Abstract
The mechanisms that mediate the cardiovascular protective effects of omega 3 (n-3) polyunsaturated fatty acids (PUFAs) have not been fully elucidated. Cytochrome P450 1A1 efficiently metabolizes n-3 PUFAs to potent vasodilators. Thus, we hypothesized that dietary n-3 PUFAs increase nitric oxide (NO)–dependent blood pressure regulation and vasodilation in a CYP1A1-dependent manner. CYP1A1 wild-type (WT) and knockout (KO) mice were fed an n-3 or n-6 PUFA–enriched diet for 8 weeks and were analyzed for tissue fatty acids and metabolites, NO-dependent blood pressure regulation, NO-dependent vasodilation of acetylcholine (ACh) in mesenteric resistance arterioles, and endothelial NO synthase (eNOS) and phospho-Ser1177-eNOS expression in the aorta. All mice fed the n-3 PUFA diet showed significantly higher levels of n-3 PUFAs and their metabolites, and significantly lower levels of n-6 PUFAs and their metabolites. In addition, KO mice on the n-3 PUFA diet accumulated significantly higher levels of n-3 PUFAs in the aorta and kidney without a parallel increase in the levels of their metabolites. Moreover, KO mice exhibited significantly less NO-dependent regulation of blood pressure on the n-3 PUFA diet and significantly less NO-dependent, ACh-mediated vasodilation in mesenteric arterioles on both diets. Finally, the n-3 PUFA diet significantly increased aortic phospho-Ser1177-eNOS/eNOS ratio in the WT compared with KO mice. These data demonstrate that CYP1A1 contributes to eNOS activation, NO bioavailability, and NO-dependent blood pressure regulation mediated by dietary n-3 PUFAs.
Introduction
Evidence from epidemiology studies as well as randomized clinical trials has shown that omega 3 (n-3) polyunsaturated fatty acids (PUFAs), specifically eicosapentaenoic acid (EPA; 20:5n-3) and docosahexanoic acid (DHA; 22:6n-3), reduce the incidence of cardiovascular diseases, including reducing cardiac events, decreasing the progression of atherosclerosis, and reducing hypertension (Morris et al., 1993; von Schacky et al., 1999; Kris-Etherton et al., 2002; de Oliveira Otto et al., 2013). Furthermore, increasing evidence supports the use of the omega-3 index (EPA+DHA as the percentage of fatty acids in red blood cells) as a biomarker and potential risk factor for cardiovascular disease (Harris, 2010; Marchioli and Levantesi, 2013). These cardiovascular benefits may be derived, in part, by the ability of n-3 PUFAs to improve endothelial function and reduce blood pressure. EPA and DHA are found at high concentrations in oily fish, and studies show that dietary fish oil supplementation significantly improves nitric oxide (NO)–dependent vasodilation in healthy subjects and in individuals with congestive heart failure, type 2 diabetes mellitus, and hypercholesterolemia (Goodfellow et al., 2000; Khan et al., 2003; Morgan et al., 2006; Stirban et al., 2010). Additionally, a meta-analysis of 31 controlled trials showed that fish oil supplementation is associated with a statistically significant, dose-dependent reduction in blood pressure in individuals with hypertension (Morris et al., 1993).
The mechanisms by which n-3 PUFAs mediate their vascular and blood pressure benefits have not been fully elucidated. It has been demonstrated that n-3 PUFAs can increase NO synthase (NOS) activity and NO bioavailability (Omura et al., 2001; Lopez et al., 2004; Li et al., 2007a,b; Singh et al., 2010; Wu et al., 2012). In addition, EPA and DHA are endogenous substrates for cytochrome P450 (P450) epoxygenases, which result in the generation of potent vasodilators, including 17,18-epoxyeicosatetraenoic acid (17,18-EEQ) and 19,20-epoxydocosapentaenoic acid (19,20-EDP), respectively (Barbosa-Sicard et al., 2005; Muller et al., 2007; Westphal et al., 2011). It has been shown that the vasodilatory action of n-3 PUFAs requires P450 metabolism (Wang et al., 2011), but it is not clear whether this is mediated by increases in NO. We have shown that genetic deletion of CYP1A1, a P450 that efficiently metabolizes EPA and DHA, significantly reduces the vasodilation responses to EPA and DHA and results in hypertension without an apparent change in NO bioavailability (Agbor et al., 2012). However, this work was conducted in mice fed a standard chow diet that was relatively low in n-3 PUFAs (0.3% α-linolenic acid, 18:3n-3; 0% EPA and DHA). Thus, we hypothesized that an n-3 PUFA-enriched diet would significantly increase EPA and DHA and their metabolites in the vasculature, and this would be associated with a CYP1A1-dependent increase in NOS activation and in NO-dependent vasodilation and blood pressure regulation.
Materials and Methods
An expanded methods section is available in the online Supplemental Methods.
Chemicals.
Acetylcholine, potassium chloride (KCl), N5-(nitroamidino)-l-2,5-diaminopentanoic acid (LNNA), and all ingredients of physiologic saline solution and homogenation buffer were purchased from Sigma-Aldrich (St. Louis, MO). The 9,11-dideoxy-9α,11α-methanoepoxy-prosta-5Z,13E-dien-1-oic acid (U46619) was purchased from Cayman Chemical (Ann Arbor, MI). Ionomycin was purchased from EMD Millipore Chemicals (San Diego, CA).
Animals and Diets.
CYP1A1 knockout mice backcrossed more than eight generations to C57Bl/6J background were generously provided by Dr. Daniel Nebert (University of Cincinnati) (Dalton et al., 2000). Age-matched C57BL/6J mice served as wild-type (WT) controls. Animals were housed in a temperature-controlled environment and fed standard 2020X Rodent Diet (Teklad Diets; Harlan Laboratories, Madison, WI). At 4 months of age, male mice were fed either an n-3 PUFA–enriched chow (150 g menhaden fish oil/kg diet; Teklad Diets) or an n-6 PUFA–enriched chow (150 g safflower oil/kg diet; Teklad Diets) for 8 weeks (see the Supplemental Methods and Supplemental Fig. 1 for additional details). Before the tissues were harvested, mice were anesthetized with a single intraperitoneal injection of ketamine/xylazine (80/4 mg/kg) and were euthanized by exsanguination. All animal protocols were approved by the University of New Mexico Animal Care and Use Committee (100749), and the investigations conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Tissue Analysis of Cytochrome P450 Eicosanoids and Fatty Acids.
Cytochrome P450–generated eicosanoids, including epoxyeicosatrienoic acids (EETs) from arachidonic acid (AA; 20:4n-6), and EEQs and EDPs from EPA and DHA, respectively, were determined from selected tissues of CYP1A1 WT and KO mice fed an n-3 or n-6 PUFA–enriched diet (n = 5 per genotype/diet) as described previously elsewhere (Arnold et al., 2010). Briefly, plasma (100 µl) or homogenized tissue samples (30 mg aliquots) were mixed with internal standards [10 ng of each 20-hydroxyeicosatetraenoic acid (HETE)-d6; 14,15-EET-d8; 14,15-dihydroxyeicosatrienoic acid-d11; and 15-HETE-d8 (Cayman Chemical)] and subjected to alkaline hydrolysis. The metabolites were extracted by solid-phase extraction and quantified by liquid chromatography with tandem mass spectrometry. Fatty acid analysis was conducted by liquid chromatography mass spectrometry; details are described in the Supplemental Methods and in Supplemental Tables 1 and 2.
Assessment of Mesenteric Arteriolar Vasoreactivity.
Mesenteric arteriolar vasoreactivity was assessed using a pressure myograph system (DMT-110 systems; Danish Myo Technology, Ann Arbor, MI) as described previously elsewhere (Agbor et al., 2012). Briefly, mesenteric arterioles were cleaned of connective tissue and adventitial fat, and were cannulated and pressurized to 60 mm Hg. Arteries from CYP1A1 WT and KO mice fed an n-3 or n-6 PUFA–enriched diet were preconstricted with U46619; vasodilation to ACh (10−9 to 10−4 M) ± preincubation with 100 µM LNNA was assessed (n = 6 per genotype/diet). The passive internal diameter (WT 172.3 ± 4.1 μm; KO 178.4 ± 3.0 μm) was determined after preincubation with the calcium ionophore ionomycin (10−5 M) for 15 minutes.
Measurement of Blood Pressure, Heart Rate, and Activity by Radiotelemetry.
Mean arterial blood pressure (MAP), heart rate (HR), and activity were measured using radiotelemetry (Data Sciences International, St. Paul, MN) in CYP1A1 WT and KO mice fed an n-3 or n-6 PUFA–enriched diet as previously described elsewhere (n = 4–6 per genotype/diet) (Lund et al., 2008; Kopf et al., 2010; Agbor et al., 2012). Seven days after surgery, we recorded MAP, HR, and activity for 10 seconds every 15 minutes for 7 days before treatment with LNNA in the drinking water (250 mg/l) for 3 days (Duling et al., 2006).
Immunoprecipitation and Western Blot Analysis of Endothelial Nitric Oxide Synthase and Phospho-Ser1177-eNOS.
Thoracic and abdominal aortas from CYP1A1 WT and KO mice fed an n-3 or n-6 PUFA–enriched diet were cleaned of connective tissue and perivascular fat, then were homogenized and supernatant frozen after centrifugation at 12,000g for 10 minutes (n = 3 per genotype/diet). The protein concentration was determined with a bicinchoninic acid assay (Thermo Scientific, Rockford, IL). Endothelial nitric oxide synthase (eNOS) was immunoprecipitated from 250 μg of aortic protein by incubating with 1.0 µg anti-eNOS primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 24 hours at 4°C followed by the addition of protein A-agarose beads (Life Technologies, Grand Island, NY) and a 2-hour incubation at 4°C. The beads were isolated by centrifugation (2000g at 4°C for 1 minute) and washed 5 times.
Protein was eluted in Laemmli sample buffer (Bio-Rad Laboratories, Hercules, CA) by heating at 100°C for 5 minutes, resolved on a 7.5% SDS-polyacrylamide gel (Bio-Rad Laboratories), and transferred to polyvinylidene fluoride membrane. Membranes were blocked, probed with anti-eNOS (1:1000) or anti–phospho-Ser1177-eNOS (1:1000; Cell Signaling Technology, Danvers, MA), washed and probed with anti-rabbit-IgG (1:4000). Proteins were detected using SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific), and the band signal intensity was obtained by use of a FluorChem R Imager (Protein Simple, Santa Clara, CA).
Statistical Analysis.
Body and organ weights were compared between genotypes by Student’s t-test. Tissue composition of fatty acids and metabolites was analyzed by two-way analysis of variance (ANOVA) with post hoc Holm-Sidak comparisons and Bonferroni correction. Diet and genotype differences in total eNOS and the ratio of phospho-Ser1177-eNOS/eNOS were analyzed by two-way ANOVA. Hourly MAP, HR and vasoreactivity were compared among groups using two-way repeated measures ANOVA, and the 24-hour MAP and HR were compared among groups using two-way ANOVA. When data were statistically significant by ANOVA, post hoc Holm-Sidak comparisons were used. P < 0.05 was considered statistically significant.
Results
Effect of CYP1A1 Genotype and PUFA Diet on Tissue Fatty Acid Composition and Generation of Cytochrome P450–Dependent Metabolites.
To interpret the physiologic changes associated with feeding an n-3 or n-6 PUFA–enriched diet, we investigated the degree to which the fatty acid composition and P450-dependent metabolites in tissues differed. We found that both CYP1A1 WT and KO mice fed an n-3 PUFA–enriched diet exhibited a significantly higher percentage of EPA and DHA and a significantly lower percentage of linoleic acid (18:2n-6) and AA in all tissues analyzed (Fig. 1). Notably, CYP1A1 KO mice accumulated a greater percentage of EPA and DHA in the aorta and kidney, compared with WT mice. In addition, the higher percentages of EPA and DHA were associated with higher levels of P450-derived EPA and DHA metabolites (EEQs and EDPs) in all tissues and lower levels of P450-derived AA metabolites (EETs; Fig. 2). The only genotype-dependent difference in metabolites occurred in the kidney, where CYP1A1 KO mice had significantly higher levels of EDPs when fed an n-6 PUFA diet.
Fig. 1.
Effect of CYP1A1 on fatty acid composition in selected organs from mice fed an n-3 or n-6 PUFA–enriched diet. (A–G) Fatty acid profiles from selected organs were analyzed from CYP1A1 WT and KO mice after feeding an n-3 PUFA or n-6 PUFA–enriched diet for 8 weeks. Data represent the mean ± S.E.M. (n = 5 per genotype/diet). *Above a horizontal line, indicates a statistically significant difference between n-3 and n-6 PUFA diet, P < 0.05. *Above a vertical bar indicates a significant difference between WT and KO mice within a diet, P < 0.05. ∑Cx:0, sum of saturated fatty acids; ∑Cx:1, sum of monounsaturated fatty acids; ALA, α-linolenic acid; DPA, docosapentaenoic acid (20:5); LA, linoleic acid (18:2n-6); RBC, red blood cell.
Fig. 2.
Effect of CYP1A1 on the profile of AA-, EPA-, and DHA-derived monoepoxides in selected organs from mice fed an n-3 or n-6 PUFA–enriched diet. (A–G) AA-derived EETs, EPA-derived EEQs, and DHA-derived EDPs were determined by liquid chromatography with tandem mass spectrometry in selected organs of CYP1A1 and KO mice. Data represent the mean ± S.E.M. (n = 5–6 per genotype/diet). *Above a horizontal line, indicates a statistically significant difference between n-3 and n-6 PUFA diet, P < 0.05. *Above a vertical bar, indicates a significant difference between WT and KO mice within a diet, P < 0.05. RBC, red blood cell.
Effect CYP1A1 Genotype and PUFA Diet on Body and Organ Weights, and Activity.
Body and organ weights from CYP1A1 KO mice fed an n-3 PUFA–enriched diet were not different compared with WT mice. In contrast, CYP1A1 KO mice fed an n-6 PUFA–enriched diet exhibited significantly lower body, heart, and liver weights, compared with WT mice. However, when liver and heart weights were normalized to body weight, there was no difference between genotypes (Table 1). Food consumption did not differ between genotypes or diets (data not shown); however, CYP1A1 KO mice exhibited significantly lower activity levels on the n-3 PUFA diet (Supplemental Fig. 2; Supplemental Methods).
TABLE 1.
Body and organ weights of 8-month-old CYP1A1 WT and KO mice on an n-3 or n-6 PUFA–enriched diet
Values are expressed as mean ± S.E.M., n = 8.
| Weight | n-3 PUFA Diet |
n-6 PUFA Diet |
||
|---|---|---|---|---|
| CYP1A1 WT | CYP1A1 KO | CYP1A1 WT | CYP1A1 KO | |
| Body (g) | 32.5 ± 1.1 | 31.2 ± 0.9 | 31.9 ± 0.8 | 28.2 ± 1.0* |
| Heart (mg) | 128 ± 4 (0.40 ± 0.26)a | 120 ± 4 (0.39 ± 0.24) | 122 ± 2 (0.39 ± 0.18) | 110 ± 0.9 (0.39 ± 0.16)* |
| Liver (mg) | 1661 ± 95 (5.0 ± 0.2) | 1553 ± 43 (5.0 ± 0.2) | 1382 ± 27 (4.3 ± 0.1) | 1245 ± 37 (4.4 ± 0.1)* |
| Kidney (mg) | 403 ± 13 (1.2 ± 0.1) | 369 ± 13 (1.2 ± 0.1) | 329 ± 7 (1.0 ± 0.1) | 348 ± 9 (1.2 ± 0.1) |
(Organ/body weight × 100).
P < 0.05 versus WT on the same diet.
Role of CYP1A1 Genotype and PUFA Diet on NO-Dependent Blood Pressure and HR.
To investigate the interaction of CYP1A1 and dietary PUFA composition on NO-dependent blood pressure regulation, we assessed MAP and HR ± NOS inhibition in CYP1A1 WT and KO mice fed an n-3 or n-6 PUFA diet. On the n-3 PUFA diet, hourly MAP was comparable between genotypes and exhibited a normal circadian pattern of increasing during the dark cycle when the mice were active (Fig. 3A). After 3 days of NOS inhibition, hourly MAP in mice on the n-3 PUFA diet increased significantly in both genotypes; however, the increase in WT mice was greater than that observed in KO mice, resulting in a genotype-dependent difference (P < 0.05) (Fig. 3B). In addition, the increase in MAP in WT mice was significantly greater than in KO mice during the dark cycle, resulting in a genotype–time-dependent interaction (P < 0.02). In contrast, on the n-6 PUFA diet CYP1A1 WT and KO mice failed to exhibit any differences in hourly MAP either before (Fig. 3C) or during NOS inhibition (Fig. 3D).
Fig. 3.
Effect of CYP1A1 on NO-dependent blood pressure regulation in mice fed an n-3 or n-6 PUFA–enriched diet. Hourly mean arterial pressure (MAP mm Hg) measured by radiotelemetry ± LNNA (250 mg/l) in drinking water for 3 days from CYP1A1 WT and KO mice fed an n-3 PUFA diet (A and B) or an n-6 PUFA diet for (C and D) 8 weeks. (E) A 24-hour MAP from CYP1A1 WT and KO mice fed an n-3 or n-6 PUFA diet. (F) Mean change in MAP of CYP1A1 WT and KO mice fed an n-3 or n-6 PUFA diet and treated with LNNA (250 mg/l) in drinking water for 3 days. Data represent the mean ± S.E.M. (n = 4 per genotype/diet). (A, C, and D) Two-way repeated measures ANOVA, time (P < 0.001). (B) Two-way repeated measures ANOVA, time (P < 0.001), genotype (P < 0.05), time-genotype interaction (P < 0.02). (E) Two-way ANOVA, diet (P < 0.001). (F) Two-way ANOVA, genotype (P < 0.05), diet (P < 0.02), genotype-diet interaction (P < 0.002). Post hoc comparisons: *P < 0.05 versus WT mice fed the same diet; †P < 0.05 versus the same genotype fed n-3 PUFA diet.
When the baseline 24-hour MAP was compared among groups, both WT and KO mice exhibited significantly lower MAP on an n-3 PUFA diet (MAP mm Hg, WTn-3 PUFA, 107.6 ± 1.2; KOn-3 PUFA, 104.6 ± 1.3) compared with an n-6 PUFA diet (MAP mm Hg, WTn-6 PUFA, 115.3 ± 1.0; KOn-6 PUFA, 117.4 ± 2.0) (diet-dependent effect P < 0.001; Fig. 3E). When the increase in MAP resulting from NOS inhibition was compared among groups, there was a genotype- and diet-dependent effect (P < 0.05 and P < 0.02, respectively) and a genotype-diet interaction (P < 0.02) (Fig. 3F). CYP1A1 WT mice on an n-3 PUFA diet exhibited a significantly greater increase in MAP during NOS inhibition compared with KO mice on an n-3 PUFA diet (change Δ in MAP + LNNA mm Hg: WTn-3 PUFA, 16.0 ± 0.9; KOn-3 PUFA, 11.4 ± 0.9; P < 0.05) and compared with WT mice on an n-6 PUFA diet (ΔMAP + LNNA mm Hg: WTn–6 PUFA, 10.9 ± 1.0; P < 0.05). However, CYP1A1 WT and KO mice responded with an equivalent increase in MAP on an n-6 PUFA diet (ΔMAP + LNNA mm Hg: WTn-6 PUFA, 10.9 ± 1.0; KOn-6 PUFA, 11.3 ± 1.0).
In addition to MAP, hourly HR also was recorded in mice by radiotelemetry. Hourly HR was significantly lower in KO mice on an n-3 PUFA diet (genotype effect P < 0.006; Fig. 4A). After 3 days of NOS inhibition, hourly HR decreased significantly in both genotypes; however, the HR of the KO mice remained significantly lower than in the WT mice (Fig. 4B). In contrast, CYP1A1 WT and KO mice on an n-6 PUFA diet failed to exhibit any differences in HR either before (Fig. 4C) or during NOS inhibition (Fig. 4D). When the baseline 24-hour HR was compared among groups, there was a genotype-dependent effect (P < 0.02), with the CYP1A1 KO mice exhibiting a significantly lower HR on the n-3 PUFA diet (Fig. 4E). When the decrease in HR resulting from NOS inhibition was compared among groups, there was a diet-dependent effect (P < 0.001), with the decrease in HR being significantly greater in mice on the n-6 PUFA diet (Fig. 4F).
Fig. 4.
Effect of CYP1A1 on HR in mice fed an n-3 or n-6 PUFA–enriched diet. Hourly heart rate measured by radiotelemetry ± LNNA (250 mg/l) in drinking water for 3 days from CYP1A1 WT and KO mice fed an n-3 PUFA diet (A and B) or an n-6 PUFA diet (C and D) for 8 weeks. (E) Twenty-four-hour HR from CYP1A1 WT and KO mice fed an n-3 or n-6 PUFA diet. (F) Mean change in HR of CYP1A1 WT and KO mice fed an n-3 or n-6 PUFA diet and treated with LNNA (250 mg/l) in drinking water for 3 days. Data represent the mean ± S.E.M. (n = 4 per genotype/diet). (A) Two-way repeated measures ANOVA, time (P < 0.001), genotype (P < 0.006). (B) Two-way repeated measures ANOVA, time (P < 0.001), genotype (P < 0.013). (C and D) Two-way repeated measures ANOVA, time (P < 0.001). (E) Two-way ANOVA, genotype (P < 0.019). (F) Two-way ANOVA, diet (P < 0.001). Post hoc comparisons: *P < 0.05 versus WT mice fed the same diet; †P < 0.05 versus the same genotype fed n-3 PUFA diet.
Role of CYP1A1 Genotype and PUFA Diet on NO-Dependent, ACh-Mediated Vasodilation of Mesenteric Arterioles.
To investigate the role of CYP1A1 and dietary PUFA composition on NO-dependent, ACh-mediated dilation, we assessed ACh vasodilation ± NOS inhibition in mesenteric arterioles from CYP1A1 WT and KO mice fed an n-3 or n-6 PUFA diet. CYP1A1 KO mice fed an n-3 PUFA diet exhibited significantly less ACh dilation compared with WT mice (genotype effect P < 0.0.02; Fig. 5A). Treatment with the NOS inhibitor LNNA significantly attenuated ACh vasodilation in both genotypes and normalized the response in KO arterioles to WT levels (Fig. 5B). In contrast, CYP1A1 KO mice fed an n-6 PUFA diet exhibited the same degree of ACh dilation as CYP1A1 WT mice (Fig. 5C). But while NOS inhibition significantly attenuated ACh dilation in WT mice, it had no effect in the KO mice (genotype effect P < 0.01; Fig. 5D). When the amount of NO-dependent ACh dilation was compared among groups, there was a genotype- and diet-dependent effect (P < 0.004 and P < 0.001, respectively) but no genotype-diet interaction. Both genotypes exhibited significantly less NO-dependent dilation on the n-6 PUFA diet, and KO mice exhibited significantly less NO-dependent dilation than WT mice on both diets (Fig. 5E).
Fig. 5.
Effect of CYP1A1 on NO-dependent, ACh-vasodilation in mesenteric arterioles from mice fed an n-3 or n-6 PUFA–enriched diet. ACh vasodilation ± LNNA (100 µM) in mesenteric arterioles preconstricted with U46619 from CYP1A1 WT and KO mice fed an n-3 PUFA diet (A and B) or an n-6 PUFA diet (C and D) for 8 weeks. (E) NO-dependent ACh vasodilation expressed as area under the curve (AUC, arbitrary units). Data represent the mean ± S.E.M. (n = 6 per genotype/diet). (A) Two-way repeated measures ANOVA, dose (P < 0.001) and genotype (P < 0.02). (D) Two-way repeated measures ANOVA, time (P < 0.001) and genotype (P < 0.01). (E) Two-way ANOVA, genotype (P < 0.004) and diet (P < 0.001). Post hoc comparisons: *P < 0.05 versus WT mice fed the same diet; †P < 0.05 versus the same genotype fed n-3 PUFA diet.
Effect of CYP1A1 Genotype and PUFA Diet on eNOS Expression and Activation.
To determine whether CYP1A1 alters the expression and/or activation of eNOS and whether this is influenced by dietary fatty acids, we analyzed expression of aortic eNOS and phosphorylation at serine-1177, associated with activation of eNOS, from mice fed an n-3 or n-6 PUFA–enriched diet. The aortic total eNOS expression did not differ between genotypes or diets. However, both WT and KO mice fed an n-3 PUFA diet exhibited significantly higher levels of activated eNOS as reflected by an increased ratio of phospho-Ser1177-eNOS to total eNOS, compared with an n-6 PUFA diet (Fig. 6, A and B). Notably, CYP1A1 KO mice fed an n-3 PUFA diet showed a significantly lower ratio of phospho-Ser1177-eNOS to total eNOS, compared with WT mice on the n-3 PUFA diet.
Fig. 6.
Effects of CYP1A1 on expression of eNOS and phospho-Ser1177-eNOS in the aorta of mice fed an n-3 or n-6 PUFA–enriched diet. (A) Representative Western blots and (B) densitometric quantification of Western blots of aortic eNOS and phospho-Ser1177-eNOS after immunoprecipitation of eNOS from CYP1A1 WT and KO mice fed an n-3 or n-6 PUFA diet (n = 3 per genotype/diet). *P < 0.05 versus WT fed n-3 PUFA diet; †P < 0.05 the same genotype fed n-3 PUFA diet.
Discussion
This study is the first to investigate the interaction of dietary PUFA composition and CYP1A1 on eNOS activation and NO-dependent regulation of blood pressure and vasoreactivity. Our results show that CYP1A1 contributed to the n-3 PUFA–dependent increase in aortic eNOS activation, NO-dependent ACh-mediated dilation in mesenteric resistance arterioles, and NO-dependent regulation of blood pressure (Fig. 7). Additionally, CYP1A1 played a role in NO-dependent ACh-mediated dilation in mesenteric resistance arterioles of mice on an n-6 PUFA diet. Our data establish that physiologic mechanisms underlying the vascular benefits of n-3 PUFA supplementation observed in human epidemiology studies likely include increased vascular NO bioavailability and NO-dependent reduction in blood pressure and that these benefits are mediated, in part, by CYP1A1.
Fig. 7.

Proposed summary scheme of the role of CYP1A1 in modulating vascular and blood pressure effects of dietary PUFAs. Previously published studies show that CYP1A1 can metabolize n-3 and n-6 PUFAs to vasoactive products that can increase NO bioavailability, and that n-3 PUFAs are preferred endogenous substrates. Our data show that CYP1A1 contributed to the n-3 PUFA–dependent increase in eNOS activation, NO-dependent vasodilation, and NO-dependent regulation of blood pressure as well as n-6 PUFA–dependent NO-dependent vasodilation.
Although CYP1A1 classically is considered to be a xenobiotic metabolizing enzyme as a result of activation of the aryl hydrocarbon receptor (AHR), a number of studies have shown that CYP1A1 can metabolize endogenous PUFAs. In reconstituted systems, human CYP1A1 acts as a hydroxylase of AA with ∼90% of the products being a mixture of monohydroxylated metabolites (i.e., HETEs) with limited cardiovascular protective effects and as a epoxygenase with ∼10% of the products being vasodilatory and anti-inflammatory epoxides (i.e., EETs) (Choudhary et al., 2004; Schwarz et al., 2004). In contrast, CYP1A1 acts primarily as a stereospecific epoxygenase of EPA and DHA, generating potent vasodilatory and anti-inflammatory epoxides, 14,15- and 17,18-EEQ, and 19,20-EDP, respectively (Schwarz et al., 2004, 2005; Fer et al., 2008; Lucas et al., 2010). Furthermore, studies show EPA is a preferred endogenous substrate for CYP1A1-generated epoxide metabolites, with enzymatic conversion rates 8 times higher than for AA. Our results in mice suggest that CYP1A1 may be important in the epoxygenase metabolism of EPA and DHA, but not of AA, in vivo. CYP1A1 KO mice on an n-3 PUFA diet showed a higher level of DHA in the aorta and a higher level of DHA and EPA in the kidney compared with WT mice. Furthermore, these increases in the parent EPA and DHA fatty acids were not associated with parallel increases in their metabolites, suggesting that the loss of CYP1A1 results in an overall decrease in the metabolism of DHA and EPA in these tissues and the subsequent accumulation of the parent fatty acids to higher levels. In contrast, CYP1A1 KO mice on an n-6 PUFA diet failed to exhibit any differences in the level of AA or AA epoxide metabolites in any tissues, suggesting that CYP1A1 may play a limited role in AA epoxygenase metabolism in vivo. It is notable that common CYP1A1 polymorphisms in humans significantly differ in their capacity to catalyze EPA to epoxide products, which could contribute to individual differences in generating vascular protective metabolites after n-3 PUFA supplementation (Schwarz et al., 2005).
It is well documented that dietary n-3 PUFA supplementation decreases blood pressure in human subjects who are hypertensive as well as those who are normotensive (Bonaa et al., 1990; Mori et al., 1999). In one study, a 10-week supplementation with dietary EPA and DHA reduced systolic blood pressure by 4.6 mm Hg and diastolic blood pressure by 3.0 mm Hg in individuals with hypertension (Bonaa et al., 1990). Similar magnitude decreases in blood pressure were observed in normotensive individuals in a double-blind, placebo-controlled trial of a 6-week DHA supplementation (Mori et al., 1999). The magnitude of these decreases can have significant impact on cardiovascular health. For example, it has been demonstrated that decreases in blood pressure of this magnitude, using antihypertensive medication, can reduce the risk of future cardiovascular events by 15–25% (Turnbull, 2003).
Preclinical models of hypertension used to investigate the pharmacologic mechanisms underlying n-3 PUFA supplementation show even larger changes in blood pressure than those observed in human studies, which may result from the use of inbred models and more tightly controlled diets. In hypertensive rat models, n-3 PUFA supplementation reduces blood pressure 20–30 mm Hg, while a dietary deficiency of n-3 PUFAs increases blood pressure by 15–20 mm Hg (Begg et al., 2012). The results of our study confirm for the first time that n-3 PUFA supplementation also decreases blood pressure in a mouse model by ∼10 mm Hg, compared with an n-6 PUFA diet. Because dietary formulations differ significantly among studies and neither parent fatty acids nor metabolites were measured in earlier studies, it is not possible to establish dose-dependent effects of n-3 PUFAs. Nonetheless, our results confirm that a mouse model responds in a similar manner as other preclinical rodent models, supporting the future use of other genetically modified mouse models to further elucidate pharmacologic mechanisms of n-3 PUFAs.
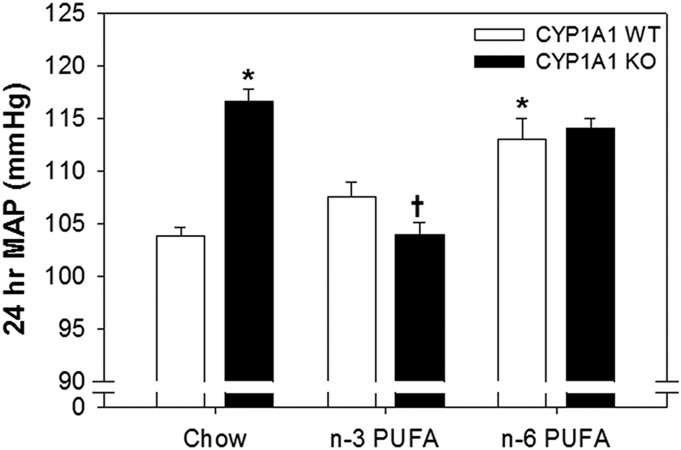
Notably, we made a number of novel observations regarding the mechanisms by which n-3 PUFAs and CYP1A1 contribute to blood pressure regulation. First, we found that the n-3 PUFA diet significantly increased the contribution of NO to blood pressure regulation and that this was mediated, in part, by CYP1A1. When NOS was inhibited, blood pressure increased significantly more in CYP1A1 WT mice on an n-3 PUFA diet, compared with WT mice on an n-6 PUFA diet and KO mice on an n-3 PUFA diet. In addition, we revealed a genotype and genotype-diet interaction in blood pressure regulation when MAP was compared among CYP1A1 WT and KO mice fed an n-3, n-6 or a normal chow diet (Fig. 8). We have reported previously that CYP1A1 KO mice fed a normal chow diet are modestly hypertensive compared with CYP1A1 WT mice (Kopf et al., 2010; Agbor et al., 2012). When CYP1A1 WT and KO mice were fed an n-3 PUFA diet, MAP in KO mice was reduced to WT levels. In contrast, when both genotypes were fed an n-6 PUFA diet, MAP in WT mice was increased to KO levels. These data suggest that when n-3 PUFAs are present in lower amounts, such as in normal chow, CYP1A1 may be an important contributor to maintaining normal blood pressure and its genetic deletion results in hypertension. We did not assess the time course of the decrease in blood pressure nor its reversibility, but one recent study in humans shows that a significant decrease in blood pressure occurs after 8 weeks of dietary n-3 PUFA supplementation and this decrease is completely reversed 8 weeks after stopping the supplementation (Fischer et al., 2014).
Fig. 8.
Effects of CYP1A1 on blood pressure in mice fed standard chow, or n-3 or n-6 PUFA–enriched diet. 24-hour MAP from CYP1A1 WT and KO measured by radiotelemetry (n = 4–6 per genotype/diet). Two-way ANOVA, diet (P < 0.02), genotype-diet interaction (P < 0.01). Post hoc comparisons: *P < 0.05 versus WT mice fed a chow diet; †P < 0.05 versus KO mice fed a chow diet or fed an n-6 PUFA diet. Data from chow fed mice was reported previously (Agbor et al., 2012).
Although the specific mechanism by which CYP1A1 contributes to blood pressure regulation has not been fully elucidated, a number of studies suggest that it may play a role in the regulation of vascular tone. CYP1A1 is induced in endothelial cells by physiologic levels of laminar shear stress that stimulate flow-mediated vasodilation (Han et al., 2008; Conway et al., 2009). CYP1A1 also is expressed in mouse mesenteric resistance arterioles (Kopf et al., 2010), and CYP1A1-generated metabolites of EPA and DHA are potent vasodilators (Zhang et al., 2001; Ye et al., 2002; Hercule et al., 2007; Morin et al., 2010). Our previous work shows that CYP1A1 KO mice exhibit significantly attenuated vasodilation responses to both EPA and DHA in mesenteric arterioles, which may contribute to the elevated blood pressure (Agbor et al., 2012). In this study, we found that CYP1A1 contributes to NO-dependent ACh dilation, independently of dietary PUFA composition. CYP1A1 KO mice on an n-3 PUFA diet were significantly less responsive than WT mice to ACh-mediated dilation, and this difference was eliminated by NOS inhibition, suggesting that the attenuated response in KO mice results from a loss of NO. Although CYP1A1 KO mice on an n-6 PUFA diet exhibited a normal dilation response to ACh, none of it was NO-dependent, suggesting that in KO mice there is a loss of NO and an increase in the contribution from compensatory mechanisms. We did not investigate the mediators of ACh dilation in CYP1A1 KO mice on an n-6 PUFA diet; however, increases in endothelial-derived hyperpolarizing factors via AA metabolism represent likely candidates (Hercule et al., 2009; Fujiwara et al., 2012).
Our data show that the n-3 PUFA diet significantly increased aortic eNOS phosphorylation at Ser1177, the site associated with eNOS activation, and this was mediated, in part, by CYP1A1. While published studies show that n-3 PUFAs increase eNOS expression, phosphorylation at Ser1177, translocation from caveolae to the cytosol, and enzyme activity, leading to increases in NO production (Okuda et al., 1997; Omura et al., 2001; Lopez et al., 2004; Stebbins et al., 2008; Wu et al., 2012), the mechanism by which CYP1A1 leads to eNOS activation is not known. It has been shown that treatment of cultured endothelial cells with nanomolar concentrations of various EET regioisomers, the P450-dependent metabolites of AA, increases eNOS expression, phosphorylation, and activity (Hercule et al., 2009). Thus, it is possible that CYP1A1-derived metabolites of EPA and DHA could also lead to eNOS activation and increases in NO bioavailability.
We have previously reported that CYP1A1 KO mice exhibit lower body, heart, liver, and kidney weights than age-matched CYP1A1 WT mice when fed a normal chow diet (Kopf et al., 2010; Agbor et al., 2012). Consistent with these observations, in our current study CYP1A1 KO mice fed an n-6 PUFA diet exhibited lower body, heart and liver weights than the WT mice. Interestingly, however, CYP1A1 KO mice exhibited normal body and organ weights when fed an n-3 PUFA diet. Other investigators have reported that high doses of n-3 PUFAs increase body weight and reduce activity of C57BL/6 mice (Rockett et al., 2010, 2012). Although we did not observe any changes in weight gain or activity in CYP1A1 WT mice on the n-3 PUFA diet, the KO mice did exhibit significantly lower activity on the n-3 PUFA diet, which could contribute to normalization of body and organ weight.
Finally, the question arises as to the role of the AHR, the primary transcriptional regulator of CYP1A1, in PUFA metabolism and cardiovascular function and disease. Studies demonstrate that sustained activation of the AHR by xenobiotics promotes the development of cardiovascular disease, including increasing blood pressure and increasing the progression of atherosclerosis (Dalton et al., 2001; Korashy and El-Kadi, 2006; Kopf et al., 2010). In contrast, genetic deletion of AHR results in low blood pressure (Zhang et al., 2010; Agbor et al., 2011, 2012). Thus, it has been proposed that constitutive (i.e., physiologic) AHR signaling via an endogenous ligand is cardiovascular protective, whereas sustained (i.e., toxicologic) AHR signaling via xenobiotic ligands promotes cardiovascular disease pathogenesis. The results from our study support the idea that constitutive levels of CYP1A1, resulting from physiologic AHR signaling, are vascular protective. However, future studies will be needed to identify the specific tissue sites of CYP1A1 metabolism as well as the potential role of other CYP isozymes that are most critical to the vascular benefits of n-3 PUFAs.
In summary, our study has demonstrated that dietary n-3 PUFAs significantly increase EPA and DHA and their metabolites in all tissues analyzed. Further, these changes were associated with decreases in blood pressure, increases in vascular eNOS activation, and increases in the contribution of NO to blood pressure regulation and vasodilation. Notably, CYP1A1 was one mediator, in part, of vascular eNOS activation and the NO contribution to blood pressure regulation, suggesting that P450-dependent n-3 PUFA metabolites play a role in the cardiovascular benefits of dietary n-3 PUFAs.
Supplementary Material
Acknowledgments
The authors thank Mary T. Walsh for expert technical assistance.
Abbreviations
- AA
arachidonic acid
- ACh
acetylcholine
- AHR
aryl hydrocarbon receptor
- ANOVA
analysis of variance
- DHA
docosahexanoic acid
- EDP
epoxydocosapentaenoic acid
- EET
epoxyeicosatrienoic acid
- EEQ
epoxyeicosatetraenoic acid
- eNOS
endothelial nitric oxide synthase
- EPA
eicosapentaenoic acid
- HETE
hydroxyeicosatetraenoic acid
- HR
heart rate
- KO
knockout
- LNNA
N5-(nitroamidino)-l-2,5-diaminopentanoic acid
- MAP
mean arterial pressure
- n-3
omega 3
- NO
nitric oxide
- P450
cytochrome P450
- PUFA
polyunsaturated fatty acid
- U46619
9,11-dideoxy-9α,11α-methanoepoxy-prosta-5Z,13E-dien-1-oic acid
- WT
wild type
Authorship Contributions
Participated in research design: Agbor, Walker.
Conducted experiments: Agbor, Wiest.
Contributed new reagents or analytic tools: Rothe, Schunck, Walker.
Performed data analysis: Agbor, Wiest, Walker.
Wrote or contributed to the writing of the manuscript: Agbor, Wiest, Schunck, Walker.
Footnotes
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grant R21-HL107133]; and the National Institutes of Health National Institute of Environmental Health Sciences [Grant R15-ES021896].
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Agbor LN, Elased KM, Walker MK. (2011) Endothelial cell-specific aryl hydrocarbon receptor knockout mice exhibit hypotension mediated, in part, by an attenuated angiotensin II responsiveness. Biochem Pharmacol 82:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agbor LN, Walsh MT, Boberg JR, Walker MK. (2012) Elevated blood pressure in cytochrome P4501A1 knockout mice is associated with reduced vasodilation to omega-3 polyunsaturated fatty acids. Toxicol Appl Pharmacol 264:351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold C, Markovic M, Blossey K, Wallukat G, Fischer R, Dechend R, Konkel A, von Schacky C, Luft FC, Muller DN, et al. (2010) Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of omega-3 fatty acids. J Biol Chem 285:32720–32733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa-Sicard E, Markovic M, Honeck H, Christ B, Muller DN, Schunck WH. (2005) Eicosapentaenoic acid metabolism by cytochrome P450 enzymes of the CYP2C subfamily. Biochem Biophys Res Commun 329:1275–1281. [DOI] [PubMed] [Google Scholar]
- Begg DP, Puskás LG, Kitajka K, Ménesi D, Allen AM, Li D, Mathai ML, Shi JR, Sinclair AJ, Weisinger RS. (2012) Hypothalamic gene expression in ω-3 PUFA-deficient male rats before, and following, development of hypertension. Hypertens Res 35:381–387. [DOI] [PubMed] [Google Scholar]
- Bønaa KH, Bjerve KS, Straume B, Gram IT, Thelle D. (1990) Effect of eicosapentaenoic and docosahexaenoic acids on blood pressure in hypertension. A population-based intervention trial from the Tromsø study. N Engl J Med 322:795–801. [DOI] [PubMed] [Google Scholar]
- Choudhary D, Jansson I, Stoilov I, Sarfarazi M, Schenkman JB. (2004) Metabolism of retinoids and arachidonic acid by human and mouse cytochrome P450 1b1. Drug Metab Dispos 32:840–847. [DOI] [PubMed] [Google Scholar]
- Conway DE, Sakurai Y, Weiss D, Vega JD, Taylor WR, Jo H, Eskin SG, Marcus CB, McIntire LV. (2009) Expression of CYP1A1 and CYP1B1 in human endothelial cells: regulation by fluid shear stress. Cardiovasc Res 81:669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton TP, Dieter MZ, Matlib RS, Childs NL, Shertzer HG, Genter MB, Nebert DW. (2000) Targeted knockout of Cyp1a1 gene does not alter hepatic constitutive expression of other genes in the mouse [Ah] battery. Biochem Biophys Res Commun 267:184–189. [DOI] [PubMed] [Google Scholar]
- Dalton TP, Kerzee JK, Wang B, Miller M, Dieter MZ, Lorenz JN, Shertzer HG, Nerbert DW, Puga A. (2001) Dioxin exposure is an environmental risk factor for ischemic heart disease. Cardiovasc Toxicol 1:285–298. [DOI] [PubMed] [Google Scholar]
- de Oliveira Otto MC, Wu JH, Baylin A, Vaidya D, Rich SS, Tsai MY, Jacobs DR, Jr, Mozaffarian D. (2013) Circulating and dietary omega-3 and omega-6 polyunsaturated fatty acids and incidence of CVD in the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc 2:e000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duling LC, Cherng TW, Griego JR, Perrine MF, Kanagy NL. (2006) Loss of alpha2B-adrenoceptors increases magnitude of hypertension following nitric oxide synthase inhibition. Am J Physiol Heart Circ Physiol 291:H2403–H2408. [DOI] [PubMed] [Google Scholar]
- Fer M, Dréano Y, Lucas D, Corcos L, Salaün JP, Berthou F, Amet Y. (2008) Metabolism of eicosapentaenoic and docosahexaenoic acids by recombinant human cytochromes P450. Arch Biochem Biophys 471:116–125. [DOI] [PubMed] [Google Scholar]
- Fischer R, Konkel A, Mehling H, Blossey K, Gapelyuk A, Wessel N, von Schacky C, Dechend R, Muller DN, Rothe M, et al. (2014) Dietary omega-3 fatty acids modulate the eicosanoid profile in man primarily via the CYP-epoxygenase pathway. J Lipid Res 55:1150–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H, Wake Y, Hashikawa-Hobara N, Makino K, Takatori S, Zamami Y, Kitamura Y, Kawasaki H. (2012) Endothelium-derived relaxing factor-mediated vasodilation in mouse mesenteric vascular beds. J Pharmacol Sci 118:373–381. [DOI] [PubMed] [Google Scholar]
- Goodfellow J, Bellamy MF, Ramsey MW, Jones CJ, Lewis MJ. (2000) Dietary supplementation with marine omega-3 fatty acids improve systemic large artery endothelial function in subjects with hypercholesterolemia. J Am Coll Cardiol 35:265–270. [DOI] [PubMed] [Google Scholar]
- Han Z, Miwa Y, Obikane H, Mitsumata M, Takahashi-Yanaga F, Morimoto S, Sasaguri T. (2008) Aryl hydrocarbon receptor mediates laminar fluid shear stress-induced CYP1A1 activation and cell cycle arrest in vascular endothelial cells. Cardiovasc Res 77:809–818. [DOI] [PubMed] [Google Scholar]
- Harris WS. (2010) The omega-3 index: clinical utility for therapeutic intervention. Curr Cardiol Rep 12:503–508. [DOI] [PubMed] [Google Scholar]
- Hercule HC, Salanova B, Essin K, Honeck H, Falck JR, Sausbier M, Ruth P, Schunck WH, Luft FC, Gollasch M. (2007) The vasodilator 17,18-epoxyeicosatetraenoic acid targets the pore-forming BK alpha channel subunit in rodents. Exp Physiol 92:1067–1076. [DOI] [PubMed] [Google Scholar]
- Hercule HC, Schunck WH, Gross V, Seringer J, Leung FP, Weldon SM, da Costa Goncalves ACh, Huang Y, Luft FC, Gollasch M. (2009) Interaction between P450 eicosanoids and nitric oxide in the control of arterial tone in mice. Arterioscler Thromb Vasc Biol 29:54–60. [DOI] [PubMed] [Google Scholar]
- Khan F, Elherik K, Bolton-Smith C, Barr R, Hill A, Murrie I, Belch JJ. (2003) The effects of dietary fatty acid supplementation on endothelial function and vascular tone in healthy subjects. Cardiovasc Res 59:955–962. [DOI] [PubMed] [Google Scholar]
- Kopf PG, Scott JA, Agbor LN, Boberg JR, Elased KM, Huwe JK, Walker MK. (2010) Cytochrome P4501A1 is required for vascular dysfunction and hypertension induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci 117:537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korashy HM, El-Kadi AO. (2006) The role of aryl hydrocarbon receptor in the pathogenesis of cardiovascular diseases. Drug Metab Rev 38:411–450. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton PM, Harris WS, Appel LJ, American Heart Association. Nutrition Committee (2002) Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 106:2747–2757. [DOI] [PubMed] [Google Scholar]
- Li Q, Zhang Q, Wang M, Liu F, Zhao S, Ma J, Luo N, Li N, Li Y, Xu G, et al. (2007a) Docosahexaenoic acid affects endothelial nitric oxide synthase in caveolae. Arch Biochem Biophys 466:250–259. [DOI] [PubMed] [Google Scholar]
- Li Q, Zhang Q, Wang M, Zhao S, Ma J, Luo N, Li N, Li Y, Xu G, Li J. (2007b) Eicosapentaenoic acid modifies lipid composition in caveolae and induces translocation of endothelial nitric oxide synthase. Biochimie 89:169–177. [DOI] [PubMed] [Google Scholar]
- López D, Orta X, Casós K, Sáiz MP, Puig-Parellada P, Farriol M, Mitjavila MT. (2004) Upregulation of endothelial nitric oxide synthase in rat aorta after ingestion of fish oil-rich diet. Am J Physiol Heart Circ Physiol 287:H567–H572. [DOI] [PubMed] [Google Scholar]
- Lucas D, Goulitquer S, Marienhagen J, Fer M, Dreano Y, Schwaneberg U, Amet Y, Corcos L. (2010) Stereoselective epoxidation of the last double bond of polyunsaturated fatty acids by human cytochromes P450. J Lipid Res 51:1125–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund AK, Agbor LN, Zhang N, Baker A, Zhao H, Fink GD, Kanagy NL, Walker MK. (2008) Loss of the aryl hydrocarbon receptor induces hypoxemia, endothelin-1, and systemic hypertension at modest altitude. Hypertension 51:803–809. [DOI] [PubMed] [Google Scholar]
- Marchioli R, Levantesi G. (2013) n-3 PUFAs in cardiovascular disease. Int J Cardiol 170(2, Suppl 1)S33–S38. [DOI] [PubMed] [Google Scholar]
- Morgan DR, Dixon LJ, Hanratty CG, El-Sherbeeny N, Hamilton PB, McGrath LT, Leahey WJ, Johnston GD, McVeigh GE. (2006) Effects of dietary omega-3 fatty acid supplementation on endothelium-dependent vasodilation in patients with chronic heart failure. Am J Cardiol 97:547–551. [DOI] [PubMed] [Google Scholar]
- Mori TA, Bao DQ, Burke V, Puddey IB, Beilin LJ. (1999) Docosahexaenoic acid but not eicosapentaenoic acid lowers ambulatory blood pressure and heart rate in humans. Hypertension 34:253–260. [DOI] [PubMed] [Google Scholar]
- Morin C, Sirois M, Echavé V, Albadine R, Rousseau E. (2010) 17,18-epoxyeicosatetraenoic acid targets PPARγ and p38 mitogen-activated protein kinase to mediate its anti-inflammatory effects in the lung: role of soluble epoxide hydrolase. Am J Respir Cell Mol Biol 43:564–575. [DOI] [PubMed] [Google Scholar]
- Morris MC, Sacks F, Rosner B. (1993) Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation 88:523–533. [DOI] [PubMed] [Google Scholar]
- Muller DN, Schmidt C, Barbosa-Sicard E, Wellner M, Gross V, Hercule H, Markovic M, Honeck H, Luft FC, Schunck WH. (2007) Mouse Cyp4a isoforms: enzymatic properties, gender- and strain-specific expression, and role in renal 20-hydroxyeicosatetraenoic acid formation. Biochem J 403:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda Y, Kawashima K, Sawada T, Tsurumaru K, Asano M, Suzuki S, Soma M, Nakajima T, Yamashita K. (1997) Eicosapentaenoic acid enhances nitric oxide production by cultured human endothelial cells. Biochem Biophys Res Commun 232:487–491. [DOI] [PubMed] [Google Scholar]
- Omura M, Kobayashi S, Mizukami Y, Mogami K, Todoroki-Ikeda N, Miyake T, Matsuzaki M. (2001) Eicosapentaenoic acid (EPA) induces Ca(2+)-independent activation and translocation of endothelial nitric oxide synthase and endothelium-dependent vasorelaxation. FEBS Lett 487:361–366. [DOI] [PubMed] [Google Scholar]
- Rockett BD, Harris M, Raza Shaikh S. (2012) High dose of an n-3 polyunsaturated fatty acid diet lowers activity of C57BL/6 mice. Prostaglandins Leukot Essent Fatty Acids 86:137–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockett BD, Salameh M, Carraway K, Morrison K, Shaikh SR. (2010) n-3 PUFA improves fatty acid composition, prevents palmitate-induced apoptosis, and differentially modifies B cell cytokine secretion in vitro and ex vivo. J Lipid Res 51:1284–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz D, Kisselev P, Chernogolov A, Schunck WH, Roots I. (2005) Human CYP1A1 variants lead to differential eicosapentaenoic acid metabolite patterns. Biochem Biophys Res Commun 336:779–783. [DOI] [PubMed] [Google Scholar]
- Schwarz D, Kisselev P, Ericksen SS, Szklarz GD, Chernogolov A, Honeck H, Schunck WH, Roots I. (2004) Arachidonic and eicosapentaenoic acid metabolism by human CYP1A1: highly stereoselective formation of 17(R),18(S)-epoxyeicosatetraenoic acid. Biochem Pharmacol 67:1445–1457. [DOI] [PubMed] [Google Scholar]
- Singh TU, Kathirvel K, Choudhury S, Garg SK, Mishra SK. (2010) Eicosapentaenoic acid-induced endothelium-dependent and -independent relaxation of sheep pulmonary artery. Eur J Pharmacol 636:108–113. [DOI] [PubMed] [Google Scholar]
- Stebbins CL, Stice JP, Hart CM, Mbai FN, Knowlton AA. (2008) Effects of dietary decosahexaenoic acid (DHA) on eNOS in human coronary artery endothelial cells. J Cardiovasc Pharmacol Ther 13:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirban A, Nandrean S, Götting C, Tamler R, Pop A, Negrean M, Gawlowski T, Stratmann B, Tschoepe D. (2010) Effects of n-3 fatty acids on macro- and microvascular function in subjects with type 2 diabetes mellitus. Am J Clin Nutr 91:808–813. [DOI] [PubMed] [Google Scholar]
- Turnbull F, Blood Pressure Lowering Treatment Trialists’ Collaboration (2003) Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 362:1527–1535. [DOI] [PubMed] [Google Scholar]
- von Schacky C, Angerer P, Kothny W, Theisen K, Mudra H. (1999) The effect of dietary omega-3 fatty acids on coronary atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 130:554–562. [DOI] [PubMed] [Google Scholar]
- Wang RX, Chai Q, Lu T, Lee HC. (2011) Activation of vascular BK channels by docosahexaenoic acid is dependent on cytochrome P450 epoxygenase activity. Cardiovasc Res 90:344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal C, Konkel A, Schunck WH. (2011) CYP-eicosanoids—a new link between omega-3 fatty acids and cardiac disease? Prostaglandins Other Lipid Mediat 96:99–108. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhang C, Dong Y, Wang S, Song P, Viollet B, Zou MH. (2012) Activation of the AMP-activated protein kinase by eicosapentaenoic acid (EPA, 20:5 n-3) improves endothelial function in vivo. PLoS ONE 7:e35508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye D, Zhang D, Oltman C, Dellsperger K, Lee HC, VanRollins M. (2002) Cytochrome p-450 epoxygenase metabolites of docosahexaenoate potently dilate coronary arterioles by activating large-conductance calcium-activated potassium channels. J Pharmacol Exp Ther 303:768–776. [DOI] [PubMed] [Google Scholar]
- Zhang N, Agbor LN, Scott JA, Zalobowski T, Elased KM, Trujillo A, Duke MS, Wolf V, Walsh MT, Born JL, et al. (2010) An activated renin-angiotensin system maintains normal blood pressure in aryl hydrocarbon receptor heterozygous mice but not in null mice. Biochem Pharmacol 80:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Oltman CL, Lu T, Lee HC, Dellsperger KC, VanRollins M. (2001) EET homologs potently dilate coronary microvessels and activate BK(Ca) channels. Am J Physiol Heart Circ Physiol 280:H2430–H2440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.