Abstract
Prostatic inflammation is of considerable importance to urologic research because of its association with benign prostatic hyperplasia and prostate cancer. However, the mechanisms by which inflammation leads to proliferation and growth remain obscure. Here, we show that insulin-like growth factors (IGFs), previously known as critical developmental growth factors during prostate organogenesis, are induced by inflammation as part of the proliferative recovery to inflammation. Using genetic models and in vivo IGF receptor blockade, we demonstrate that the hyperplastic response to inflammation depends on interleukin-1–driven IGF signaling. We show that human prostatic hyperplasia is associated with IGF pathway activation specifically localized to foci of inflammation. This demonstrates that mechanisms of inflammation-induced epithelial proliferation and hyperplasia involve the induction of developmental growth factors, further establishing a link between inflammatory and developmental signals and providing a mechanistic basis for the management of proliferative diseases by IGF pathway modulation.
Introduction
Asymptomatic prostatic inflammation is of considerable importance to urologic research because of its association with two of the most common health concerns in urology: prostate cancer and benign prostatic hyperplasia (BPH). Inflammation in the human prostate is extremely common and is associated with dysplastic changes including focal disruption of the epithelium, polymorphisms of epithelial cell nuclei, and increased epithelial proliferation (McNeal 1968; Cotran et al., 1999; DeMarzo et al., 2003). Inflammation is manifested by leukocytic infiltration and the release of proinflammatory cytokines, chemokines, prostanoids, and growth factors. The origins of inflammation in the prostate, which remain a subject of debate, are likely multifactorial. Infection from culturable and nonculturable organisms causes inflammation in acute and chronic bacterial prostatitis, but these conditions are relatively uncommon (Hochreiter et al., 2000; Krieger and Riley, 2002). Numerous nonbacterial potential causes of inflammation have been investigated, including viruses, environmental and dietary components, systemic steroid action (especially estrogens), oxidative stress, systemic inflammation associated with the metabolic syndrome, and urinary reflux of noxious stimuli into the prostatic ducts (De Marzo et al., 2007). Though data in this area are still sparse, the number of potential chemicals in urine may represent a major inflammatory stimulus in the prostate. Whatever the causes, the mechanistic understanding of how prostatic inflammation promotes the genesis and growth of prostate cancer and benign prostatic growth is a major gap in the development of superior treatment of these two common urologic conditions.
BPH is defined as a benign enlargement of the prostate gland (Roehrborn, 2008). Although the clinical manifestation of the disease is a characterized symptom profile known as lower urinary tract symptoms, the disease clearly also includes induced proliferation of both benign epithelial and stromal compartments (Roehrborn, 2008). Recent reports describe a clear association of BPH with histologically evident inflammation, with the overall prevalence of inflammation in BPH specimens ranging from 75 to 100%. A recent prospective study of autopsy specimens found chronic inflammation in 75% of prostates obtained from 93 men with histologic evidence of BPH compared with 50% of prostates not affected by BPH (Delongchamps et al., 2008). A second study found substantial prostatic inflammation in 100% of 80 men undergoing prostatectomy for treatment of BPH (Nickel et al., 1999). Further, prostate biopsies of 8224 men enrolled in the Reduction by Dutasteride of Cancer Events (REDUCE) trial revealed inflammation in 78% of specimens, and survey studies have characterized BPH-associated inflammation as having an abundance of T cells, high expression of a variety of inflammatory cytokines, including interleukin-1 (IL-1), IL-6, and IL-8, which are well characterized inducers of prostatic proliferation and growth (Steiner et al., 2003; Nickel et al., 2007). Perhaps most importantly, the Medical Therapy of Prostate Symptoms (MTOPS) study found that the most tightly correlated histologic finding to prostate symptomatology and growth is the presence of prostatic inflammation (Nickel et al., 2008). These reports clearly suggest a significant role for inflammation in BPH and define the need for a mechanistic understanding.
Previous work from our laboratory has shown that inflammatory mediators play a critical role in organogenesis of the prostate by inducing the axis of developmental growth factors, particularly the insulin-like growth factor (IGF) pathway (Jerde and Bushman, 2009). There is solid evidence that IGF signaling plays a role in epithelial proliferation and growth of the prostate during development (Ruan et al., 1999), and IGF-1 expression is reactivated in cancerous and BPH prostate tissues and is believed to promote cell proliferation (Monti et al., 2001; McLaren et al., 2011; Savvani et al., 2013). These reports suggested to us that IGF signaling may serve as a bridge between inflammatory signaling and the induction of cell proliferation. Here, we show that inflammation of the prostate causes rapid and substantial induction of IGF signaling in the mouse prostate, that the proliferative response of the tissue to inflammation depends on IGF signaling, and that human prostatic hyperplasia is associated with IGF pathway activation that is specifically localized to foci of inflammation. This demonstrates that the mechanism of inflammation-induced epithelial proliferation and prostatic hyperplasia involves the induction of developmental growth factors providing a mechanistic basis for the management of proliferative prostatic diseases such as BPH.
Materials and Methods
In Vivo Induction of Inflammation, Proliferation, and Assessment of Reactive Hyperplasia.
All animal experiments were conducted under the approval and supervision of the Indiana University School of Medicine Animal Care and Use Committee, and in accordance of National Institutes of Health guidelines for animal research. Escherichia coli strain 1677 (2 × 106/ml, 100 μl per mouse) was instilled through catheters into the urinary tract of C57BK wild-type and IL-1 receptor 1 [IL-1R1(–/–)] mice (The Jackson Laboratory, Bar Harbor, ME; verified by genotyping) at 8 weeks of age as described elsewhere (Jerde and Bushman, 2009). Mice were inoculated with 100 mg of bromidated deoxyuridine (BrdU; Roche Applied Science, Indianapolis, IN) 2 hours before sacrifice, and groups were sacrificed daily 1–7 days after induction. Phosphate-buffered saline (PBS)–instilled animals were used as naïve controls. Prostate tissues were either paraffin-embedded for histologic and immunohistochemical analysis, snap-frozen for molecular analysis, or incubated in Krebs buffer for release experiments as described herein.
The severity of the inflammatory response was graded as previously described (Boehm et al., 2012) in three random 20× fields of H&E sections according to four criteria: inflammatory infiltrate, tissue damage, hyperplastic response, and hemorrhage. Leukocytes were counted and each field given a score as follows: 0, no leukocytes; 1 (mild) less than 10 leukocytes; 2 (moderate) 10–30 leukocytes; or 3 (severe) greater than 30 leukocytes. The focality of tissue damage based on the integrity of the epithelium and the presence of visibly damaged cells (or pyknotic nuclei was scored as: 0, full epithelial integrity and no damaged cells; 1 (mild) sloughed epithelium in less than 25% of a ductal cross section and/or 1–20 damaged cells; 2 (moderate) sloughed epithelium in greater than 25% of ductal cross section and/or more than 20 damaged cell; or 3 (severe) complete epithelial slough. Hyperplasia was scored as: 0, normal pseudostratified epithelium; 1 (mild) basal cell expansion resulting in a stratified bilayer; 2 (moderate) thickened epithelium (3–5 cell layers); or 3 (severe) thickened epithelium (greater than five cell layers). Finally, each field was scored for hemorrhage based on the presence and number of extravascular erythrocytes: 0, no free erythrocytes; 1 (mild) less than 10 erythrocytes; 2 (moderate) 10–30 erythrocytes; or 3 (severe) greater than 30 erythrocytes.
Focality for each criterion was then assessed as: 0, no evidence of the criterion occurring; 1 (focal) criterion occurs in one location; 2 (limited) criterion occurs in less than 25% of the field; 3 (intermediate) criterion occurs in 25–50% of the field; 4 (widespread) criterion found in greater than 50% of the field. The severity and focality scores were multiplied together for each criterion and a single score per 20× field (and the average of three determinations per field were averaged per data point. Data are presented as the mean ± S.E.M. Because the dorsal-lateral prostate responded with the most pronounced and consistent inflammation, we chose this lobe for further characterization of the IGF signaling pathway in mouse prostates.
A subset of mice received the IGF receptor antagonist picropodophyllin (PPP) (for synthesis and structure, see Berkowitz et al., 2000) concurrent with inflammation. This was performed with a protocol adapted from previous reports (Razuvaev et al., 2007). In this experiment, C57 wild-type or IL-1R1(−/−) mice were injected twice daily (every 12 hours ± 30 minutes) with PPP (10 mg/kg i.p.) in a total volume of 50 μl 75% corn oil, 25% dimethylsulfoxide (DMSO) before and during the inflammatory period. The first two treatments for each animal were 24 and 12 hours before induction of inflammation, and the twice-daily injections continued throughout the inflammation period of 3 to 5 days. Complete inhibition of IGF signaling was verified by immunoblotting for activated IGF receptor (phospho-Y-1161) as described here.
In Vivo Activation of IGF Signaling by IGF-1–Coated Affi-Gel Beads.
IGF signaling was activated specifically to test its ability to induce hyperplasia independent of other inflammation-induced factors. Affi-Gel beads (Bio-Rad Laboratories, Hercules, CA) were washed 3 times for 5 minutes with sterile PBS-Tween. Beads were then incubated with excess 100 ng/ml IGF-1 (EMD Millipore, Billerica, MA) in PBS for 1 hour to adhere IGF to the beads. The beads were then quickly centrifuged to pellet and were resuspended in 100 μl of sterile PBS per animal. Suspended beads were administered to anesthetized animals via intraurethral catheter similar to the method for administering bacteria. Mice remained anesthetized for 30 minutes to allow adhering of the beads to the urinary tract tissue. Trial experiments confirmed by gross inspection that the beads effectively adhered to all prostatic ducts and the bladder. Effective induction of the IGF pathway was verified by induction of IGFR1 activation via immunoblotting for phospho-Y-1161 IGFR1 as described here.
Colocalization of IGF-1 Activation and Inflammation in Human BPH Specimens.
Human specimens were provided generously by Dr. Wade Bushman and Dr. Wei Huang at the University of Wisconsin School of Medicine and Public Health, with appropriate minimal risk institutional review board approval. Sections were cut from pre-existing paraffin-embedded human prostate tissues obtained as part of a transurethral resection of the prostate (TURP) or from prostate specimens removed collaterally from bladder cancer patients undergoing radical cystectomy (cystoprostatectomy) as control human specimens. These controls were age-matched (average 68 years of age versus 64 years of age) to the BPH-TURP specimens and were verified by record to be naïve for bacterial infection or pretreatment with Bacillus Calmette-Guérin as first-line therapy because these patients had presented with muscle invasive bladder cancer. Further, the controls were not exhibiting benign prostatic hyperplasia symptoms and were verified by pathology to be prostate cancer free. Sections were stained with H&E in a routine manner, and adjacent sections were costained immunofluorescently for CD45 to identify inflammatory cells, and phospho-Y-1161 IGFR1 as described here. Colocalization of inflammation and IGF pathway activation were verified.
mRNA Expression of IGF Family Members by Reverse-Transcription Polymerase Chain Reaction.
Data for mRNA expression of IGF family members as reported in Fig. 1, A and B, were obtained by real-time reverse-transcription polymerase chain reaction. Prostate lobes were snap-frozen in liquid nitrogen immediately upon dissection and stored at −80°C. RNA extraction was performed via RNeasy columns in conditions recommended by the manufacturer for whole tissues (Qiagen, Valencia, CA). RNA yield was measured by 260/280 nm ratio. We synthesized cDNA via reverse transcription as previously described elsewhere (Jerde and Bushman, 2009). Real-time PCR analysis was performed on all samples for the IGF family members Igf-1, Igf-2, Igfbp1, Igfbp2, Igfbp3, Igfbp4, Igfbp5, and Igfbp6 (primers, Table 1). PCR reactions were run and amplicons detected via SYBR green fluorescence MyiQ thermocycler system (Bio-Rad Laboratories) Expression levels were normalized to ribosomal S27 and reported as a ratio of the IGF family gene of interest to S27 relative difference over PBS-instilled time-point equivalent controls.
Fig. 1.
Expression of IGF pathway components shifts to a proproliferative direction during prostatic inflammation. (A) mRNA expression of Igf-1 and Igf-2 at the time point of inflammation as indicated on the x-axis; data shown are the ratios to PBS-instilled control mouse prostates harvested at equivalent time points, where inflamed prostates are shaded and control prostates are not. (B) Igfbp pathway modulators (insulin-like growth factor binding proteins) by reverse-transcription polymerase chain reaction after induction of inflammation at the time points shown (in days after induction) relative to vehicle-treated control prostates (shading reflects different days). (C and D) IGF-1 (C) and IGF-2 (D) peptide release (dark shading) and tissue concentration (without shading) show a regulated pattern of expression and release. Data shown for total tissue content are pg/mg protein and for released peptide are pg/mg protein per 30 minutes; all data are expressed as mean ± S.E.M. *P < 0.05 versus PBS-instilled prostate; comparisons using analysis of variance, n = 6.
TABLE 1.
Primers used for reverse-transcription polymerase chain reaction calculations of gene expression of IGF and related genes
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Igf1 | AGAGACCCTTTGCGGGGC | CGGATAGAGCGGGCTGCTT |
| Igf2 | GATCCCAGTGGGGAAGTCG | GCTGGACATCTCCGAAGAGGCTC |
| Igfbp1 | CAAAGGCTGCTGTGGTCTC | TAGGTGCTGATGGCGTTCC |
| Igfbp2 | GCAGGTTGCAGACAGTGATG | AACACAGCCAGCTCCTTCAT |
| Igfbp3 | CGATTCCAAGTTCCATCCAC | TTCTGGGTGTCTGTGCTTTG |
| Igfbp4 | AGAGCGAACATCCCAACAAC | CCCACGATCTTCATCTTGCT |
| Igfbp5 | CCTACTCCCCCAAGGTCTTC | TTGGACTGGGTCAGCTTCTT |
| Igfbp6 | GAAGAATCCACGGACCTCTG | CTCGGAAGACCTCAGTCTGG |
Protein Content and Release of IGF Family Members by Enzyme-Linked Immunosorbent Assay.
Data reported in Fig. 1, C and D, were obtained by enzyme-linked immunosorbent assay. Prostate tissues from inflamed and naïve mice were collected after inflammation induction, and the mediator release was analyzed as described elsewhere (Jerde et al., 2000). Tissues were equilibrated for 1 hour in aerated Krebs physiologic salt solution, with buffer changes every 15 minutes. At the end of the equilibration period, tissues were incubated in fresh aerated Krebs solution for 30 minutes. After the experiment, Krebs was collected and frozen as the “released” fraction. Tissues were then homogenized in fresh buffer; the resulting slurry was incubated with Triton X-100 at a final concentration of 0.1% and incubated on ice for 30 minutes. The homogenate was centrifuged at 16,000g for 30 minutes, and the supernatant was collected as “total tissue” content. All collections were analyzed by enzyme-linked immunosorbent assay for IGF-1 and IGF-2 concentrations as recommended by the manufacturer (R&D Systems, Minneapolis, MN). Absorbance readings for each concentration were analyzed relative to a standard curve of known IGF concentrations, and are normalized to total protein concentrations and reported as picograms of IGF per mg total protein—either released or tissue total. Comparisons between inflamed and PBS-instilled control prostates at each time point of inflammation were made with analysis of variance, with P < 0.05 considered statistically significant.
Protein Quantification by Immunoblotting.
Total protein quantification was obtained by immunoblotting. Whole prostate tissues were homogenized in lysis buffer containing protease inhibitor (150 mM NaCl, 10 mM Tris, 1 mM EDTA, 1 mM benzene sulfonyl fluoride, and 10 μg/ml each of aprotinin, bestatin, l-leucine, and pepstatin A). Triton X-100 was added to a concentration of 1%, and the homogenate was incubated on ice for 60 minutes, followed by centrifugation for 20 minutes at 14,100g at 4°C. The supernatant was collected, and the total protein concentration was determined by bicinchoninic acid assay (Pierce, Rockford, IL). Proteins (20 μg/well) were resolved by electrophoresis in 4 to 20% gradient SDS–polyacrylamide electrophoresis gels (Bio-Rad Laboratories), and resolved proteins were transferred to polyvinylidene difluoride membranes, blocked overnight [nonfat dry milk (10 g/l), bovine serum albumin (10 g/l), and NaN3 (0.5 g/l)] in 1× PBS (2.7 mM KCl, 1.5 mM KH2PO4, 136 mM NaCl, 8 mM Na2HPO4) + 0.05% (v/v) Tween 20. After the blocking period, membranes were incubated for 16 hours with one of the following primary antibodies diluted as indicated in blocking buffer: P-Y-1161 IGFR1 (1:750; Abcam, Cambridge, MA); total Akt, P-T308-Akt and P-S473-Akt (1:500; Cell Signaling Technologies, Danvers, MA). After blots were washed 6 times in PBS + 0.05% Tween 20, the blots were incubated with donkey antibody against rabbit IgG conjugated to horseradish peroxidase for 1 hour (1:500,000 dilution; Pierce) in nonfat dry milk (2.5 g/liter), PBS, and 0.05% Tween 20. Peroxidase activity was detected via West Femto chemiluminescence reagent as directed by the manufacturer (Pierce). Photo images were analyzed by densitometry, and ratios of protein of interest to glyceraldehyde-3-phosphate dehydrogenase were determined and compared between treatments.
Protein Localization by Immunofluorescence.
Inflamed and naïve prostate specimens were fixed in 10% phosphate-buffered formalin overnight, processed and embedded in paraffin, and cut into 5-μm sections with a microtome. Tissues were mounted and heat-fixed to glass slides, deparaffinized in xylene and methanol gradient, and subjected to heat-induced antigen retrieval in citrate buffer for 15 minutes. Sections were blocked with a bovine serum albumin (1% BSA)–serum (10%) mixture for 3 hours and incubated with primary antibody overnight at 4°C. Antibodies and dilutions used for this study were as follows: rabbit-P-Y1161-IGF-1R (1:200; Abcam); P-T308-Akt or P-S473-Akt (1:100; Cell Signaling Technologies); mouse anti-BrdU antibody from BrdU labeling Detection Kit II (1:100; Roche). Sections were washed with PBS-Tween and incubated with Alexa 488 or 595–conjugated secondary antibodies against rabbit or mouse for 1 hour at 20 to 25°C. Sections were washed and incubated with Hoechst counterstain (4 mg/ml) for 10 minutes. Tissues were washed and covered with aqueous medium and glass coverslips. Tissue sections were analyzed by immunofluorescence and the number of positive- and negative-stained cells was determined by counting individual visually evident positive cells. Briefly, we counted cells with positive staining for the protein of interest (IGF-1R, Akt) in the epithelial compartment of each tissue. The epithelial compartment was made evident with PanCK immunofluorescence on the red channel, not shown). We then counted all positive cells (made evident by intact nuclei visible by Hoechst counterstain) and generated a percentage of epithelial cells that were positive for activation of the protein of interest. Three randomly selected fields at 20× magnification were chosen for analysis from each tissue and averaged for one data point.
Statistical Analysis.
Two-way analysis of variance was performed to determine the significance of treatment group and day of sacrifice (SAS 9.1.3; SAS Institute, Cary, NC). When appropriate (two-sided P < 0.05), pairwise comparisons applying Fisher’s exact test were calculated.
Ethics Approval Declarations.
All animal studies and acquisition of human sections were approved by the appropriate institutional review board(s). All animal studies were conducted in strict adherence to protocols approved by Indiana University School of Medicine and University of Wisconsin–Madison Animal Care and Use Committees, and in accordance of National Institutes of Health guidelines for animal research. Human specimens were assessed as part of collaboration with Dr. Wade Bushman and Dr. Wei Huang at the University of Wisconsin School of Medicine and Public Health, with appropriate minimal risk institutional review board approval. Sections were cut from pre-existing paraffin-embedded tissues in the pathology bank. Informed consent was received from participants before collecting tissues for the tissue bank, and no patient-identifying information was available to the authors.
Results
Inflammation Induces a Proproliferative Shift in the Expression of IGF Pathway Components in the Prostate.
We induced inflammation in mouse prostates using our established model of uropathogenic E. coli 1677 instilled via catheter into the urinary tract (Boehm et al., 2012). This model induces a reproducible inflammatory response characterized by tissue damage, hyperplastic response, and reliable induction of inflammatory mediators including interleukins-1, -2, -4, -6, -8, -12, -17, tumor necrosis factor-α, interferon-γ, transforming growth factor-β, and the prostanoid producing enzyme cyclooxygenase-2. Hyperplasia in this model is maximal 3 days after instillation, and inflammation is generally resolved by day 7 (Jerde and Bushman, 2009; Boehm et al., 2012). IGF-1 mRNA expression is increased by inflammation in this model (Fig. 1A), beginning at day 2 and maximizing at 3 days after instillation with a 4-fold induction. Correspondingly, IGF-1 peptide and release is also substantially increased by inflammation in this model. Tissue accumulation of IGF-1 peptide maximizes 5 days after inflammation, demonstrating regulated peptide release and a retention of peptide during the resolution phase (Fig. 1C). IGF-2 in contrast, does not show an increase in mRNA expression or total IGF-2 synthesis but does show an increase in peptide release (Fig. 1D). The action of IGF on the tissue is highly dependent upon the activity of six known binding proteins, insulin-like growth factor binding proteins 1–6. Inflammation of the prostate was not associated with regulated mRNA expression of Igfbp1 or 4, but Igfbp 2, 3 5, and 6 all showed a conserved expression pattern of decreased expression 1 to 3 days after induction of inflammation, a distinct rebound of expression at day 5, and a normalization of expression by day 7 (Fig. 1B). This change in Igfbp expression may, along with IGF ligand induction, represent a coordinate shift in IGF pathway modulators in a proproliferative direction.
IGF Signaling Pathway Is Activated during Prostatic Inflammation.
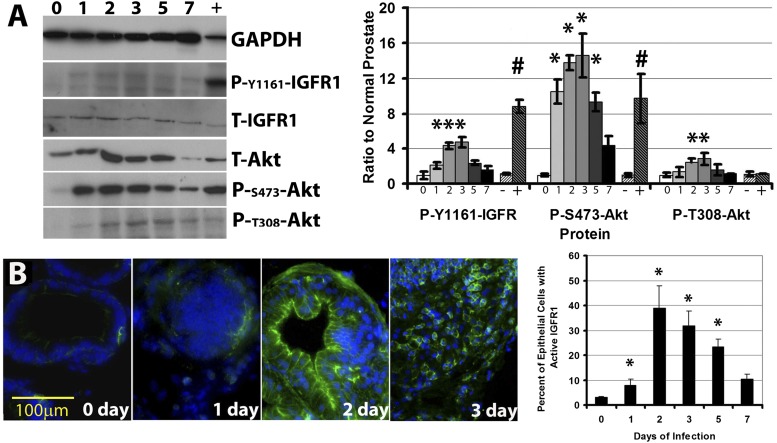
Upon activation by IGF ligand, the dimerized IGF receptor (IGF-R1) is phosphorylated at tyrosine 1161 in its intracellular SH1 domain (Vincent and Feldman, 2002). As such, phosphorylation at this residue is a readout of receptor activation and subsequent activation of downstream signaling pathways. Induced inflammation of the mouse prostate is associated with 5-fold increase in activated IGFR1 relative to control prostates, maximizing 3 days after induction of inflammation (Fig. 2). Receptor activation is returned to baseline levels by 7 days after instillation. Inflammation also causes a rapid increase in activation of the phosphoinositol-3-phosphate kinase pathway, as measured by phosphorylation of its signaling intermediate Akt on residues serine 473 and threonine 308 (Hemmings and Restuccia, 2012). Phosphoinositol-3-phosphate kinase-Akt is the primary signaling pathway induced by IGFs in epithelial cells. However, Akt activation is more rapidly induced during inflammation than is IGF itself induced, suggesting multiple pathways may contribute to Akt activation. Control prostates were incubated with 10 ng/ml IGF-1 in Krebs physiologic salt solution for 2 hours (positive control) or Krebs alone (negative control) to demonstrate the maximal inducibility of the IGF pathway in isolated prostate tissues.
Fig. 2.
The IGF signaling pathway is activated during prostatic inflammation. Immunoblotting (A) for activated IGF-1 receptor (PO4-Y-1161-IGFR1), total Akt (T-Akt), and phosphorylated (activated) Akt at serine 473 and threonine 308 at 0–7 days of prostatic inflammation. Right: Quantified results from immunoblots expressed as ratio to naïve (normal) mouse prostates; positive control lanes (marked “+” reflect tissues treated in vitro with 100 ng/ml IGF-1 (in Krebs buffer) for 2 hours before harvest to endogenously activate the pathway; negative control samples (marked “−”) were incubated in Krebs alone. Data are mean ± S.E.M. *P < 0.05 versus PBS-instilled prostate; #P < 0.05 IGF-1 treatment versus vehicle-treated controls; comparisons using analysis of variance (ANOVA), n = 5. (B) The IGF signaling pathway is activated in the prostatic epithelium. Immunofluorescence (left) for activated IGF-1 receptor (PO4-Y-1161-IGFR1-green) demonstrates substantial IGF-1 receptor activation located nearly exclusively in the epithelium after 1 to 3 days of inflammation. (Right) quantified results from immunofluorescence, shown as number of positive cells per total epithelial cells; n = 5, *P < 0.05, versus PBS-instilled prostate; comparisons using ANOVA, n = 5. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
IGF Pathway Activation Occurs in the Prostatic Epithelium.
Normal rodent prostate histology includes a pseudostratified epithelial bilayer of the prostatic ducts, surrounded by supporting stroma consisting of a smooth muscle dense layer and a fibroblastic connective stroma. The epithelium is pseudostratified into luminal and basal cell layers as all epithelial cells contact the basement membrane. IGF pathway activation in normal mouse prostates is limited to a small number of epithelial cells that are exclusive to the basal layer (Fig. 2B, 0 day). These basally activated cells routinely exist as groups of two to four connected cells. Induced inflammation of the mouse prostate is associated with a rapid increase in the number of epithelial cells with activated IGFR1 (Fig. 2B).
At day 1 of inflammation, an expansion of the limited number of IGFR-activated cells occurs, and this is limited primarily to the basal compartment of the epithelium (Fig. 2B). By the second day of inflammation, histologic hyperplasia begins, and both the luminal and basal epithelial cell populations exhibit IGFR1 activation, accounting for 39% of the total epithelial cells. Histologic activation of hyperplasia is most pronounced at 3 days after inflammation in this model, and IGF signaling is evident in these specimens. However, the total IGF-activated cell population begins to decline at this point in the model, as the luminal cells are no longer evident of active induction and positive cells are sporadic throughout the multilayered hyperplastic epithelium at this point. This may indicate that actively dividing cells exhibit active IGF signaling, but cells acquiring quiescence no longer have activated pathway, at least as measured by RTK phosphorylation of this receptor. At 5 days after induction of inflammation, hyperplasia begins its resolution and IGFR1-activated cells return to a more basal localization though most basal cells remain activated. By 7 days after induction, IGF signaling returns to its normal state. IGF pathway activation was not observed in the stroma compartment or inflammatory cells in this model.
Hyperplastic Human Prostates Exhibit IGF Pathway Activation Juxtaposed to Foci of Intense Inflammation.
We analyzed prostate specimens from six prostates removed for relief of BPH symptoms by TURP and six control prostates removed as part of a cystoprostatectomy procedure in men with invasive bladder cancer. The control prostates were age-matched (65 years BPH, 62 years control), and the control prostates had no previous exposure to Bacillus Calmette-Guerin treatment. In both groups, IGFR1 activation was observed specifically juxtaposed to areas of inflammatory cell infiltrate (Fig. 3). Confirming previous reports (Delongchamps et al., 2008; Nickel et al., 2008; Bostanci et al., 2013), BPH specimens exhibited significantly more areas of inflammation than control prostates. All six TURP specimens analyzed exhibited widespread and severe inflammation; control prostates exhibited focal and moderate to slight inflammation. Regardless, the presence of inflammation juxtaposed to prostatic inflammation was directly correlated to IGFR1 activation, often resulting in histologic reactive hyperplasia of the epithelium (Fig. 3D), and the difference in IGF activation between BPH and control was a direct function of inflammatory intensity.
Fig. 3.
The IGF signaling pathway is activated in the prostatic epithelium during human BPH in epithelium juxtaposed to prostatic inflammation. Immunofluorescence for activated IGF-1 receptor (PO4-Y-1161-IGFR1-green; and CD45-red) in (A) normal human prostate without inflammation, (B) normal human prostate juxtaposed to inflammation, (C) BPH human prostate area lacking inflammation, and (D) BPH ducts juxtaposed to inflammation.
Genetic IL-1R Knockdown Attenuates Inflammation-Induced IGF Signaling.
Inflammation-induced IGF-1 activity is reduced in IL-1R1(−/−) mice. We infected IL-1R1(−/−) and wild-type controls for 2 days (maximal for IGF-1 induction) and measured IGF-1 expression by reverse-transcription polymerase chain reaction and IGF pathway activity by immunoblotting and immunofluorescence for activated (PO4-Y-1161) IGF receptor. We found that IGF-1 RNA induction was 4-fold in inflamed wild-type prostates but less than doubled in IL-1R1(−/−) prostates (n = 4, P = 0.05). We found that activation of the IGF-R1 was also attenuated by 60% in immunoblots, and that IGF-R1 phosphorylation was evident in a significantly reduced number of cells by immunofluorescence (Fig. 4). Quantified data from four inflamed dorsal-lateral prostates showed that IGF-R1 was active in 24% of cells in the wild-type inflamed prostates and only 3.2% of IL-1R1(−/−) prostates.
Fig. 4.
Inflammation-induced IGF signaling is dependent upon interleukin-1 signaling. Immunofluorescence for activated IGF-1 receptor (PO4-Y-1161-IGFR1-green) occurs in the epithelium of 3-day inflamed wild-type mice (A), but significantly less IGFR1 activation occurs in 3-day inflamed IL-1R1(−/−) mice (B). IL-1R1(−/−) mice exhibit significantly less reactive hyperplasia in response to inflammation. Immunoblotting for IGFR1 (C and D) demonstrates significantly increased IGFR1 activation in inflamed prostates as measured by phosphor-Y1161, and IGF-induced Akt signaling as measured by both phosphor-S473 and phosphor-T308. Representative blots (C), and quantified data (D), n = 4. Inflammation failed to induce IGFR1 activation and Akt signaling in IL-1R1(−/−) mice. In addition, inflammation-induced activity of STAT3 in wild-type mice but not IL-1R1(−/−) mice suggests that an IL-1 to IGF-1 signaling loop involves STAT3 signaling, as in development (Jerde and Bushman, 2009). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
We had previously published that IL-1–induced IGF expression depended on signal transducer and activator of transcription 3 (STAT3) signaling in developing prostates, and that STAT3 activation occurs in the stroma of developing and inflamed prostates (figure 5, D and E, in Jerde and Bushman, 2009). To investigate whether this STAT3 dependence is conserved in inflammation-induced IGF-1 expression, we analyzed STAT3 activation in inflamed and control wild-type and IL-1R1(−/−) mice, and found that inflammation-induced STAT3 activation (as measured by P-Y705) is abrogated in IL-R1(−/−) mice relative to wild type (Fig. 4C). These data demonstrate that IGF induction and signaling depend on IL-1 signaling, and suggest that STAT3 signaling may mediate this effect as it does in organ development.
Fig. 5.
IGF inhibition attenuates inflammatory reactive hyperplasia. H&E (A–C) and immunofluorescence (PanCK-red; BrdU-green) (D–F) on normal control mouse prostates (A and D); 3-day inflamed mouse prostates (B and E), and inflamed mouse prostates cotreated in vivo with 10 mg/kg b.i.d. PPP [IGF inhibitor (C and F)]. Inflammatory index scores from H&E specimens (G) as previously published (Boehm et al., 2012) reflect no change in inflammatory status by PPP but a substantial decrease in hyperplasia. This is confirmed by BrdU proliferative index (H). Data are mean ± S.E.M.; *P < 0.05 3-day inflamed versus PBS-instilled (control) prostate; #P < 0.05 PPP i.p. treatment versus vehicle-treated controls; comparisons using analysis of variance, n = 8.
Pharmacologic IGFR1 Antagonism Attenuated Inflammation-Induced Hyperplasia and Epithelial Proliferation In Vivo.
Prostatic inflammation results in pronounced reactive hyperplasia of the prostate epithelium 3 days after induction, characterized by multilayering of the epithelium and the presence of atypical cuboidal cells with pleomorphic nuclei (Jerde and Bushman, 2009; Boehm et al., 2012). Mice treated with the IGF-R1 antagonist PPP (10 mg/kg i.p.) before and during the inflammatory period (twice daily) exhibited an inflammatory response characterized by inflammatory infiltrate and tissue damage but failed to exhibit the cellular hyperplasia evident in vehicle-treated (50 μl 75% corn oil, 25% DMSO i.p.) animals (Fig. 5, A–C). The epithelial cells did not form a multilayered epithelium, and luminal cells retained their columnar morphology. Though the epithelial cells still showed some atypical signs of damage, such as pyknotic nuclei and epithelial crowding, hyperplasia was attenuated by PPP treatment. This was quantified by analysis of histologic grading as previously reported elsewhere (Jerde and Bushman, 2009; Boehm et al., 2012) and performed by a blinded scorer (S.H.L.) (Fig. 5G).
These data demonstrate that the hyperplasic component of the inflammatory response is attenuated by PPP, but the infiltrate and vascular components are not affected by PPP. We confirmed an inhibition of proliferative response by BrdU-labeling animals 2 hours before sacrifice and determining the percent of epithelial cells proliferating; inflammation induces substantial epithelial proliferation that is attenuated by PPP treatment (Fig. 5, D–F). BrdU quantification is represented in Fig. 5H.
Direct Administration of IGF-1 to the Prostate Induces Epithelial Proliferation and Hyperplasia.
To confirm IGF as causative in the hyperplastic response of the prostate, we instilled IGF-1 coated Affi-Gel beads (Macias et al., 1996; Ferguson et al., 2004) into the prostate via catheter and measured histologic evidence of hyperplasia and inflammation. IGF-coated beads induce epithelial proliferation resulting in hyperplasia of the prostate epithelium above that observed by instilling PBS-incubated beads (Fig. 6, A and B). This effect was attenuated by PPP treatment (Fig. 6, C and D). In addition, administration of IGF-1–coated beads had a similar inducing effect on IL-1R1(−/−) mice, indicating that IGF can suffice to induce the hyperplastic effect in the absence of IL-1 signaling (Fig. 6, E and F).
Fig. 6.
IGF-coated Affi-Gel beads instilled in the prostate induce hyperplasia. H&E sections of dorsal-lateral prostates instilled with PBS-treated beads (A), IGF-1 coated beads (B), PBS-treated beads in PPP-treated animals (C), IGF-coated beads in PPP-treated animals (D), PBS-coated beads in IL-1R1(−/−) mouse prostates (E), and IGF-coated beads in IL-1R1(−/−) mouse prostates (F). Gross analysis of prostate lobes instilled with Affi-Gel beads (G) indicates that all three lobes and the bladder maintain the presence of beads for the 2-day duration of the experiment. Inflammatory index scores from H&E specimens reflect a significant increase in hyperplasia by instillation of IGF-1–coated beads, but no significant change in inflammation is conferred relative to PBS-treated beads. (H) However, the instillation of beads into the prostate induces a slight inflammatory effect relative to naïve mouse prostates. Histologically evident hyperplasia was confirmed by an increase in the number of BrdU-labeled cells by IGF-coated beads (I), an increase that was prevented by systemic treatment with PPP. Data are mean ± S.E.M.; *P < 0.05 2-day IGF-1–coated beads versus PBS-treated (control) beads instilled in the prostate; #P < 0.05 PPP i.p. treatment versus vehicle-treated controls; comparisons using analysis of variance, n = 4. BL/U, bladder/ureter; CG, coagulating gland; DLP, dorsal lateral prostate; VP, ventral prostate.
Effective instillation of beads into all lobes of the prostate was confirmed by gross analysis of the dissected lobes that revealed the presence of Affi-Gel beads in all prostatic lobes and the bladder 2 days after instillation. The beads were adhered to the epithelium (Fig. 6G). We histologically scored hyperplasia and inflammation as described in the methods, and found that IGF-loaded beads increased the hyperplasia score beyond that of PBS-treated beads, but IGF did not significantly promote inflammation (Fig. 6H) BrdU labeling confirmed the induction of proliferation by IGF-1 administration (Fig. 6I). Although the inflammatory response itself was not affected by IGF-1 or by PPP treatment, instillation of beads did induce a slight inflammatory reaction whether the beads were coated with IGF or not.
Discussion
The IGF pathway is regulated in a proproliferative direction during prostatic inflammation, resulting in downstream pathway activation and epithelial proliferation and reactive hyperplasia. Specifically, the IGF-1 pathway ligand exhibits mRNA induction that is evident the second day after induction of inflammation, and maximizes at 3 days after induction. Peptide production of IGF-1 ligand corresponds to this induction. IGF-1 peptide secretion appears to follow a regulated pattern in which increased IGF-1 is actively released, but retention of peptide occurs upon the resolution phase of the inflammatory process at day 5. Conversely, IGF-2 expression does not appear to be induced in our model of prostatic inflammation, but the regulated release of the peptide does appear to increase, suggesting that peptide release is an active point of IGF regulation in response to inflammation. Concentrations of both IGF peptides and IGF signaling pathway activation return to normal after 7 days of inflammation in this model.
Our data further indicate that pharmacologic IGF pathway inhibition attenuates inflammatory reactive hyperplasia in the mouse prostate. It is important to note that IGF and IGF receptor null mice are available but would not be applicable to use in the prostate for these studies due to the lack of prostatic tissue, given that IGF is indispensable for prostate development (Ruan et al., 1999). Previous reports indicate that the pharmacologic inhibitor PPP is effective in vivo at attenuating intimal hyperplasia (Cohen et al., 1991). We adapted this protocol to be applicable toward inflammation-induced hyperplasia of the prostate because the higher DMSO volumes used in the previous study produced unacceptable toxicity when given concurrently with prostatic inflammation. The adaptation of reduced DMSO allowed PPP to yield significant results at inhibiting inflammation-induced IGF signaling and resulting proliferation and hyperplasia, with minimal toxic effects. Further, PPP inhibited hyperplasia induced by exogenous instillation of IGF-1 delivered by IGF-1–coated Affi-Gel beads. These experiments indicate that IGF signaling plays a role in the hyperplastic response of the prostatic epithelium to inflammatory stimuli, and provide a mechanistic basis toward future clinical studies assessing the IGF signaling pathway as a therapeutic target for prostatic diseases including BPH and prostate cancer.
Our data also indicate that the IL-1 to IGF signaling loop that we have previously identified in the developing prostate as critical to IGF-induced prostatic growth (Jerde and Bushman, 2009) is reactivated in inflammation-driven reactive hyperplasia. The finding that IL-1R1(−/−) mice do not exhibit inflammation-induced hyperplasia while still exhibiting consistent inflammatory cell infiltrate demonstrates a role for this cytokine in hyperplasia specifically, and the finding that these mice do not exhibit IGF induction or evident IGF signaling points to IGF as an induced growth factor that mediates this effect. Further, administration of IGF-1 via Affi-Gel beads to the prostate activates IGF signaling in both wild-type and IL-1–null mice similarly, demonstrating that IGF is downstream of IL-1 in its hyperplastic role. In addition, IL-1R1–null mice fail to exhibit STAT3 induction as measured by phosphorylation at tyrosine 705, suggesting that, as in development, IL-1 is working via a STAT3-dependent mechanism. Future work in this area will be directed at comprehensively evaluating the role of STAT3 in IL-1–induced hyperplasia.
Additionally, IL-1 may not be the only cytokine involved in inflammation-induced IGF-driven proliferation; other cytokines induced in this model include IL-6, IL-8, tumor necrosis factor, or IL-18 (Boehm et al., 2012), and others that may induce IGF expression or may sit in between IL-1 and IGF (O’Connor et al., 2008). These cytokines are induced in our model, and other cytokines can be induced by IL-1 (Garlanda et al., 2013). Therefore, future work in this area would include further investigation of the role of inflammatory mediators in growth factor–induced hyperplasia. Therefore, in summary (Supplemental Fig. 1), our data propose the model that IL-1 produced during prostatic inflammation signals to the IL-1R1 receptor on responsive stromal cells, resulting in IGF production and proliferative signaling in the prostatic epithelium, resulting in hyperplasia. This model recapitulates the events that occur during prostate development.
While inflammation in the mouse model is transient and thus IGF-1 induction is transient, the chronic and recurrent inflammation observed in human BPH specimens results in sustained IGF pathway activation. Activation of the IGF signaling pathway occurs in the epithelium of human BPH specimens specifically juxtaposed to areas of inflammation. An important observation from our data is that inflammation when present in nondiseased prostates also induces IGF signaling concurrent with proliferation. The primary difference between BPH specimens and nondiseased specimens involving IGF signaling does not seem to be how the prostatic microenvironment responds to inflammation but rather how much inflammation occurs in each situation. While nondiseased specimens exhibit inflammation rarely and in limited focal pattern, inflammation in highly symptomatic TURP-removed BPH specimens was severe and widespread. As reported by Nickel and others (Delongchamps et al., 2008; Nickel et al., 2008; Bostanci et al., 2013), our observations are that inflammation is a uniform pathology of BPH specimens but is much less prevalent in normal conditions. This severe and widespread inflammation results in activated IGF signaling and the promotion of hyperplasia. Therefore, these data add to the growing evidence suggesting a proliferative role for inflammation in the progression of BPH.
The critical role for IGF signaling in prostatic growth is well established, but a pathologic role for IGF in BPH has been difficult to elucidate. IGF promotes epithelial growth in tissue culture studies and in vivo (Cohen et al., 1991; Plymate et al., 1996). IGF overexpression exhibits a potent growth-promoting effect for IGF in the prostate and results in hyperplasia (DiGiovanni et al., 2000; Kaplan-Lefko et al., 2008). Further, Ruan and Kleinberg demonstrated that IGF-I is required for prostate development (Ruan et al., 1999). However, conflicting reports exist regarding in vivo effects of IGF in humans. Circulating IGF serum concentrations have been analyzed by several groups with no consensus, casting doubt upon a critical role for IGF in BPH pathology (Khosravi et al., 2001; Stattin et al., 2001; Latif et al., 2002). These data analyzing circulating serum IGF concentrations ignore the role of locally induced IGF on growth, and a careful analysis of TURP specimens removed for BPH symptoms was clearly warranted. In developing prostatic growth, locally expressed IGF is indispensable for prostate growth (Ruan et al., 1999), and IGF is highly induced by local inflammatory mediators during development (Jerde and Bushman, 2009). Given the intensity of inflammation in BPH in previous reports and our own analysis, a recapitulation of this IL-1–IGF signaling loop seemed plausible. The present data therefore shed significant new light by identifying that locally produced IGF serves as a critical intermediary between inflammation and proliferation in the prostate.
While the present study is primarily focused on a role for inflammation-induced proliferation in a benign setting, the relevance of these results may extend into malignant prostate pathology as well (Nelson et al, 2003). A rapidly accumulating body of evidence links the presence and activity of inflammation to the development of cancer in the prostate (Rokman et al., 2002). Two prostate cancer susceptibility genes, ribonuclease L (RNase L) and macrophage scavenger receptor 1 (MSR1), function in host immune responses and protection against genome damage during inflammation (Rokman et al., 2002; Xu et al., 2002). Further, histopathology studies of human prostatectomy specimens have identified lesions characterized by proliferating epithelial cells and activated inflammatory cells (proliferative inflammatory atrophy) in juxtaposition to areas of neoplasia (De Marzo et al., 1999; Nelson et al., 2004). The sustained cell proliferation in an environment rich in inflammatory cells, growth factors, activated stroma, and DNA damage–promoting agents could potentiate and/or promote neoplasia (De Marzo et al., 1999). Cytokines are induced locally in prostate cancer microenvironment, and are known to induce proliferation of prostate cancer cells in vitro and in vivo (Culig, 2011; Karkera et al., 2011). In addition, proliferative inflammatory atrophy, a histologic lesion characterized by proliferating epithelial cells and activated inflammatory cells, is often found in association with prostatic intraepithelial neoplasia and prostate cancer and has been postulated to represent a premalignant lesion (Nelson et al., 2004). Loss of glutathione-S-transferase-π1 gene product is detected in a high proportion of prostatic intraepithelial neoplasia and cancer specimens and is considered a critical event that renders prostatic epithelial cells more vulnerable to genomic damage mediated by reactive oxygen and nitrogen species (Nelson et al., 2001). These findings have prompted the hypothesis that chronic inflammation is involved in the genesis and/or progression of prostate cancer, and that an activation of growth factor signaling may be one mechanism for how inflammation orchestrates a proproliferative microenvironment.
Taken in total, these cell culture, animal, and human data demonstrate a significant role for IGF in the proliferative response of the prostate to inflammation. IGF-induced epithelial proliferation is dependent on induction from IL-1 signaling, recapitulating a similar signaling loop found in prostate development. Further, inflammation-associated hyperplasia in human prostates is tightly associated and histologically juxtaposed to IGF signaling activation, suggesting a correlation of our animal data to human pathology in BPH. These data confirm a role of IGF in prostatic epithelial growth and suggest a reactivation of developmental-like signaling as part of the proliferative response to inflammatory injury that occurs in the promotion of prostate disease.
Supplementary Material
Acknowledgments
The authors thank Drs. Wade Bushman and Wei Huang (University of Wisconsin-Madison) for providing human BPH sections for immunofluorescence staining and expertise in BPH clinical histology and pathology. The authors thank Kai-Ming Chou and Yeh-Wen Chen for use of and expertise in fluorescent microscopy.
Abbreviations
- BPH
benign prostatic hyperplasia
- BrdU
bromidated deoxyuridine
- DMSO
dimethylsulfoxide
- IGF
insulin-like growth factor
- IL
interleukin
- IL-1R1
interleukin-1 receptor 1
- PBS
phosphate-buffered saline
- PPP
picropodophyllin
- STAT3
signal transducer and activator of transcription 3
- TURP
transurethral resection of the prostate
Authorship Contributions
Participated in research design: Hahn, McFarland, Lee, Jerde.
Conducted experiments: Hahn, Myers, McFarland, Lee, Jerde.
Performed data analysis: Hahn, Lee, Jerde.
Wrote or contributed to the writing of the manuscript: Hahn, Myers, Jerde.
Footnotes
This work was funded by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK092366-01A1]; and also partially funded by the Department of Molecular and Environmental Toxicology through a training grant from the National Institutes of Health National Institute of Environmental Health Sciences [Grant T32-ES007015] (to T.J.J.).
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Berkowitz DB, Choi S, Maeng JH. (2000) Enzyme-assisted asymmetric total synthesis of (−)-podophyllotoxin and (−)-picropodophyllin. J Org Chem 65:847–860. [DOI] [PubMed] [Google Scholar]
- Boehm BJ, Colopy SA, Jerde TJ, Loftus CJ, Bushman W. (2012) Acute bacterial inflammation of the mouse prostate. Prostate 72:307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostanci Y, Kazzazi A, Momtahen S, Laze J, Djavan B. (2013) Correlation between benign prostatic hyperplasia and inflammation. Curr Opin Urol 23:5–10. [DOI] [PubMed] [Google Scholar]
- Cohen P, Peehl DM, Lamson G, Rosenfeld RG. (1991) Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins in primary cultures of prostate epithelial cells. J Clin Endocrinol Metab 73:401–407. [DOI] [PubMed] [Google Scholar]
- Cotran RS, Kumar V, Robbins SL. (1999) Prostatitis, in Pathologic Basis of Disease, 6th ed (Robbins SL, ed) pp 1025–1027, WB Saunders, Philadelphia. [Google Scholar]
- Culig Z. (2011) Cytokine disbalance in common human cancers. Biochim Biophys Acta 1813:308–314. [DOI] [PubMed] [Google Scholar]
- De Marzo AM, Marchi VL, Epstein JI, Nelson WG. (1999) Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol 155:1985–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marzo AM, Meeker AK, Zha S, Luo J, Nakayama M, Platz EA, Isaacs WB, Nelson WG. (2003) Human prostate cancer precursors and pathobiology. Urology 62(5, Suppl 1)55–62. [DOI] [PubMed] [Google Scholar]
- De Marzo AM, Platz EA, Sutcliffe S, Xu J, Grönberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG. (2007) Inflammation in prostate carcinogenesis. Nat Rev Cancer 7:256–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delongchamps NB, de la Roza G, Chandan V, Jones R, Sunheimer R, Threatte G, Jumbelic M, Haas GP. (2008) Evaluation of prostatitis in autopsied prostates—is chronic inflammation more associated with benign prostatic hyperplasia or cancer? J Urol 179:1736–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiovanni J, Bol DK, Wilker E, Beltrán L, Carbajal S, Moats S, Ramirez A, Jorcano J, Kiguchi K. (2000) Constitutive expression of insulin-like growth factor-1 in epidermal basal cells of transgenic mice leads to spontaneous tumor promotion. Cancer Res 60:1561–1570. [PubMed] [Google Scholar]
- Ferguson CM, Schwarz EM, Puzas JE, Zuscik MJ, Drissi H, O’Keefe RJ. (2004) Transforming growth factor-beta1 induced alteration of skeletal morphogenesis in vivo. J Orthop Res 22:687–696. [DOI] [PubMed] [Google Scholar]
- Garlanda C, Dinarello CA, Mantovani A. (2013) The interleukin-1 family: back to the future. Immunity 39:1003–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings BA, Restuccia DF. (2012) PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol 4:a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochreiter WW, Duncan JL, Schaeffer AJ. (2000) Evaluation of the bacterial flora of the prostate using a 16S rRNA gene based polymerase chain reaction. J Urol 163:127–130. [PubMed] [Google Scholar]
- Jerde TJ, Bushman W. (2009) IL-1 induces IGF-dependent epithelial proliferation in prostate development and reactive hyperplasia. Sci Signal 2:ra49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerde TJ, Saban R, Bjorling DE, Steinberg H, Nakada SY. (2000) Distribution of neuropeptides, histamine content, and inflammatory cells in the ureter. Urology 56:173–178. [DOI] [PubMed] [Google Scholar]
- Karkera J, Steiner H, Li W, Skradski V, Moser PL, Riethdorf S, Reddy M, Puchalski T, Safer K, Prabhakar U, et al. (2011) The anti-interleukin-6 antibody siltuximab down-regulates genes implicated in tumorigenesis in prostate cancer patients from a phase I study. Prostate 71:1455–1465. [DOI] [PubMed] [Google Scholar]
- Kaplan-Lefko PJ, Sutherland BW, Evangelou AI, Hadsell DL, Barrios RJ, Foster BA, Demayo F, Greenberg NM. (2008) Enforced epithelial expression of IGF-1 causes hyperplastic prostate growth while negative selection is requisite for spontaneous metastogenesis. Oncogene 27:2868–2876. [DOI] [PubMed] [Google Scholar]
- Khosravi J, Diamandi A, Mistry J, Scorilas A. (2001) Insulin-like growth factor I (IGF-I) and IGF-binding protein-3 in benign prostatic hyperplasia and prostate cancer. J Clin Endocrinol Metab 86:694–699. [DOI] [PubMed] [Google Scholar]
- Krieger JN, Riley DE. (2002) Prostatitis: what is the role of infection. Int J Antimicrob Agents 19:475–479. [DOI] [PubMed] [Google Scholar]
- Latif Z, McMillan DC, Wallace AM, Sattar N, Mir K, Jones G, Underwood MA. (2002) The relationship of circulating insulin-like growth factor 1, its binding protein-3, prostate-specific antigen and C-reactive protein with disease stage in prostate cancer. BJU Int 89:396–399. [DOI] [PubMed] [Google Scholar]
- Macias D, Gañan Y, Ros MA, Hurle JM. (1996) In vivo inhibition of programmed cell death by local administration of FGF-2 and FGF-4 in the interdigital areas of the embryonic chick leg bud. Anat Embryol (Berl) 193:533–541. [DOI] [PubMed] [Google Scholar]
- McLaren ID, Jerde TJ, Bushman W. (2011) Role of interleukins, IGF and stem cells in BPH. Differentiation 82:237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeal JE. (1968) Regional morphology and pathology of the prostate. Am J Clin Pathol 49:347–357. [DOI] [PubMed] [Google Scholar]
- Monti S, Di Silverio F, Iraci R, Martini C, Lanzara S, Falasca P, Poggi M, Stigliano A, Sciarra F, Toscano V. (2001) Regional variations of insulin-like growth factor I (IGF-I), IGF-II, and receptor type I in benign prostatic hyperplasia tissue and their correlation with intraprostatic androgens. J Clin Endocrinol Metab 86:1700–1706. [DOI] [PubMed] [Google Scholar]
- Nelson WG, De Marzo AM, DeWeese TL. (2001) The molecular pathogenesis of prostate cancer: Implications for prostate cancer prevention. Urology 57(4, Suppl 1)39–45. [DOI] [PubMed] [Google Scholar]
- Nelson WG, De Marzo AM, DeWeese TL, Isaacs WB. (2004) The role of inflammation in the pathogenesis of prostate cancer. J Urol 172:S6–S11, discussion S11–S12. [DOI] [PubMed] [Google Scholar]
- Nelson WG, De Marzo AM, andIsaacs WB. (2003) Prostate cancer. N Engl J Med 349:366–381. [DOI] [PubMed] [Google Scholar]
- Nickel JC, Downey J, Young I, Boag S. (1999) Asymptomatic inflammation and/or infection in benign prostatic hyperplasia. BJU Int 84:976–981. [DOI] [PubMed] [Google Scholar]
- Nickel JC, Roehrborn CG, O’Leary MP, Bostwick DG, Somerville MC, Rittmaster RS. (2007) Examination of the relationship between symptoms of prostatitis and histological inflammation: baseline data from the REDUCE chemoprevention trial. J Urol 178:896–900, discussion 900–901. [DOI] [PubMed] [Google Scholar]
- Nickel JC, Roehrborn CG, O’Leary MP, Bostwick DG, Somerville MC, Rittmaster RS. (2008) The relationship between prostate inflammation and lower urinary tract symptoms: examination of baseline data from the REDUCE trial. Eur Urol 54:1379–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, McCusker RH, Strle K, Johnson RW, Dantzer R, Kelley KW. (2008) Regulation of IGF-I function by proinflammatory cytokines: at the interface of immunology and endocrinology. Cell Immunol 252:91–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plymate SR, Tennant M, Birnbaum RS, Thrasher JB, Chatta G, Ware JL. (1996) The effect on the insulin-like growth factor system in human prostate epithelial cells of immortalization and transformation by simian virus-40 T antigen. J Clin Endocrinol Metab 81:3709–3716. [DOI] [PubMed] [Google Scholar]
- Razuvaev A, Henderson B, Girnita L, Larsson O, Axelson M, Hedin U, Roy J. (2007) The cyclolignan picropodophyllin attenuates intimal hyperplasia after rat carotid balloon injury by blocking insulin-like growth factor-1 receptor signaling. J Vasc Surg 46:108–115. [DOI] [PubMed] [Google Scholar]
- Roehrborn CG. (2008) Pathology of benign prostatic hyperplasia. Int J Impot Res 20(Suppl 3):S11–S18. [DOI] [PubMed] [Google Scholar]
- Rökman A, Ikonen T, Seppälä EH, Nupponen N, Autio V, Mononen N, Bailey-Wilson J, Trent J, Carpten J, Matikainen MP, et al. (2002) Germline alterations of the RNASEL gene, a candidate HPC1 gene at 1q25, in patients and families with prostate cancer. Am J Hum Genet 70:1299–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan W, Powell-Braxton L, Kopchick JJ, Kleinberg DL. (1999) Evidence that insulin-like growth factor I and growth hormone are required for prostate gland development. Endocrinology 140:1984–1989. [DOI] [PubMed] [Google Scholar]
- Savvani A, Petraki C, Msaouel P, Diamanti E, Xoxakos I, Koutsilieris M. (2013) IGF-IEc expression is associated with advanced clinical and pathological stage of prostate cancer. Anticancer Res 33:2441–2445. [PubMed] [Google Scholar]
- Stattin P, Kaaks R, Riboli E, Ferrari P, Dechaud H, Hallmans G. (2001) Circulating insulin-like growth factor-I and benign prostatic hyperplasia—a prospective study. Scand J Urol Nephrol 35:122–126. [DOI] [PubMed] [Google Scholar]
- Steiner GE, Stix U, Handisurya A, Willheim M, Haitel A, Reithmayr F, Paikl D, Ecker RC, Hrachowitz K, Kramer G, et al. (2003) Cytokine expression pattern in benign prostatic hyperplasia infiltrating T cells and impact of lymphocytic infiltration on cytokine mRNA profile in prostatic tissue. Lab Invest 83:1131–1146. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Feldman EL. (2002) Control of cell survival by IGF signaling pathways. Growth Horm IGF Res 12:193–197. [DOI] [PubMed] [Google Scholar]
- Xu J, Zheng SL, Komiya A, Mychaleckyj JC, Isaacs SD, Hu JJ, Sterling D, Lange EM, Hawkins GA, Turner A, et al. (2002) Germline mutations and sequence variants of the macrophage scavenger receptor 1 gene are associated with prostate cancer risk. Nat Genet 32:321–325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.