cell Quest Program.
Immunohistochemistry
To quantify Tim-3 cells in large numbers of patients, paraffin-embedded PCa samples were processed for immunohistochemistry. Specimens were fixed in 10 % neutral buffered formalin, embedded in paraffin, and cut into serial sections at a thickness of 3 μm. Paraffin-embedded tissues were dewaxed in xylene, rehydrated by serial concentrations of ethanol, and then rinsed in phosphate buffer solution (PBS) followed by treatment with 3 % H2O2 to refrain endogenous peroxidase. After being heated in a microwave at 750 W for 15 min to repair the tissue antigen, the sections were incubated with 10 % normal goat serum at room temperature for 10 min to block non-specific reactions. This was followed by a PBS wash and incubation with primary rat monoclonal anti-human Tim-3 (clone 344823, 1/200, IgG2a, R&D Systems) for 12 h at 4 °C, and then with HRP-conjugated goat anti-rat IgG (1/500, Invitrogen). After a PBS wash, the sections were developed in diaminobenzidine (DAB) substrate. The sections were then counter-stained in hematoxylin for 2 min and then dehydrated in ethanol and xylene before being mounted. Sections were re-prepared by EnVision immunohistochemical staining. PBS instead of primary antibodies was as negative control. Visualization was achieved with ABC-Elite Reagent (Sigma). The sections were counter-stained with Mayer’s hematoxylin (Sigma). The nuclei were stained with 1 % ammonium hydroxide. The numbers of Tim-3 cells were counted in five fields at ×400 magnification.
Statistical analysis
Data analyses were performed using SPSS statistical package 15.0 (SPSS Inc, USA). Patient characteristics were expressed as the mean ± SD for continuous variables, and as the count and percent for discrete variables. Data were analyzed using Student’s t test, Mann–Whitney non-parametric U test, and standard chi-square analysis. The Pearson correlation analysis was used to calculate the correlation coefficient. A P value less than 0.05 was considered significant.
Results
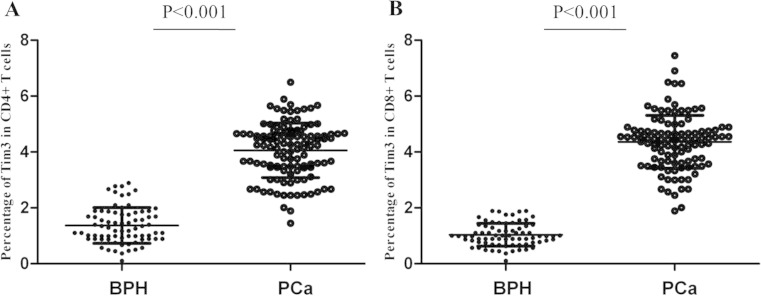
Selected characteristics of the 116 PCa patients and 92 BPH controls are presented in Table 1. We investigated the expression of Tim-3 on CD4+ T cells and CD8+ T cells from peripheral blood of 116 PCa patients and 92 BPH controls. As shown in Fig. 1a, increased proportion of Tim-3+ cells was detected on CD4+ T cells in PCa patients than that in BPH patients (mean ± SEM 4.02 ± 0.46 % vs 1.22 ± 0.32 %, P < 0.001). Similarly, the expression of Tim-3 on CD8+ T cells was also significantly elevated in PCa patients compared to BPH patients (4.46 ± 0.32 % vs 0.82 ± 0.20 %, P < 0.001) (Fig. 1b). We further investigated the correlation between Tim-3 on CD4+ T cells and Tim-3 on CD8+ T cells in PCa patients. Data revealed that the expression of Tim-3 on CD4+ T cells was positively correlated with the level of Tim-3 on CD8+ T cells in our patient group (r = 0.646, P < 0.001). These results suggest that Tim-3 maybe involved in the pathogenesis of PCa by its regulation on various immune cells.
Table 1.
Correlations of Tim-3 expression with the clinicopathological features of PCa
| Variable | No. of case | Tim-3 expression | P value | |
|---|---|---|---|---|
| Low | High | |||
| Age (years) | ||||
| ≤60 | 39 | 20 | 19 | 0.396 |
| >60 | 77 | 32 | 45 | |
| Preoperative PSA level (ng/ml) | ||||
| ≤20 | 67 | 46 | 21 | 0.038 |
| >20 | 49 | 17 | 32 | |
| Gleason score | ||||
| 2–4 | 32 | 22 | 10 | 0.009 |
| 5–7 | 25 | 8 | 17 | |
| 8–10 | 31 | 7 | 24 | |
| Metastases | ||||
| With | 27 | 3 | 24 | P < 0.001 |
| Without | 89 | 31 | 58 | |
Fig. 1.
Percentage of Tim-3 expression on CD4+ and CD8+ T cells in PCa patients and BPH patients. a Percentage of Tim-3 expression on CD4+ T cells. b Percentage of Tim-3 expression on CD8+ T cells
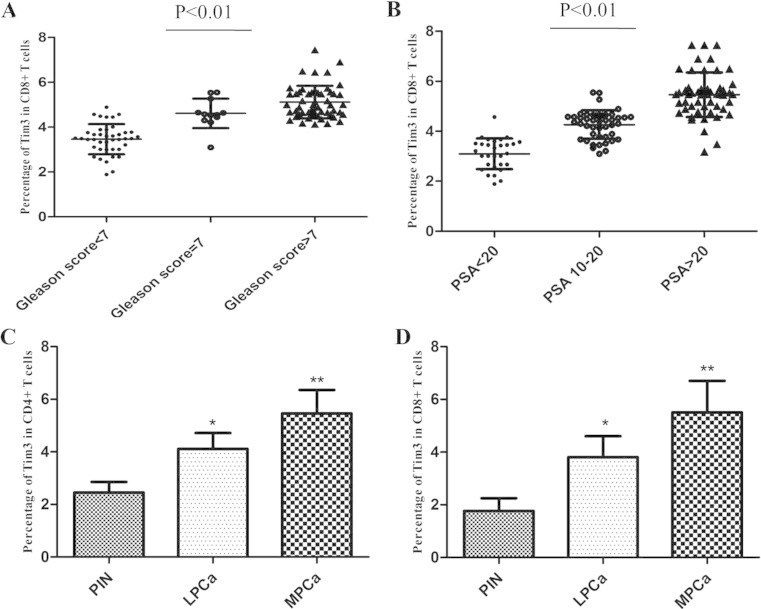
We further investigated the levels of Tim-3 on CD4+ T cells and CD8+ T cells in the different groups of PCa patients. Data showed that the levels of Tim-3 on CD4+ T cells and CD8+ T cells exhibited different expression patterns in terms of localization depending on pathological category of PCa and metastasis. We stratified localized PCa by the Gleason score into three subgroups, Gleason score <7, =7, and >7. In the localized PCa samples, levels of Tim-3 on CD4+ T cells and CD8+ T cells appeared to be associated with a higher Gleason score, which reached its predominance in Gleason >7 cases (Fig. 2a). We further stratified localized PCa by three subcategories on the basis of preoperative PSA levels: <10 ng/ml as low risk, 10–20 ng/ml as intermediate risk, and >20 ng/ml as high risk. The analysis revealed that patients with the higher PSA level presented significantly higher Tim-3 expression on these cells, in which PCa patients with >20 ng/ml PSA levels revealed significantly upregulated level of Tim-3 than the other stages (Fig. 2b). Next, we analyzed for Gleason score and preoperative PSA simultaneously. The analysis revealed that in the high-risk subcategory (PSA > 20 ng/ml) higher levels of Tim-3 on CD4+ T cells and CD8+ T cells were associated with Gleason >7, and lower levels of Tim-3 on these cells were associated with Gleason <7 (P < 0.05).
Fig. 2.
a Percentage of Tim-3 expression on CD8+ T cells in the three subgroups of localized PCa samples, Gleason score <7, =7, and >7. b Percentage of Tim-3 expression on CD8+ T cells in the in the three subgroups of localized PCa samples, PSA levels <10 ng/ml, 10–20 ng/ml, and > 20 ng/ml. c Percentage of Tim-3 expression on CD4+ T cells in PIN, localized PCa, and metastatic PCa. d Percentage of Tim-3 expression on CD8+ T cells in PIN, localized PCa, and metastatic PCa (*P < 0.05; **P < 0.01)
In addition, the difference of levels of Tim-3 on both CD4+ T cells and CD8+ T cells between tissues with or not with all types of metastasis (lymphnode, central nervous system, or bone) also exists statistical significance (P < 0.01). No significant correlation, however, was found between levels of Tim-3 and age. Altogether, levels of Tim-3 on both CD4+ T cells and CD8+ T cells revealed significant elevation from PIN to localized and to metastatic PCa (Fig. 2c, d).
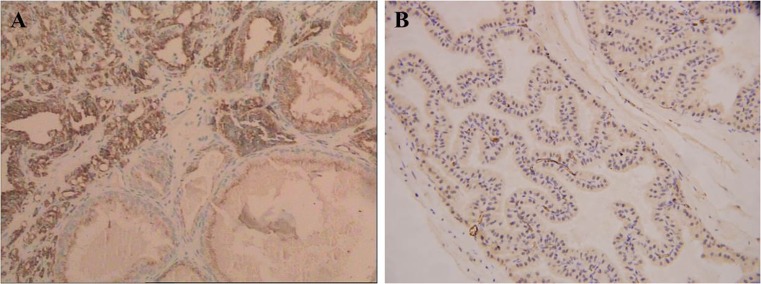
As levels of Tim-3 on both CD4+ T cells and CD8+ T cells were basically detected in peripheral blood of PCa and BPH patients, we extended our studies further to include large numbers of paraffin-fixed prostate tissues with immunohistochemistry. Immunohistochemical expression of Tim-3 was examined in 92 BPH tissues and 116 PCa tissues. We observed positive staining of Tim-3 in PCa; however, little or no staining of Tim-3 was observed in BPH epithelium (Fig. 3). A total of 82 of 116 (70.69 %) malignant cases showed positive staining for Tim-3, 18 of 92 (19.57 %) benign cases showed positive, and the difference of Tim-3 expression between PCa and BPH was statistically significant (P < 0.001). There were higher numbers of Tim-3 cells in PCa tissues than BPH tissues. These results indicate that Tim-3 expression is increased on T cells infiltrating the PCa microenvironment.
Fig. 3.
Immunohistochemistry. a Positive staining of Tim-3 was observed in prostate cancer. b Little or no staining of Tim-3 was observed in the cytoplasm of benign prostate epithelium (Envision × 40)
A total of 116 archival PCa samples with intact clinicopathological data were identified for Tim-3 expression by immunohistochemistry and correlated with clinicopathological parameters. As a result, Tim-3 expression was positively correlated with the Gleason score and the concentration of PSA in blood serum and metastasis, while there was no significant relationship between Tim-3 level and variables such as age (Table 1).
Discussion
Tim-3 has emerged as a promising target for cancer immunotherapy [11]. Recent studies have focused on the role of Tim-3 expression in multiple pathological scenarios. Tim-3 is a molecule expressed on terminally differentiated Th1 cells but not on Th2 cells, which negatively regulate Th1 immunity [11]. It is also a phosphatidylserine receptor to mediate phagocytosis of apoptotic cells [15]. Recent studies showed that Tim-3 plays a significant role in tumor progression by maintaining the tumor immunosuppressive environment via regulatory T cells (T regs). Many studies have shown that dysregulation of Tim-3 expression on CD4+ T cells and CD8+ T cells is closely related to various tumors. For example, Wu et al. showed that Tim-3 expression on CD4+ T cells and CD8+ T cells was elevated in ovarian cancer [16]. Han et al. reported that the level of Tim-3 on CD4+ T cells was increased in glioma patients and was correlated with disease progression [17]. Recent studies have focused on the role of Tim-3 expression on CD8+ T cells in peripheral blood as well as within tumors. Therefore, Tim-3 has emerged as a promising target for cancer immunotherapy. However, the nature of the Tim-3 signaling pathway remains undefined in patients with PCa. In this study, we evaluated the expression and clinical relevance of the Tim-3 signaling pathway in a large set of prostate samples, including BPH, PIN, localized PCa, and metastatic PCa.
We first examined Tim-3 expression on various immune cells in PCa patients and further explored its correlation with disease activity. We investigated the expression of Tim-3 on CD4+ T cells and CD8+ T cells from peripheral blood of 116 PCa patients and 92 BPH controls. Our results show that the expression of Tim-3 on CD4+ T cells and CD8+ T cells was significantly elevated in PCa patients compared to BPH patients and a significantly positive correlation of Tim-3 expression on CD4+ T cells and Tim-3 on CD8+ T cells exists in PCa patients. Our data provided direct evidence for the first time that Tim-3 was involved in the pathogenesis of PCa by its regulation on CD4+ and CD8+ T cell subsets.
Furthermore, we found that there was a significantly positive correlation between the level of Tim-3 on CD4+ T cells and the level of Tim-3 on CD8+ T cells in PCa patients. It is interesting that we have found the levels of Tim-3 on CD4+ T cells and CD8+ T cells exhibited different expression patterns in terms of localization depending on pathological category of PCa and metastasis. In the localized PCa samples, levels of Tim-3 on CD4+ T cells and CD8+ T cells appeared to be associated with higher Gleason score and the higher preoperative PSA levels. In line with previous analysis, the level of Tim-3 on CD4+ T cells could be positively correlated with disease progression. In our PCa patients, levels of Tim-3 on both CD4+ T cells and CD8+ T cells revealed significant elevation from PIN to localized and to metastatic PCa, suggesting that Tim-3 may also act as an indicator of the disease progression in PCa. In addition, the difference of levels of Tim-3 on both CD4+ T cells and CD8+ T cells between tissues with or not with all types of metastasis (lymphnode, central nervous system, or bone) also exists statistical significance (P < 0.01).
Immunohistochemical analysis revealed that positive staining of Tim-3 in PCa but little or no staining of Tim-3 was observed in BPH epithelium. It is possible that Tim-3 expression is increased on T cells infiltrating the PCa microenvironment. Tim-3 expression was positively correlated with the Gleason score, the concentration of PSA in blood serum, and the bone metastasis in PCa patients, while there was no significant relationship between Tim-3 level and variables such as age.
In conclusion, our study identified an increased level of Tim-3 on both CD4+ and CD8+ T cells in peripheral blood as well as within tumors of PCa patients. Levels of Tim-3 on both CD4+ T cells and CD8+ T cells closely correlate with advanced disease stage, which predicts a poorer prognosis. However, further studies will be needed to understand the mechanism on how Tim-3 may affect the development and progression of PCa, which may provide knowledge for using Tim-3 as a novel therapy for effective tumor management.
Acknowledgments
Conflicts of interest
None.
Footnotes
The Publisher and Editor retract this article in accordance with the recommendations of the Committee on Publication Ethics (COPE). After a thorough investigation we have strong reason to believe that the peer review process was compromised.
An erratum to this article can be found online at 10.1007/s13277-017-5487-6.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Ren SC, Chen R, Sun YH. Prostate cancer research in China. Asian J Androl. 2013;15:350–3. doi: 10.1038/aja.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saad F, Pantel K. The current role of circulating tumor cells in the diagnosis and management of bone metastases in advanced prostate cancer. Future Oncol. 2012;8:321–31. doi: 10.2217/fon.12.3. [DOI] [PubMed] [Google Scholar]
- 4.Raheem O, Kulidjian AA, Wu C, Jeong YB, Yamaguchi T, Smith KM, et al. A novel patient-derived intra-femoral xenograft model of bone metastatic prostate cancer that recapitulates mixed osteolytic and osteoblastic lesions. J Transl Med. 2011;9:185. doi: 10.1186/1479-5876-9-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localized disease. Eur Urol. 2011;59:61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 6.Pontes-Junior J, Reis ST, de Oliveira LC, Sant’anna AC, Dall’oglio MF, Antunes AA, et al. Association between integrin expression and prognosis in localized prostate cancer. Prostate. 2010;70:1189–95. doi: 10.1002/pros.21153. [DOI] [PubMed] [Google Scholar]
- 7.Anderson AC. Tim-3, a negative regulator of anti-tumor immunity. Curr Opin Immunol. 2012;24:213–6. doi: 10.1016/j.coi.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, et al. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318:1141–3. doi: 10.1126/science.1148536. [DOI] [PubMed] [Google Scholar]
- 9.Kikushige Y, Akashi K. TIM-3 as a therapeutic target for malignant stem cells in acute myelogenous leukemia. Ann N Y Acad Sci. 2012;1266:118–23. doi: 10.1111/j.1749-6632.2012.06550.x. [DOI] [PubMed] [Google Scholar]
- 10.Kozlowska A, Mackiewicz J, Mackiewicz A. Therapeutic gene modified cell based cancer vaccines. Gene. 2013;525:200–7. doi: 10.1016/j.gene.2013.03.056. [DOI] [PubMed] [Google Scholar]
- 11.Sakuishi K, Jayaraman P, Behar SM, Anderson AC, Kuchroo VK. Emerging Tim-3 functions in antimicrobial and tumor immunity. Trends Immunol. 2011;32:345–9. doi: 10.1016/j.it.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang F, et al. TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PLoS One. 2012;7(2):e30676. doi: 10.1371/journal.pone.0030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–86. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–79. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–52. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 16.Wu J, Liu C, Qian S, Hou H. The expression of Tim-3 in peripheral blood of ovarian cancer. DNA Cell Biol. 2013;32:648–53. doi: 10.1089/dna.2013.2116. [DOI] [PubMed] [Google Scholar]
- 17.Han S, Feng S, Xu L, Shi W, Wang X, Wang H, et al. Tim-3 on peripheral CD4(+) and CD8(+) T cells is involved in the development of glioma. DNA Cell Biol. 2014;33:245–50. doi: 10.1089/dna.2013.2306. [DOI] [PubMed] [Google Scholar]