Abstract
Blood-brain barrier (BBB) integrity is compromised in many central nervous system disorders. Complex astrocyte and vascular endothelial cell interactions that regulate BBB integrity may be disturbed in these disorders. We previously showed that systemic administration of 3-chloropropanediol [(S)-(+)-3-chloro-1,2-propanediol] induces a transitory glial fibrillary acidic protein-astrocyte loss, reversible loss of tight junction complexes, and BBB integrity disruption. However, the intracellular signaling mechanisms that induce BBB integrity marker loss are unclear. We hypothesize that 3-chloropropanediol–induced modulation of tight junction protein expression is mediated through the phosphoinositide-3-kinase (PI3K)/AKT pathway. To test this hypothesis, we used a mouse brain endothelial cell line (bEnd.3) exposed to 3-chloropropanediol for up to 3 days. Results showed early reversible loss of sharp paracellular claudin-5 expression 90, 105, and 120 minutes after 3-chloropropanediol (500 μM) treatment. Sharp paracellular claudin-5 profiles were later restored, but lost again by 2 and 3 days after 3-chloropropanediol treatment. Western blot and immunofluorescence studies showed increased p85-PI3K expression and transitory increased AKT (Thr308) phosphorylation at 15 and 30 minutes after 3-chloropropanediol administration. PI3K inhibitors LY294002 [2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one hydrochloride; 2.5–25 μM] and PI-828 [2-(4-morpholinyl)-8-(4-aminopheny)l-4H-1-benzopyran-4-one; 0.1–10 μM] prevented the 3-chloropropanediol–induced AKT (Thr308) phosphorylation and both early and late loss of paracellular claudin-5. However, AKT inhibitors only prevented the early changes in claudin-5 expression. This mechanistic study provides a greater understanding of the intracellular signaling pathways mediating tight junction protein expression and supports a hypothesis that two independent pathways triggered by PI3K mediate early and late loss of paracellular claudin-5 expression.
Introduction
The blood-brain barrier (BBB) is a highly dynamic structure composed of endothelial cells, astrocytes and their end-feet, and extracellular matrix components. Together, these form a physical and metabolic barrier between the central nervous system and circulating blood. In health, the BBB acts as a crucial homeostatic regulator for promoting neuronal survival and restricts peripherally circulating toxins, blood proteins, and micro-organisms from entering brain tissue (Abbott et al., 2010; Banks and Erickson, 2010). The low permeability of the BBB is due, in part, to tight junction complexes composed of various transmembrane proteins, including claudin, occludin, and junctional adhesion molecule subfamilies (Tsukita et al., 2001; Chiba et al., 2008; Willis et al., 2008, 2010). The integrity of the BBB is compromised in many central nervous system disorders, including demyelinating disorders, such as multiple sclerosis and in cerebral ischemia (Grossman et al., 1986; Hatashita and Hoff, 1990; Lo et al., 1994).
Despite the importance of the BBB, the factors responsible for the induction and maintenance of its properties remain unclear. Several cytoplasmic signaling molecules, such as phosphoinositide-3-kinase (PI3K), Rho kinase, protein kinase C (PKC) isozymes, and phospholipase C, have been localized to tight junction complexes and may regulate their assembly and disassembly (González-Mariscal et al., 2008). The PI3K/AKT pathway is activated by a number of receptors and intracellular signaling molecules leading to PI3K phosphorylation and generation of phosphatidylinositol (3,4,5)-triphosphate (Vivanco and Sawyers, 2002; West et al., 2002; Paez and Sellers, 2003; Murillo et al., 2004; Hemmings and Restuccia, 2012). The serine/threonine kinase AKT (also known as protein kinase B) binds to phosphatidylinositol (3,4,5)-triphosphate at the plasma membrane promoting AKT (Thr308) phosphorylation, resulting in activation of its serine/threonine kinase activity (Alessi et al., 1997). Fully active AKT shows further phosphorylation at Ser473, inducing numerous cellular functions including angiogenesis, metabolism, growth, proliferation, survival, protein synthesis, transcription, and apoptosis (Brunet et al., 2001). It has been reported that PI3K inhibitors preserve, and may increase, endothelial function, preventing the pathogenesis of many neurodegenerative disorders (Cain et al., 2010).
We previously showed in vivo that a single systemic administration of the selective gliotoxin, 3-chloropropanediol [(S)-(+)-3-chloro-1,2-propanediol], induced reversible loss of glial fibrillary acidic protein immunoreactive astrocytes, extensive remodeling of the extracellular matrix, and loss of paracellular tight junction protein (claudin-5, occludin, and zona occludens-1) expression and BBB integrity in the rat inferior colliculus over a 28-day period (Willis et al., 2004a,b, 2013). 3-Chloropropanediol has been reported to undergo bioactivation to a reactive monoaldehyde through the action of alcohol dehydrogenase III (Mori et al., 2000; Skamarauskas et al., 2007). In vivo, the selectivity of 3-chloropropanediol for astrocytes may be due to the greater capacity of these cells to bioactivate the gliotoxin (Mori et al., 2000). However, the endothelial intracellular signaling mechanisms triggered by disruption of the BBB microenvironment that resulted in the loss of paracellular tight junction protein expression were not studied. We hypothesize that claudin-5 protein modification in the vascular endothelial cell and loss of BBB integrity are mediated through modulation of the PI3K/AKT pathway. Because of the dynamic interactions in the BBB microenvironment in vivo, we tested our hypothesis using a vascular endothelial cell line monoculture (bEnd.3) treated with 3-chloropropanediol. Although these endothelial cells show poor transendothelial electrical resistance values, they do express mRNA and protein markers for tight junction proteins and form a barrier to radiolabeled sucrose (Deli et al., 2005; Brown et al., 2007). The use of bEnd.3 cells is also advantageous because they are not contaminated by other cells of the neurovascular unit and do not dedifferentiate when passaged (Brown et al., 2007). In this study, we demonstrate that claudin-5 expression shows an early and late biphasic modulation of paracellular expression after 3-chloropropanediol treatment that is modulated through the PI3K/AKT signaling pathway. There is early increased PI3K expression and AKT (Thr308), but not AKT (Ser473), phosphorylation. Pharmacologic inhibition of PI3K by LY294002 [2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one hydrochloride; 1–25 μM] or PI-828 [2-(4-morpholinyl)-8-(4-aminopheny)l-4H-1-benzopyran-4-one; 0.1–10 μM] attenuated AKT (Thr308) phosphorylation and preserved both the early and late loss of claudin-5 expression. In addition, although pharmacologic inhibition of AKT with 10-DEBC (10-[4′-(N,N-diethylamino)butyl]-2-chlorophenoxazine hydrochloride; 10 μM) or AKT1 [1L-6-hydroxymethyl-chiroinositol-2(R)-2-O-methyl-3-O-octadecyl-sn-glycerocarbonate; 10 μM] also attenuated the early transitory loss of claudin-5 expression, it did not preserve the late loss of paracellular expression, providing evidence that early and late loss of claudin-5 is mediated through two distinct pathways. These results show that the PI3K/AKT pathway plays a central role in the regulation of claudin-5 expression and suggests that regulation of the PI3K/AKT pathway could hold therapeutic potential for neurodegenerative diseases.
Materials and Methods
bEnd.3 Cell Culture.
bEnd.3 endothelial cells are BALB/c mouse brain endothelial cells transformed by a retrovirus vector that expresses polyomavirus middle T antigen (CRL-2299; American Type Culture Collection, Manassas, VA). bEnd.3 cells were grown in Dulbecco’s modified Eagle’s medium (D6046)/10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO) according to the supplier’s instructions. Cell lines were grown in a humidified cell culture incubator at 37°C and 5% CO2/95% room air following the supplier’s recommendations. For experimental procedures, cells were trypsinized and plated at a density of 0.5–1.0 × 104 cells/cm2 (Omidi et al., 2003) onto plastic culture plates or collagen I–coated (Trevigen, Gaithersburg, MD) glass coverslips. Cells were grown until confluent. Medium was changed every 3 to 4 days and 24 hours before the day of the experiment.
3-Chloropropanediol and PI3K/AKT Inhibitor Treatment.
3-Chloropropanediol (Sigma-Aldrich) was prepared in Dulbecco’s modified Eagle’s medium/10% fetal bovine serum and added to bEnd.3 cells to give a final concentration of 500 μM. Endothelial cells were continuously exposed to 3-chloropropanediol for either 0, 15, 30, 60, 90, 105, 120, or 240 minutes or 1, 2, or 3 days. Inhibitors LY294002, PI-828, 10-DEBC (Tocris Bioscience, Minneapolis, MN), and AKT1 inhibitor (Calbiochem, San Diego, CA) were added to the bEnd.3 cells 30 minutes prior to 3-chloropropanediol (500 μM) administration. After 3-chloropropanediol administration, culture plates were returned to the incubator for up to 2 hours or 3 days.
SDS-PAGE and Western Blot Analysis.
Medium was aspirated. bEnd.3 cells were washed with phosphate-buffered saline (PBS) (4°C) and then CelLytic buffer (4°C) (Sigma-Aldrich) containing protease and phosphatase inhibitor cocktails 2 and 3 (Sigma-Aldrich). Cells were removed using a plastic scraper, collected, and homogenized. Total bEnd.3 cell homogenates were cleared by centrifugation (10,000g, 10 minutes, 4°C) and used for all Western blot analysis. Protein concentration of each bEnd.3 cell homogenate was determined using the bicinchoninic acid protein assay (Pierce Biotechnology, Rockford, IL).
bEnd.3 cell homogenates were separated on SDS-polyacrylamide gels (10% Bis-Tris Criterion XT precast gels; Bio-Rad, Hercules, CA). The separated proteins were then transferred to polyvinylidene difluoride (PVDF) membranes (Immobilon PVDF, 0.45 μm; EMD Millipore, Billerica, MA). Nonspecific binding was blocked with Aquablock (EastCoast Biologics Inc., North Berwick, ME) for at least 30 minutes at room temperature. PVDF membranes were incubated on a plate rocker overnight at 4°C with primary antibodies diluted in Aquablock using 1) rabbit antibodies p85-PI3K, pAKT (Thr308), and pAKT (Ser473) (1:1000; Cell Signaling Technologies, Danvers, MA), and 2) mouse antibodies claudin-5 (2.5 μg/ml; Life Technologies, Carlsbad, CA) and glyceraldehyde 3-phosphate dehydrogenase (2.0 μg/ml; GenTex, Irvine, CA).
PVDF membranes were washed with Tris-Tween buffered saline [30 mM Tris, 150 mM NaCl and 0.5% (v/v) Tween 20 at pH 7.4] and incubated with secondary antibody, goat anti-rabbit IR Dye 800, or goat anti-mouse IR Dye 800 (0.1 μg/ml; LI-COR, Lincoln, NE) in Aquablock for at least 180 minutes at room temperature. PVDF membranes were washed in Tris-Tween buffered saline and then PBS. Protein bands were visualized by infrared laser scanning using the Odyssey Infrared Imaging system (LI-COR). Molecular weights of the protein bands were calculated using Odyssey software and were quantified and corrected for background using ImageJ densitometric software (National Institutes of Health, Bethesda, MD). Protein levels were normalized to expression of glyceraldehyde 3-phosphate dehydrogenase, which served as internal loading control.
Immunofluorescence Microscopy.
After treatment, bEnd.3 cells were washed in PBS and fixed/permeabilized in 100% ethanol for 5 minutes. Cells were then washed with PBS followed by 1% bovine serum albumin/0.2% Tween-20 in PBS (buffer) before incubating in normal goat serum (2 mg/ml in buffer; DakoA/S, Glostrup, Denmark) for 15 minutes at room temperature.
Indirect immunofluorescence was performed using the same antibodies as listed above. For double-label immunofluorescence, mouse antibodies were coincubated with rabbit antibodies at room temperature. Primary antibodies were diluted in buffer and incubated with cells for 90 minutes. Cells were washed in buffer and incubated in purified goat anti-mouse IgG and goat anti-rabbit IgG secondary antibodies conjugated to either Alexa Fluor-488 or Alexa Fluor-568 (4 μg/ml; Life Technologies) for 60 minutes in the dark. Finally, cells were washed in buffer and then PBS, and mounted in ProLong Gold antifade with 4′,6-diamidino-2-phenylindole (Life Technologies). These bEnd.3 monolayers were assessed for changes in claudin-5 and AKT (Thr308 and Ser473) expression.
bEnd.3 monolayers were examined using an Olympus BX53 (Olympus, Center Valley, PA) fluorescence microscope. Images were captured with a digital camera and CellSens image software (Olympus). Images were exported and uniformly adjusted to optimize brightness and contrast utilizing Paint Shop Pro (Jasc Software Inc., Eden Prairie, MN).
Statistical Analysis.
Western blot densitometry data are reported as means ± S.E.M. from 3–5 separate experiments in each group. Statistical significance between treatment groups and control values was determined using t test analysis. P values less than 0.05 were considered significant.
Results
Early Loss of Paracellular Expression of Claudin-5.
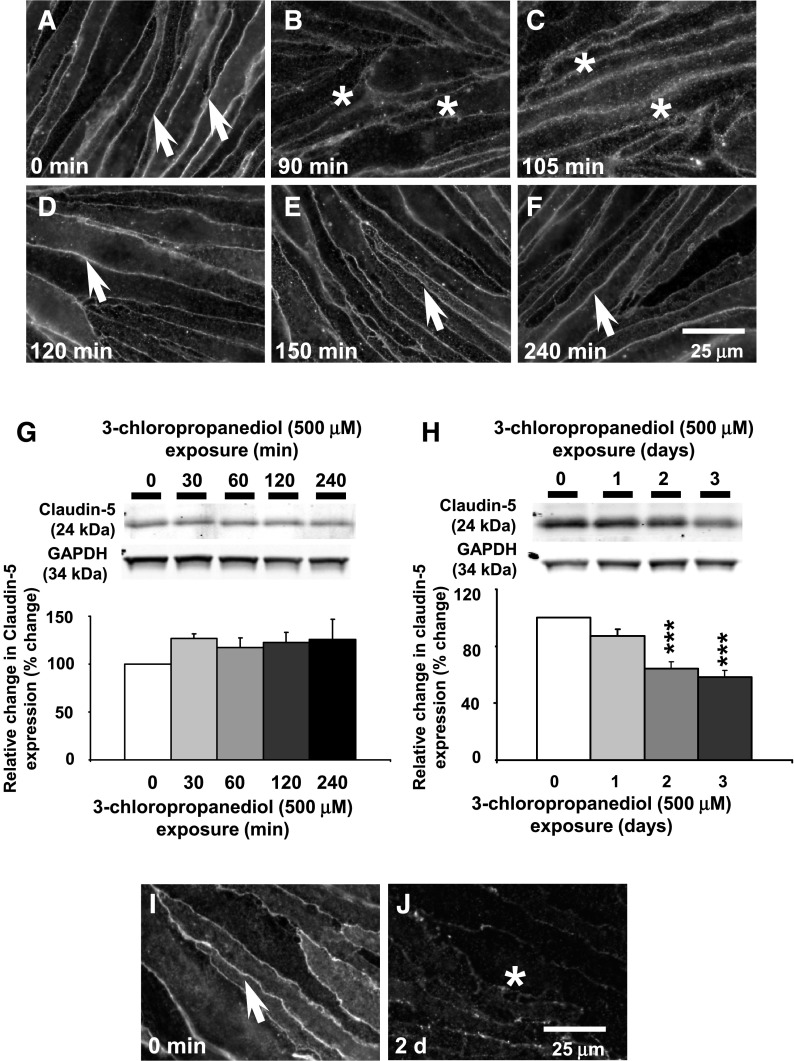
In control bEnd.3 cells, claudin-5 expression appeared as sharply defined immunoreactivity, marking the margins of the endothelial cells (Fig. 1A, arrows). After 3-chloropropanediol (500 μM) administration (90 and 105 minutes), claudin-5 immunoreactivity appeared diffuse, although still close to cell margins (Fig. 1, B and C, asterisks). By 120 and 150 minutes, claudin-5 immunoreactivity appeared sharper (Fig. 1, D and E, arrows) and by 240 minutes was comparable to that seen in control tissue (Fig. 1F, arrow). bEnd.3 cell homogenates showed no change in claudin-5 expression over this time course (Fig. 1G).
Fig. 1.
Early and late loss of paracellular claudin-5 expression. (A–F) Immunofluorescence analysis showed early reversible loss of paracellular claudin-5 expression over a 240-minute period. Arrows and asterisks show claudin-5 immunoreactivity. (G) Western blot analysis showed no loss of total claudin-5 expression over a 240-minute time course. (H) A marked loss (65%) of claudin-5 expression was observed after 3 days. (I and J) Immunofluorescence studies showed a late loss of claudin-5 expression after 3-chloropropanediol exposure for 2 days. Protein quantification data were obtained by densitometry and normalized using GAPDH as a loading control. Values are expressed as relative optical density and are represented as mean ± S.E.M. ***P < 0.001 (compared with the controls). For each column, n = 4–6 independent experiments. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. Scale bar, 25 μm in A–F, I, and J.
Delayed Loss of Paracellular Claudin-5 Expression.
After 2 and 3 days of 3-chloropropanediol administration, paracellular claudin-5 expression was again lost. The loss of claudin-5 expression at paracellular domains appears to be a loss of protein because Western blot analysis of bEnd.3 cell homogenates showed a significant (P < 0.001) decrease in claudin-5 expression at days 2 and 3 (Fig. 1H). This decrease was also seen in immunofluorescence studies because claudin-5 expression was lost at the cell margins by 2 and 3 days (Fig. 1, I and J).
Transitory Increased p85-PI3K Expression.
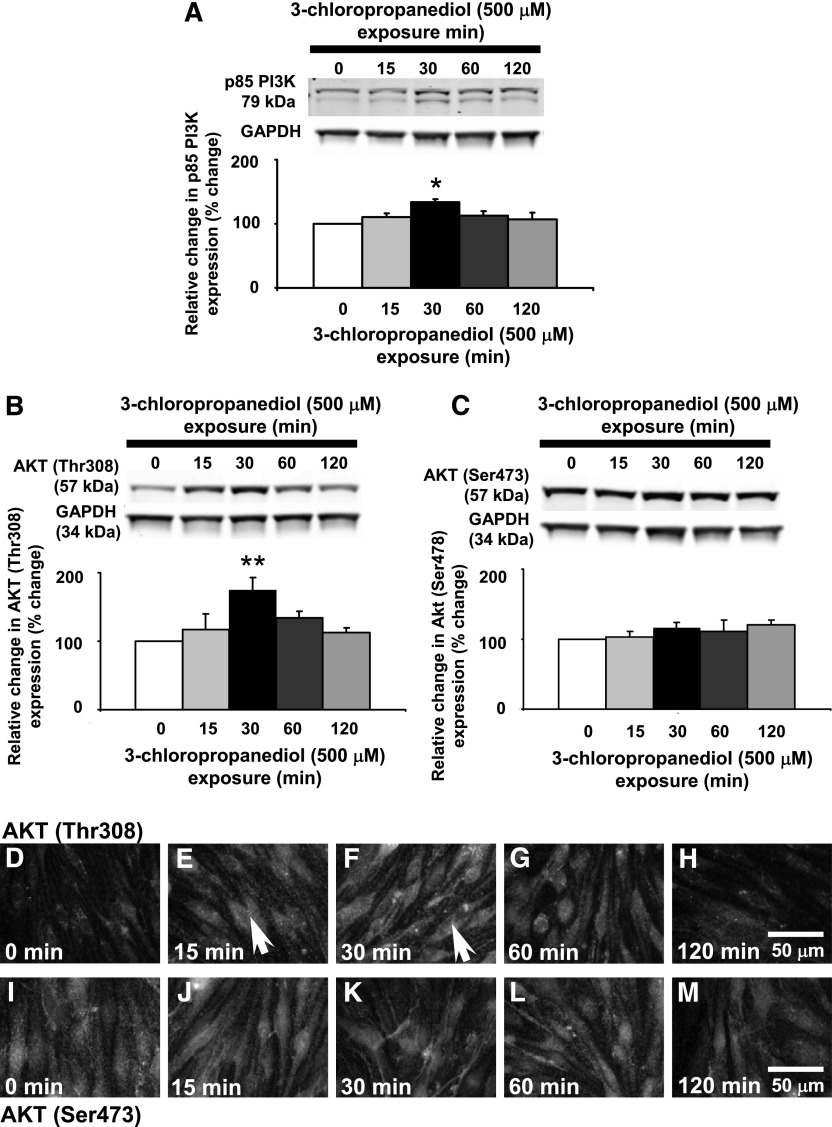
We propose that the PI3K/AKT pathway regulates the biphasic changes seen in paracellular claudin-5 expression. In Western blot analysis of bEnd.3 cell homogenates, the regulatory subunit of PI3K, p85-PI3K, appeared as two bands at approximately 79 kDa. After 3-chloropropanediol (500 μM) administration, there was a significant (P < 0.05) transitory increase in expression of both bands at 30 minutes (Fig. 2A). By 60 and 120 minutes of exposure, expression of both bands was returning to control levels.
Fig. 2.
Transitory increased p85-PI3K and AKT (Thr308) phosphorylation. Western blot analysis showed changes in p85-PI3K and AKT (Thr308) expression in bEnd.3 cells after 3-chloropropanediol (500 μM) administration. (A) p85-PI3K appeared as a 79-kDa band and showed increased expression at 30 minutes. (B) AKT (Thr308) appeared as a 57-kDa band. Over 30 minutes, there was a marked increase in expression of the 57-kDa band. The intensity of this band decreased after 60 and 120 minutes. (C) AKT (Ser473) showed no change in expression. (D–H) Immunofluorescence analysis showed increased AKT (Thr308) expression at 15 and 30 minutes after 3-chloropropanediol administration compared with controls. By 60 and 120 minutes, expression decreased and was comparable to control cells. Arrows show increased cytoplasmic immunofluorescence. (I–M) AKT (Ser473) phosphorylation showed no change in expression over the study period. Protein quantification data were obtained by densitometry and normalized using GAPDH as a loading control. Values are expressed as relative optical density and are represented as mean ± S.E.M. *P < 0.05; **P < 0.01 (compared with the controls). For each column, n = 4–6 independent experiments. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. Scale bar, 50 μm in D–M.
Transitory Increased AKT (Thr308) Phosphorylation, but Not AKT (Ser473) in bEnd.3 Cells.
Further analysis of the PI3K/AKT pathway showed theronine residue-specific AKT phosphorylation (Fig. 2, B and C). In bEnd.3 cell homogenates from control cells, AKT (Thr308) appeared as a single band at 57 kDa and showed a low level of expression. Fifteen minutes after 3-chloropropanediol administration, AKT (Thr308) phosphorylation expression increased (140%) and peaked at 30 minutes (180%), showing a significant (P < 0.01) increase compared with control levels (Fig. 2B). By 60 minutes, AKT (Thr308) expression decreased and returned to control levels after 120 minutes (Fig. 2B). There was no significant change in AKT (Ser473) phosphorylation over the 120-minute period of the study (Fig. 2C).
A similar pattern of increased AKT (Thr308) phosphorylation expression was seen in immunofluorescence microscopy studies after 3-chloropropanediol administration. Control cells showed low levels of cytoplasmic AKT (Thr308) expression (Fig. 2D). By 15 and 30 minutes, increased cytoplasmic immunofluorescence was seen (Fig. 2, E and F, arrows), which had returned to levels seen in control cells by 120 minutes (Fig. 2, G and H). There was little change in the expression of AKT (Ser473) phosphorylation over the course of the experiment (Fig. 2, I–M).
PI3K Inhibition Attenuates AKT (Thr308) Phosphorylation.
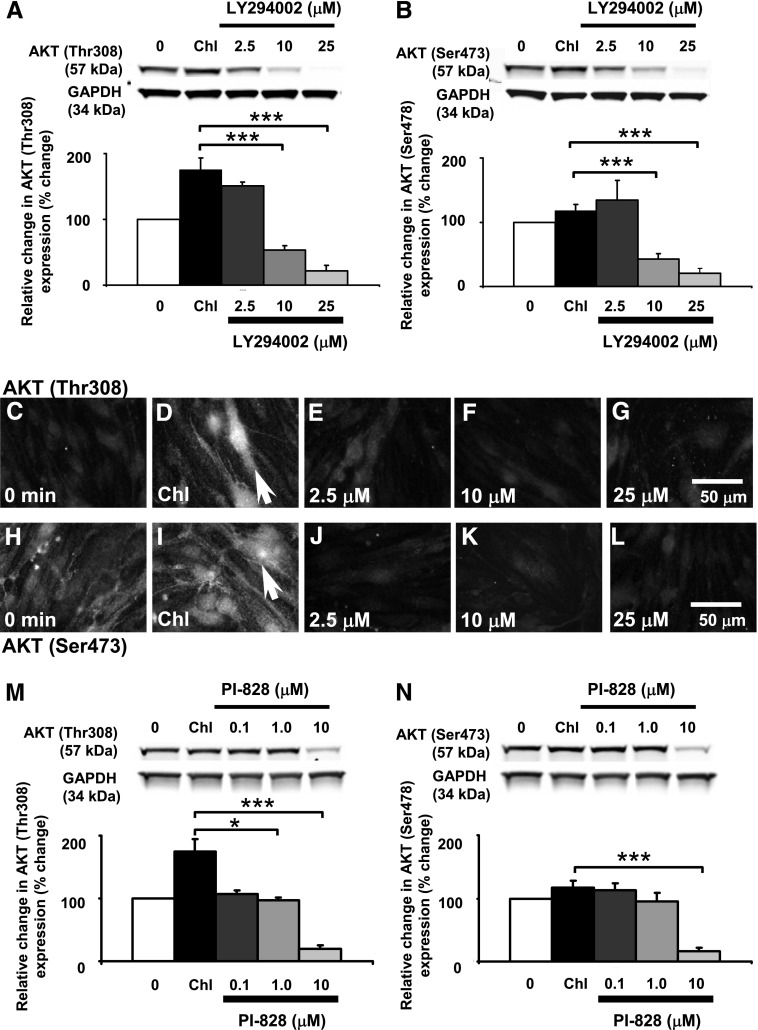
To pharmacologically test the role of the PI3K/AKT pathway in modulating paracellular claudin-5 expression, we used LY294002 (2.5–25 μM) and PI-828 (0.1–10 μM), both highly selective inhibitors of PI3K. LY294002 inhibits α, β, δ, and γ PI3K subunits with IC50 values ranging from 0.31 to 6.60 μM. PI-828 shows greater potency with IC50 values ranging from 0.098 to 1.967 μM. The inhibitors were applied to the bEnd.3 cells 30 minutes prior to 3-chloropropanediol (500 μM) administration. LY294002 treatment promoted a dose-dependent reduction in the 3-chloropropanediol–induced expression of AKT (Thr308) phosphorylation (Fig. 3A). Pretreatment with LY294002 (2.5 μM) slightly reduced the 3-chloropropanediol–induced expression of AKT (Thr308) phosphorylation from over 180% to about 150% with respect to control levels. Increased doses of LY294002 (10–25 μM) significantly (P < 0.001) reduced AKT (Thr308) phosphorylation to below control levels. Although 3-chloropropanediol treatment induced no marked effect on AKT (Ser473) phosphorylation, LY294002 pretreatment significantly (P < 0.001) reduced the expression of AKT (Ser473) phosphorylation to below control levels (Fig. 3B).
Fig. 3.
LY294002 and PI-828 attenuation of 3-chloropropanediol–induced changes in AKT (Thr308) in bEnd.3 cells. (A) Western blot analysis showed that preincubation with LY294002 (2.5–25 μM) significantly prevented the 3-chloropropanediol–induced AKT (Thr308) phosphorylation and significantly reduced AKT (Thr308) to below control levels. (B) LY294002 pretreatment also reduced AKT (Ser473) phosphorylation to below control levels of expression. Results from immunofluorescence studies paralleled the Western blot results. (C–G) AKT (Thr308) phosphorylation induced by 3-chloropropanediol incubation was prevented by 30-minute pretreatment with LY294002. (H–L) AKT (Ser473) also showed decreased levels of expression when treated with LY294002 (25 μM). Arrows (D and I) show increased cytoplasmic immunofluorescence. (M) Preincubation with PI-828 (0.1–10 μM) significantly prevented the 3-chloropropanediol–induced AKT (Thr308) phosphorylation and significantly reduced AKT (Thr308) to below control levels. (N) PI-828 pretreatment also reduced AKT (Ser473) phosphorylation to below control levels of expression. Protein quantification data were obtained by densitometry and normalized using GAPDH as a loading control. Values are expressed as relative optical density and are represented as mean ± S.E.M. *P < 0.05; ***P < 0.001 (compared with the controls). For each column, n = 4–6 independent experiments. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. Scale bar, 50 μm in C–L.
Immunofluorescence microscopy demonstrated that the 3-chloropropanediol–induced cytoplasmic AKT (Thr308) phosphorylation was inhibited in a dose-dependent manner with LY294002 (2.5–25 μM); this paralleled changes seen in Western blot analysis (Fig. 3, C–G). Cells probed for AKT (Ser473) phosphorylation showed little change in expression after 3-chloropropanediol treatment, but did show a marked decrease after pretreatment with LY294002 (Fig. 3, H–L).
PI-828 pretreatment also showed a dose-dependent attenuation of the 3-chloropropanediol–induced AKT (Thr308) phosphorylation increase (Fig. 3M). PI-828 (0.1–1 μM) restored AKT (Thr308) phosphorylation to control levels, whereas treatment with 10 μM reduced AKT (Thr308) and AKT (Ser473) expression to below control levels (P < 0.001) (Fig. 3, M and N). This is a similar pattern to that seen with the higher doses of LY294002 (Fig. 3, A and B).
Attenuation of Early and Late Biphasic Loss of Paracellular Claudin-5 Expression.
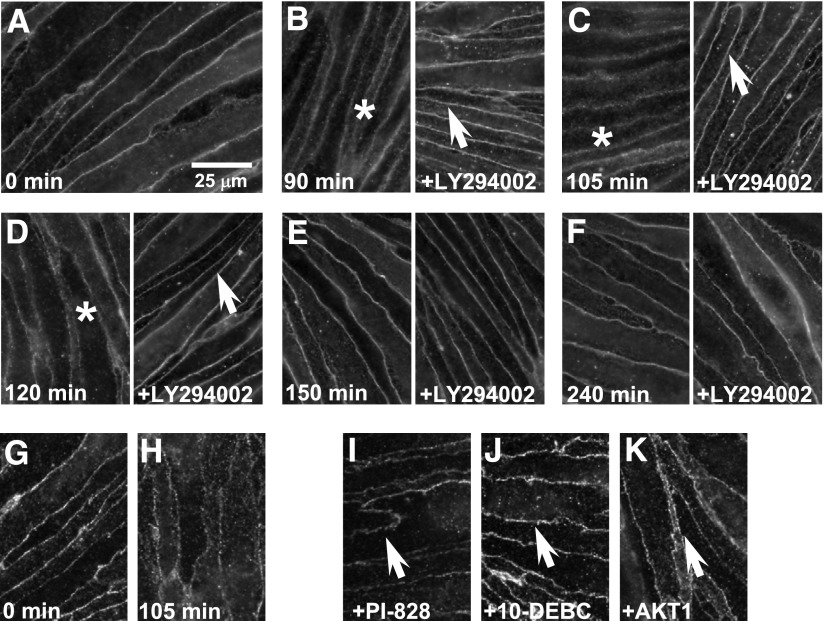
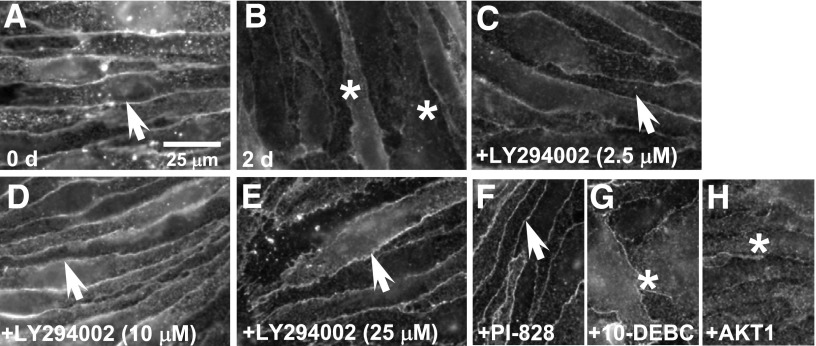
Having targeted modulation of the PI3K/AKT pathway and AKT (Thr308) phosphorylation expression, we determined the effect on the early and late loss of paracellular claudin-5 expression (Fig. 4, A–F). The early (90–120 minutes) 3-chloropropanediol–induced loss of sharply defined claudin-5 expression (Fig. 4, B–D, asterisks) was prevented by LY294002 (25 μM) treatment (Fig. 4, B–D, arrows). This early change in paracellular claudin-5 expression seen after 105 minutes compared with control cells (Fig. 4, G and H) was also prevented by PI-828 (25 μM), 10-DEBC (25 μM), and AKT1 (25 μM) treatment (Fig. 4, I–K, arrows). The late paracellular claudin-5 loss seen at 2 days was prevented by pretreatment with PI3K inhibitors PI-828 and LY294002 (Fig. 5, A–F, arrows). However, pretreatment with AKT inhibitors (10-DEBC and AKT1) did not prevent the 3-chloropropanediol–induced claudin-5 loss seen at 2 days (Fig. 5, G and H, asterisks).
Fig. 4.
Attenuation of early loss of paracellular claudin-5 expression by PI3K and AKT inhibitors. (A) Immunofluorescence studies showed sharply defined claudin-5 expression in control untreated cells. (B–D) Claudin-5 immunoreactivity rapidly (90, 105, and 120 minutes) became diffuse (asterisks) after 3-chloropropanediol treatment. (E and F) By 150 and 240 minutes, sharp claudin-5 immunoreactivity was restored. Pretreatment with LY294002 (25 μM) prevented the loss of sharp claudin-5 immunoreactivity over a 240-minute period (B–F). Pretreatment with PI-828 (25 μM) (I), 10-DEBC (25 μM) (J), and AKT1 (25 μM) (K) also prevented the early reversible loss of paracellular claudin-5 expression (arrows). Scale bar, 25 μm.
Fig. 5.
Attenuation of late loss of paracellular claudin-5 expression by PI3K, but not AKT inhibitors. (A) Immunofluorescence analysis showed sharply defined paracellular claudin-5 expression in control cells. (B) After 2 days of 3-chloropropanediol administration, sharply defined claudin-5 expression was lost (asterisks). (C–E) Pretreatment with LY294002 (2.5–25 μM, arrows) showed a dose-dependent attenuation of the late loss of paracellular claudin-5 expression. (F–H) Loss of sharp claudin-5 expression could also be prevented by PI-828 (25 μM, arrow) (F), but not by 10-DEBC (25 μM) (G) or AKT1 (25 μM) (H). Scale bar, 25 μm.
Discussion
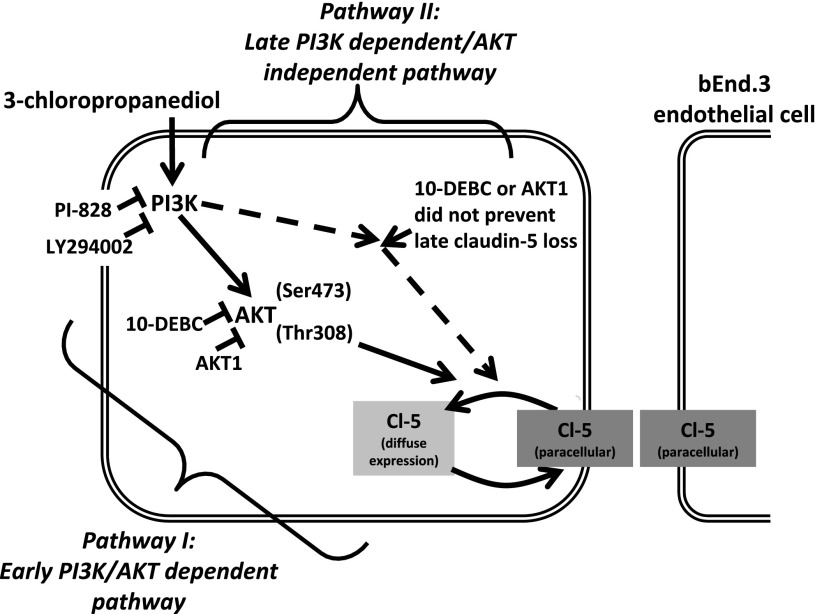
In this study, we have shown that treatment of the vascular endothelial cell line (bEnd.3) with 3-chloropropanediol induced 1) early loss of sharply defined paracellular claudin-5 expression at 90, 105, and 120 minutes; 2) late loss of paracellular claudin-5 expression at 2 and 3 days of exposure; 3) increased PI3K expression; and 4) increased AKT (Thr308) phosphorylation, but not AKT (Ser473). In addition, we have shown that use of selective PI3K or AKT inhibitors attenuated AKT (Thr308) phosphorylation and the early changes in claudin-5 expression. By contrast, late changes in claudin-5 expression were only attenuated by PI3K inhibitors LY294002 and PI-828, and not 10-DEBC or AKT1. Together, these results show that the PI3K/AKT pathway plays a critical role in modulating paracellular claudin-5 expression and may be significant in the development of neurodegenerative disorders in which there is a loss of BBB integrity (Fig. 6).
Fig. 6.
Schematic representation of intracellular signaling pathways that mediate early and late biphasic changes in paracellular claudin-5 expression. This study suggests that the early reversible and late loss of paracellular claudin-5 expression in bEnd.3 endothelial cells is mediated through two different intracellular pathways. Pathway I is PI3K/AKT-dependent. Pharmacologic inhibition of PI3K with LY294002 (25 μM) or PI-828 (10 μM) attenuated AKT (Thr308) phosphorylation and prevented early reversible loss of paracellular claudin-5 expression. The early reversible change in paracellular claudin-5 morphology was also prevented by AKT inhibitors 10-DEBC (10 μM) or AKT1 (25 μM). Pathway II is PI3K-dependent/AKT-independent. Pharmacologic inhibition of PI3K with LY294002 (25 μM) or PI-828 (10 μM) also prevented late loss of paracellular claudin-5 expression. However, this pathway is independent of AKT activation because AKT inhibitors 10-DEBC (10 μM) and AKT1 (25 μM) did not prevent the loss of paracellular claudin-5 expression seen at 1, 2, and 3 days.
A key finding of this study is the induced early and late biphasic loss of claudin-5 paracellular expression. 3-Chloropropanediol treatment induced the sharply defined paracellular claudin-5 expression to appear diffuse after 90, 105, and 120 minutes, although still localized around the cell membrane. After 120 minutes, the claudin-5 immunoreactivity appeared sharply defined again and morphology was comparable to control samples. Western blot analysis showed no change in total claudin-5 expression over this time course. We extended the time course out to 1, 2, or 3 days of 3-chloropropanediol exposure, and claudin-5 expression was again observed to be lost from paracellular domains and was accompanied by a loss of total claudin-5 protein expression. This early and late response in paracellular tight junction protein expression is consistent with previous in vivo and in vitro studies that report a similar biphasic loss of BBB integrity with increased permeability (Witt et al., 2008; Jiao et al., 2011). Jiao and colleagues (2011) used a focal cerebral ischemia model and showed a biphasic increase of BBB permeability, with an initial change at 3 hours followed by a second change at 72 hours after injury. In another study in which cerebral oxygenation, but not total cerebral blood flow, was reduced, a similar biphasic change in paracellular permeability was seen at 10 minutes after hypoxic conditions and again later at 6–18 hours (Witt et al., 2008). In vitro, generation of reactive oxygen species, notably superoxide, induced a rapid increase in endothelial permeability, and modified claudin-5 expression at the cellular junctions (Schreibelt et al., 2007). In these in vivo and in vitro models, there are differences in the timing of the biphasic response. This is probably due to differences in experimental induction of BBB disruption. However, we speculate that the intracellular signaling pathway responsible for regulation of paracellular expression of tight junction proteins and BBB integrity is common in these models.
In this study, we focused on the role of the PI3K/AKT pathway in order to understand the signaling mechanisms responsible for induction of the reversible biphasic changes seen in claudin-5 expression. This signaling pathway is activated by a number of receptors, including integrin, cytokine, G protein–coupled receptors, and intracellular signaling molecules (Vivanco and Sawyers, 2002; West et al., 2002; Paez and Sellers, 2003; Murillo et al., 2004; Hemmings and Restuccia, 2012). The PI3K/AKT pathway is thought to be a survival pathway because it plays a role in cellular angiogenesis, protein synthesis, metabolism, and proliferation (Hemmings and Restuccia, 2012). Despite the central importance of the PI3K/AKT pathway in cell homeostasis, disruption, such as overphosphorylation of AKT, can induce detrimental effects on cell health (Khan et al., 2012). We show that this pathway plays a central role in 3-chloropropanediol–induced biphasic early (90, 105, and 120 minutes) and late (1, 2, and 3 days) loss of paracellular claudin-5 expression. Western blot analysis showed that within 30 minutes of 3-chloropropanediol administration, there was rapid transitory increased p85-PI3K expression. This increase in p85-PI3K protein expression is unlikely to be due to de novo synthesis of new protein because of the acute nature of the study, but rather a transitory change in PI3K conformation upon activation that favors p85-PI3K antibody binding. Recent structural studies have shown interactions between PI3K activity and conformation that may in part be induced by phosphorylation of two serine residues (Ser361 and Ser652) on the p85α subunit (Lee et al., 2011). In addition, the use of selective cell-permeable PI3K inhibitors, LY294002 and PI-828, prevented both the early and late changes seen in bEnd.3 cells. However, we propose that after increased p85-PI3K expression, the early and late changes in claudin-5 morphology are the result of two separate pathways mediated through different downstream targets. The early loss of paracellular claudin-5 appears to be mediated through a PI3K/AKT-dependent pathway. We show a rapid increase in AKT (Thr308), but not AKT (Ser473), phosphorylation and that inhibition of AKT with 10-DEBC or AKT1 attenuated these early changes in claudin-5 morphology. However, despite inhibition of AKT with 10-DEBC or AKT1, the late (1, 2, and 3 days) loss of paracellular and total claudin-5 expression was still observed. Thus, these results suggest that the late changes in claudin-5 expression are mediated by a PI3K-dependent/AKT-independent pathway (Fig. 6). We are currently studying components of this pathway.
Several factors have been identified that regulate BBB permeability, including tight junction protein phosphorylation status (González-Mariscal et al., 2008). Other studies have shown PKC isozymes to regulate tight junction protein phosphorylation status, subcellular localization, assembly, and maintenance of paracellular integrity in endothelial cells of the BBB (Andreeva et al., 2001; Avila-Flores et al., 2001; Angelow et al., 2005; González-Mariscal et al., 2008). We previously showed in a hypoxic study that increased phosphorylation of PKC-θ and PKC-ζ were associated with BBB integrity loss (Willis et al., 2010). Furthermore, a nonisoform-specific PKC inhibitor, chelerythrine chloride, reduced the degree of hypoxic-induced sucrose permeability and leak of dextran tracers (Fleegal et al., 2005; Willis et al., 2010). Other studies have shown PKC-α, PKC-β, and PKC-δ isoform expression to increase with enhanced vascular permeability after cerebral ischemia or inflammatory stimulation (Yuan, 2002; Jiao et al., 2011). In vitro studies have also shown that PKC isozymes including PKC-ζ, PKC-βII, and PKC-δ have a role in endothelial integrity in bEnd.3 cells as well as in Caco-2 and MDCK cultures (Dodane and Kachar, 1996; Kim et al., 2010). In this study, we speculate that the transitory AKT (Thr308) phosphorylation triggers a signaling cascade that culminates in specific PKC isoform(s) activation. This induces claudin-5 phosphorylation resulting in translocation from tight junction domains to cytosolic pools or nonparacellular junction membrane domains at 90, 105, and 120 minutes while total claudin-5 expression levels remain the same.
The proposed PI3K-dependent/AKT-independent late loss of claudin-5 may be explained by protein degradation rather than a transitory change in phosphorylation state, because we show a significant decrease in total claudin-5 expression at 1, 2, and 3 days (Fig. 1H). This loss of claudin-5 expression may be mediated through a number of pathways, including 1) protein degradation by the ubiquitin–proteasome system because claudin-5 can be polyubiquitinated, triggering the proteasome-dependent degradation, 2) ubiquitin-independent breakdown, 3) induction of oxidative stress through production of heme oxygenase-1 (HSP32), or 4) indirect lysosomal-dependent breakdown (Mandel et al., 2012a,b). Claudin-5 expresses a relatively short half-life and undergoes rapid protein turnover by the ubiquitin–proteasome system (Mandel et al., 2012a,b). We propose that after 3-chloropropanediol administration, there is induction of oxidative stress and/or activation of the ubiquitin proteasome degradation pathway thus inducing loss of claudin-5 at 1, 2, and 3 days. Induction of this pathway is independent of AKT activation in our system because inhibition of AKT by 10-DEBC (25 μM) or AKT1 (25 μM) was not able to prevent this delayed loss. However, upstream inhibition of PI3K by LY294002 or PI-828 was able to preserve paracellular claudin-5 expression at 1, 2, and 3 days.
In summary, this study provides a mechanistic approach for understanding intracellular signaling cascades responsible for regulating paracellular tight junction protein expression and BBB integrity. We show an early and late biphasic loss of paracellular claudin-5 expression that is modulated through the PI3K/AKT pathway. Inhibition of PI3K preserved both the early and late loss of claudin-5 expression, whereas inhibition of AKT only prevented the early reversible loss of paracellular claudin-5 expression. We thereby propose that two independent pathways triggered by PI3K are responsible for the early and late loss of paracellular claudin-5 expression seen in this study (Fig. 6). Pathway I is PI3K/AKT (Thr308) phosphorylation-dependent and induces the early reversible loss of claudin-5 from paracellular domains. This pathway is sensitive to inhibition of PI3K or AKT because paracellular claudin-5 expression can be preserved. Pathway II is PI3K activation-dependent, but is AKT-independent and results in the delayed loss of claudin-5. Inhibition of PI3K can preserve claudin-5 expression, but inhibition downstream at AKT has no effect because claudin-5 is still lost. These results have important implications for the development of future novel therapeutics and preventative disease measures. By understanding the intracellular signaling pathways involved in BBB breakdown, we can develop agents that modify these pathways.
Abbreviations
- 3-chloropropanediol
(S)-(+)-3-chloro-1,2-propanediol
- 10-DEBC
10-[4′-(N,N-diethylamino)butyl]-2-chlorophenoxazine hydrochloride
- AKT1
1L-6-hydroxymethyl-chiroinositol-2(R)-2-O-methyl-3-O-octadecyl-sn-glycerocarbonate
- BBB
blood-brain barrier
- LY294002
2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one hydrochloride
- PBS
phosphate-buffered saline
- PI-828
2-(4-morpholinyl)-8-(4-aminopheny)l-4H-1-benzopyran-4-one
- PI3K
phosphoinositide-3-kinase
- PKC
protein kinase C
- PVDF
polyvinylidene difluoride
Authorship Contributions
Participated in research design: Camire, Beaulac, Brule, Willis.
Conducted experiments: Camire, Beaulac, Brule, McGregor, Lauria.
Performed data analysis: Camire, Beaulac, Brule, McGregor, Lauria, Willis.
Wrote or contributed to the writing of the manuscript: Camire, Beaulac, Brule, Willis.
Footnotes
This research was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant P20-GM103643]; the American Heart Association [Grant SDG2170105]; and Westbrook College of Health Professions, University of New England [Research Fellowship].
References
- Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ. (2010) Structure and function of the blood-brain barrier. Neurobiol Dis 37:13–25. [DOI] [PubMed] [Google Scholar]
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. (1997) Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol 7:261–269. [DOI] [PubMed] [Google Scholar]
- Andreeva AY, Krause E, Müller EC, Blasig IE, Utepbergenov DI. (2001) Protein kinase C regulates the phosphorylation and cellular localization of occludin. J Biol Chem 276:38480–38486. [DOI] [PubMed] [Google Scholar]
- Angelow S, Zeni P, Höhn B, Galla HJ. (2005) Phorbol ester induced short- and long-term permeabilization of the blood-CSF barrier in vitro. Brain Res 1063:168–179. [DOI] [PubMed] [Google Scholar]
- Avila-Flores A, Rendón-Huerta E, Moreno J, Islas S, Betanzos A, Robles-Flores M, González-Mariscal L. (2001) Tight-junction protein zonula occludens 2 is a target of phosphorylation by protein kinase C. Biochem J 360:295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Erickson MA. (2010) The blood-brain barrier and immune function and dysfunction. Neurobiol Dis 37:26–32. [DOI] [PubMed] [Google Scholar]
- Brown RC, Morris AP, O’Neil RG. (2007) Tight junction protein expression and barrier properties of immortalized mouse brain microvessel endothelial cells. Brain Res 1130:17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Datta SR, Greenberg ME. (2001) Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol 11:297–305. [DOI] [PubMed] [Google Scholar]
- Cain RJ, Vanhaesebroeck B, Ridley AJ. (2010) The PI3K p110alpha isoform regulates endothelial adherens junctions via Pyk2 and Rac1. J Cell Biol 188:863–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba H, Osanai M, Murata M, Kojima T, Sawada N. (2008) Transmembrane proteins of tight junctions. Biochim Biophys Acta 1778:588–600. [DOI] [PubMed] [Google Scholar]
- Deli MA, Abrahám CS, Kataoka Y, Niwa M. (2005) Permeability studies on in vitro blood-brain barrier models: physiology, pathology, and pharmacology. Cell Mol Neurobiol 25:59–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodane V, Kachar B. (1996) Identification of isoforms of G proteins and PKC that colocalize with tight junctions. J Membr Biol 149:199–209. [DOI] [PubMed] [Google Scholar]
- Fleegal MA, Hom S, Borg LK, Davis TP. (2005) Activation of PKC modulates blood-brain barrier endothelial cell permeability changes induced by hypoxia and posthypoxic reoxygenation. Am J Physiol Heart Circ Physiol 289:H2012–H2019. [DOI] [PubMed] [Google Scholar]
- González-Mariscal L, Tapia R, Chamorro D. (2008) Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta 1778:729–756. [DOI] [PubMed] [Google Scholar]
- Grossman RI, Gonzalez-Scarano F, Atlas SW, Galetta S, Silberberg DH. (1986) Multiple sclerosis: gadolinium enhancement in MR imaging. Radiology 161:721–725. [DOI] [PubMed] [Google Scholar]
- Hatashita S, Hoff JT. (1990) Brain edema and cerebrovascular permeability during cerebral ischemia in rats. Stroke 21:582–588. [DOI] [PubMed] [Google Scholar]
- Hemmings BA, Restuccia DF. (2012) PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol 4:a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao H, Wang Z, Liu Y, Wang P, Xue Y. (2011) Specific role of tight junction proteins claudin-5, occludin, and ZO-1 of the blood-brain barrier in a focal cerebral ischemic insult. J Mol Neurosci 44:130–139. [DOI] [PubMed] [Google Scholar]
- Khan N, Afaq F, Khusro FH, Mustafa Adhami V, Suh Y, Mukhtar H. (2012) Dual inhibition of phosphatidylinositol 3-kinase/Akt and mammalian target of rapamycin signaling in human nonsmall cell lung cancer cells by a dietary flavonoid fisetin. Int J Cancer 130:1695–1705 Int J Cancer 130: 1695–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YA, Park SL, Kim MY, Lee SH, Baik EJ, Moon CH, Jung YS. (2010) Role of PKCbetaII and PKCdelta in blood-brain barrier permeability during aglycemic hypoxia. Neurosci Lett 468:254–258. [DOI] [PubMed] [Google Scholar]
- Lee JY, Chiu YH, Asara J, Cantley LC. (2011) Inhibition of PI3K binding to activators by serine phosphorylation of PI3K regulatory subunit p85α Src homology-2 domains. Proc Natl Acad Sci USA 108:14157–14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo EH, Pan Y, Matsumoto K, Kowall NW. (1994) Blood-brain barrier disruption in experimental focal ischemia: comparison between in vivo MRI and immunocytochemistry. Magn Reson Imaging 12:403–411. [DOI] [PubMed] [Google Scholar]
- Mandel I, Paperna T, Glass-Marmor L, Volkowich A, Badarny S, Schwartz I, Vardi P, Koren I, Miller A. (2012a) Tight junction proteins expression and modulation in immune cells and multiple sclerosis. J Cell Mol Med 16:765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel I, Paperna T, Volkowich A, Merhav M, Glass-Marmor L, Miller A. (2012b) The ubiquitin-proteasome pathway regulates claudin 5 degradation. J Cell Biochem 113:2415–2423. [DOI] [PubMed] [Google Scholar]
- Mori O, Haseba T, Kameyama K, Shimizu H, Kudoh M, Ohaki O, Arai Y, Yamazaki M, Asano G. (2000) Histological distribution of class III alcohol dehydrogenase in human brain. Brain Res 852:186–190. [DOI] [PubMed] [Google Scholar]
- Murillo CA, Rychahou PG, Evers BM. (2004) Inhibition of α5 integrin decreases PI3K activation and cell adhesion of human colon cancers. Surgery 136:143–149. [DOI] [PubMed] [Google Scholar]
- Omidi Y, Campbell L, Barar J, Connell D, Akhtar S, Gumbleton M. (2003) Evaluation of the immortalised mouse brain capillary endothelial cell line, b.End3, as an in vitro blood-brain barrier model for drug uptake and transport studies. Brain Res 990:95–112. [DOI] [PubMed] [Google Scholar]
- Paez J, Sellers WR. (2003) PI3K/PTEN/AKT pathway. A critical mediator of oncogenic signaling. Cancer Treat Res 115:145–167. [PubMed] [Google Scholar]
- Schreibelt G, Kooij G, Reijerkerk A, van Doorn R, Gringhuis SI, van der Pol S, Weksler BB, Romero IA, Couraud P-O, Piontek J, et al. (2007) Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J 21:3666–3676. [DOI] [PubMed] [Google Scholar]
- Skamarauskas J, Carter W, Fowler M, Madjd A, Lister T, Mavroudis G, Ray DE. (2007) The selective neurotoxicity produced by 3-chloropropanediol in the rat is not a result of energy deprivation. Toxicology 232:268–276. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M, Itoh M. (2001) Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2:285–293. [DOI] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL. (2002) The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer 2:489–501. [DOI] [PubMed] [Google Scholar]
- West KA, Castillo SS, Dennis PA. (2002) Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist Updat 5:234–248. [DOI] [PubMed] [Google Scholar]
- Willis CL, Brooks TA, Davis TP. (2008) Chronic inflammatory pain and the neurovascular unit: a central role for glia in maintaining BBB integrity? Curr Pharm Des 14:1625–1643. [DOI] [PubMed] [Google Scholar]
- Willis CL, Camire RB, Brule SA, Ray DE. (2013) Partial recovery of the damaged rat blood-brain barrier is mediated by adherens junction complexes, extracellular matrix remodeling and macrophage infiltration following focal astrocyte loss. Neuroscience 250:773–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis CL, Leach L, Clarke GJ, Nolan CC, Ray DE. (2004a) Reversible disruption of tight junction complexes in the rat blood-brain barrier, following transitory focal astrocyte loss. Glia 48:1–13. [DOI] [PubMed] [Google Scholar]
- Willis CL, Meske DS, Davis TP. (2010) Protein kinase C activation modulates reversible increase in cortical blood-brain barrier permeability and tight junction protein expression during hypoxia and posthypoxic reoxygenation. J Cereb Blood Flow Metab 30:1847–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis CL, Nolan CC, Reith SN, Lister T, Prior MJ, Guerin CJ, Mavroudis G, Ray DE. (2004b) Focal astrocyte loss is followed by microvascular damage, with subsequent repair of the blood-brain barrier in the apparent absence of direct astrocytic contact. Glia 45:325–337. [DOI] [PubMed] [Google Scholar]
- Witt KA, Mark KS, Sandoval KE, Davis TP. (2008) Reoxygenation stress on blood-brain barrier paracellular permeability and edema in the rat. Microvasc Res 75:91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan SY. (2002) Protein kinase signaling in the modulation of microvascular permeability. Vascul Pharmacol 39:213–223. [DOI] [PubMed] [Google Scholar]