Abstract
The role of intestinal human pregnane X receptor (PXR) in colon cancer was determined through investigation of the chemopreventive role of rifaximin, a specific agonist of intestinal human PXR, toward azoxymethane (AOM)/dextran sulfate sodium (DSS)–induced colon cancer. Rifaximin treatment significantly decreased the number of colon tumors induced by AOM/DSS treatment in PXR-humanized mice, but not wild-type or Pxr-null mice. Additionally, rifaximin treatment markedly increased the survival rate of PXR-humanized mice, but not wild-type or Pxr-null mice. These data indicated a human PXR–dependent therapeutic chemoprevention of rifaximin toward AOM/DSS-induced colon cancer. Nuclear factor κ-light-chain-enhancer of activated B cells–mediated inflammatory signaling was upregulated in AOM/DSS-treated mice, and inhibited by rifaximin in PXR-humanized mice. Cell proliferation and apoptosis were also modulated by rifaximin treatment in the AOM/DSS model. In vitro cell-based assays further revealed that rifaximin regulated cell apoptosis and cell cycle in a human PXR-dependent manner. These results suggested that specific activation of intestinal human PXR exhibited a chemopreventive role toward AOM/DSS-induced colon cancer by mediating anti-inflammation, antiproliferation, and proapoptotic events.
Introduction
Colorectal cancer is one of the most common forms of fatal cancer in the world; however, the underlying molecular pathogenesis and effective strategies for prevention and treatment of spontaneous colorectal cancer are not fully understood (le Clercq and Sanduleanu, 2014). Chronic inflammation is a known risk factor for carcinogenesis, and accumulated data indicate that up to 15% of human cancer incidence is associated with inflammation (Drexler and Yazdi, 2013). Inflammatory bowel diseases, such as ulcerative colitis and Crohn's disease, dramatically increase the risk of colorectal cancer. Proinflammatory pathways and mediators including eicosanoids catalyzed by cyclooxygenases (COXs) or 5-lipoxygenase and cytokines/chemokines promote tumorigenesis. Anti-inflammatory strategies, such as inhibition of COXs by nonsteroidal anti-inflammatory drugs, are among the most promising approaches for the chemoprevention of colon cancer (Stolfi et al., 2013). In addition, the importance of inflammation is further highlighted by the dependence of tumor type and progression on activation of nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) (Mladenova et al., 2013). The NF-κB pathway activation observed during inflammatory bowel diseases may contribute to tumor formation by providing antiapoptotic survival signals to the colonic epithelial cells (Onizawa et al., 2009). Indeed, in mice harboring a mutation in intestinal inhibitor of κB kinase β that leads to suppression of the NF-κB pathway, colon tumorigenesis was significantly attenuated (Greten et al., 2004).
Rifaximin (Xifaxan; Salix Pharmaceuticals, Raleigh, NC), a nonsynthetic antibiotic that has low gastrointestinal absorption while retaining antibacterial activity within the intestine, was approved in 2004 for therapy of traveler’s diarrhea and in 2010 for therapy of hepatic encephalopathy due to its antibiotic-based inhibition of ammonia-producing enteric bacteria that reduces circulating gut-derived ammonia in patients with cirrhosis (Mullen et al., 2014). In addition, numerous clinical trials revealed that rifaximin has efficacy toward irritable bowel syndrome, presumably as a result of alteration of intestinal microbiota (Xu et al., 2014). Thus, rifaximin has therapeutic applications beyond its antibiotic activity, and is especially attractive due to its minimal systemic absorption and safety profile. A previous study indicated that rifaximin is the gut-specific agonist of human pregnane X receptor (PXR, NR1I2) (Ma et al., 2007) and is also a potential drug candidate for acute colitis through its anti-inflammatory activity, due in part to inhibition of the NF-κB signaling cascade through specific activation of intestinal PXR (Cheng et al., 2010). Therefore, the chemopreventive role of rifaximin toward colitis-associated colon cancer was examined in the present study.
The experimental colitis-mediated mouse colon cancer model was created through the injection of azoxymethane (AOM), a mutagenic agent, and dextran sulfate sodium (DSS), a proinflammatory chemical (Kohno et al., 2005). This model was used in the present study to determine the role of specific activation of intestinal human PXR in the suppression of AOM/DSS-induced colon cancer. The therapeutic role of rifaximin toward AOM/DSS-induced colon cancer in PXR-humanized, wild-type, and Pxr-null mice was evaluated. Furthermore, the effect of rifaximin on proinflammatory cytokines and apoptosis during development of colitis and colon carcinogenesis was also investigated. The results suggested that specific activation of intestinal PXR by rifaximin might exert a chemopreventive role in AOM/DSS-induced colon cancer through inhibition of the NF-κB pathway and induction of cellular apoptosis.
Materials and Methods
Animals and Chemicals.
Male PXR-humanized, wild-type, and Pxr-null mice were housed in temperature- and light-controlled rooms and were given water and pelleted chow ad libitum. All animal experiments were carried out in accordance with the Institute of Laboratory Animal Resources guidelines and approved by the National Cancer Institute Animal Care and Use Committee. Rifaximin (Xifaxan) was provided by Salix Pharmaceuticals, Inc. (Morrisville, NC.). AOM was purchased from Sigma-Aldrich (St. Louis, MO) and DSS (molecular mass 36,000–50,000 Da) was from MP Biochemicals (Solon, OH).
AOM/DSS-Induced Colon Cancer Model and Rifaximin Treatment.
Before starting the experiments, mice were acclimatized for 1 week. Two- to three-month-old PXR-humanized, wild-type, and Pxr-null male mice were divided into a control group, AOM/DSS group, and AOM/DSS+rifaximin group (n = 20 for each group). To induce colon cancer, PXR-humanized, wild-type, and Pxr-null mice were given an intraperitoneal injection with 10 mg/kg AOM in 0.1 ml of phosphate-buffered saline (PBS) followed by three cycles of 2% DSS in drinking water for 1 week and normal drinking water for 2 weeks. The AOM/DSS+rifaximin group was administered rifaximin in the 10 mg/kg rifaximin–containing AIN-93G diet (Dyets Inc., Bethlehem, PA), whereas mice in the AOM/DSS group and control group received the AIN-93G diet without rifaximin. This dose of rifaximin is equivalent to oral administration of 1 mg/kg per day for each mouse. All mice in the AOM/DSS+rifaximin group received rifaximin-containing diet for 1 week before the AOM/DSS treatment until the end of the experiment. Body weights and food and water consumption were monitored once per week throughout the experiment. Clinical assessment of all DSS/AOM-treated animals for body weight, stool consistency, rectal bleeding, and general appearance was performed weekly. At day 180 of the experiment, all mice were killed, and whole colorectal tissues were collected for biochemical and pathologic analyses.
Macroscopic and Histopathologic Evaluation Analysis.
After killing the mice, the colons were excised from the ileocecal junction to the anal verge and flushed with PBS. Blood was collected for the preparation of serum. Colons were weighed and colon length (from the colocecal junction to the rectum) was measured for assessment of morphologic changes. The colon was opened longitudinally. Gross examination was performed to measure the pattern of tumor development, including quantity, size, and the location of each tumor within the large bowel. The tumor incidence was defined as the number of mice with tumors/total mice in the group. For histologic analysis, the colon tissues were fixed in 10% formalin for 24 hours, followed by paraffin embedding. The incidence of tumors with a diameter >4 mm was also assessed because most large tumors with a diameter >4 mm that were examined contained carcinoma components.
Short-Term Antiproliferation Effect of Rifaximin.
To observe the short-term antiproliferation role of rifaximin, PXR-humanized (n = 9), wild-type (n = 9), and Pxr-null (n = 9) mice were given an intraperitoneal injection with 10 mg/kg AOM dissolved in 0.1 ml of PBS followed by one cycle of 2% DSS in drinking water for 1 week. Among them, four mice were used for bromodeoxyuridine (BrdU) staining experiments. Mice were injected with 200 μmol/kg body weight of BrdU. After 1 hour, the mice were killed and the colons taken for BrdU staining as detailed in a previous study (Wang et al., 2008). The BrdU labeling index was calculated using the ratio of BrdU staining–positive cell number versus the total cell number. The colons taken from the other five mice were subjected to quantitative polymerase chain reaction (qPCR) analysis for specific mRNAs.
qPCR Analysis.
Total RNA was extracted from colon tissues of each mouse using TRIzol reagent (Invitrogen, Carlsbad, CA), and qPCR was performed using cDNA generated from 1 µg of total RNA with SuperScript II Reverse Transcriptase (Invitrogen). Primers for qPCR were designed using the Primer Express software (Applied Biosystems, Foster City, CA); sequences are available upon request. qPCR reactions were carried out using SYBR Green PCR master mix (SuperArray, Frederick, MD) using an ABI Prism 7900HT Sequence Detection System (Applied Biosystems). Values were quantitated using the comparative cycle threshold method, and results were normalized to mouse β-actin.
Cell Apoptosis Assays by Flow Cytometry.
Human colonic epithelial cells, HT-29, were grown at 37°C with 5% CO2 in Dulbecco’s modified Eagle’s medium (Invitrogen, Grand Island, NY) supplemented with 10% fetal bovine serum (Gemini Bio-Products, Woodland, CA) and 1% penicillin-streptomycin (Invitrogen). HT-29 cells were seeded at a density of 5 × 104 cells/well in 24-well plates. Expression vectors for human PXR and retinoid X receptor α (RXRα) constructs were transfected into cells using Fugene transfection reagent (Roche, Indianapolis, IN). Twenty-four hours post-transfection, the cells were incubated with dimethylsulfoxide (vehicle) or rifaximin for an additional 24 hours, followed by cell apoptosis analysis as follows. Cells were treated with fluorescein isothiocyanate–conjugated annexin V in conjunction with propidium iodide (PI), which stains necrotic cells to identify apoptotic cells measured by flow cytometry (BD Biosciences, San Jose, CA). Cells were differentiated in early apoptosis (annexin V+, PI−) from those in late apoptosis (annexin V+, PI+) stage. To confirm expression of the human PXR in the transfected HT-29 cells, nuclear extracts of cells 24 hours after transfection were prepared using the NE-PER kit (Pierce/Thermo Scientific, Rockford, IL). Nuclear extracts (20 μg) were loaded on a SDS–polyacrylamide gel, and western blotting was carried out using a Human PXR Common/NR112 monoclonal antibody (mAb) (Clone H4417; R&D Systems, Minneapolis, MN) and Histone H3 (D2B12) XR rabbit mAb (Cell Signaling Technology, Danvers, MA) diluted 1:1000 in 5% skim milk. Nuclear extracts of liver tissues from PXR-humanized and Pxr-null mice were also loaded as a positive and negative control, respectively. The human PXR mAb recognizes human PXR1 and 2. The immunogen was amino acids 1–40 of human PXR. Primers used for qPCR are shown in the Supplemental Fig. 5 legend.
Statistics.
Experimental values are expressed as the mean ± S.D. Statistical analysis was performed with two-tailed Student’s t tests, and P values <0.05 were considered to be statistically significant.
Results
Rifaximin Prevents AOM/DSS-Induced Colon Carcinogenesis in a Human PXR–Dependent Manner.
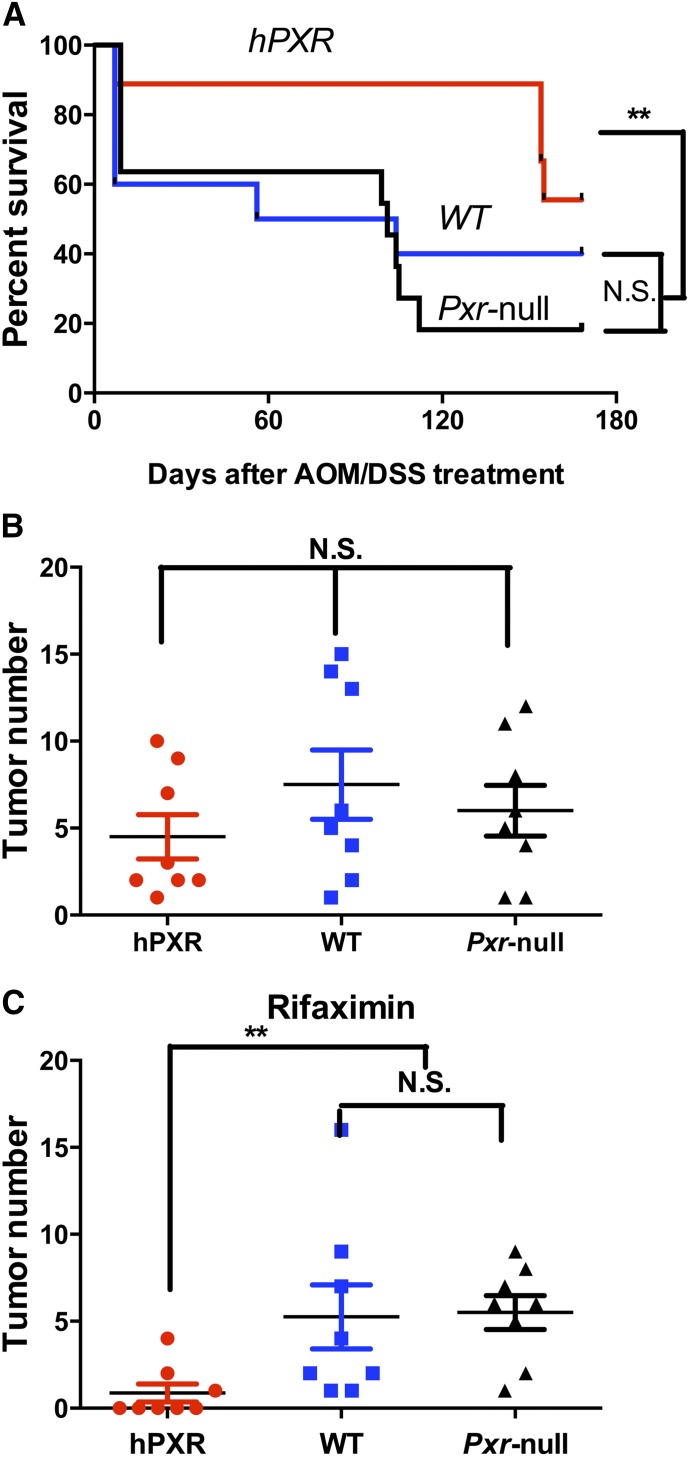
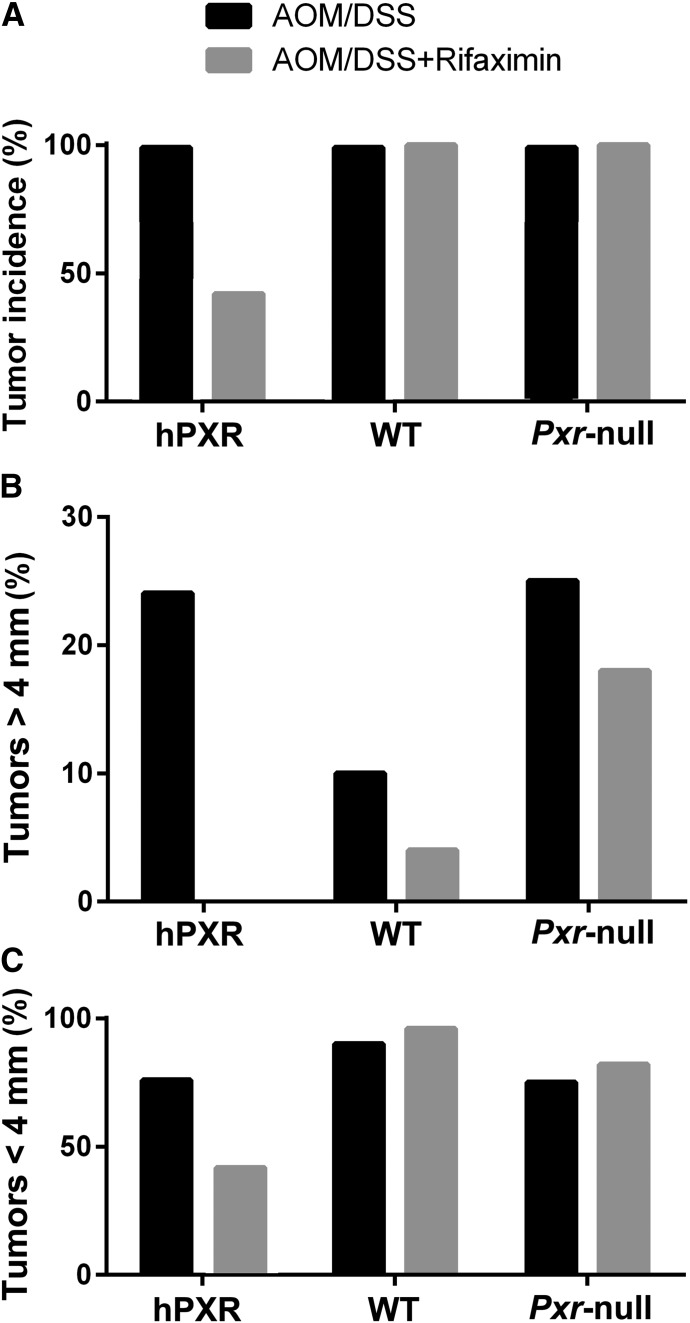
PXR-humanized mice with AOM/DSS+rifaximin treatment had the highest survival rate among PXR-humanized, wild-type, and Pxr-null mice treated with AOM/DSS+rifaximin (Fig. 1A). However, there were no significant differences in survival rates among the three mouse lines treated with AOM/DSS only (Supplemental Fig. 1). The AOM/DSS-induced colon cancer produced similar total tumor numbers per colon in PXR-humanized, wild-type, and Pxr-null mice fed a control diet (Fig. 1B). However, rifaximin treatment led to reduced tumor numbers only in PXR-humanized mice in comparison with wild-type and Pxr-null mice (Fig. 1C). Total tumor incidence in the PXR-humanized mice treated with AOM/DSS+rifaximin was 42% compared with 100% in the AOM/DSS group, and 100% in the wild-type and Pxr-null mice treated with AOM/DSS and AOM/DSS+rifaximin (Fig. 2A). Among the tumors found in the AOM/DSS group in the PXR-humanized mice, 24% had diameters >4 mm (Fig. 2B) with histologic characteristics of adenocarcinomas (Supplemental Fig. 2). All of the tumors in the PXR-humanized mice treated with AOM/DSS+rifaximin were smaller, with diameters <4 mm (Fig. 2C). Wild-type and Pxr-null mice treated with AOM/DSS had 10 and 25% >4-mm diameter tumors, respectively, and when treated with AOM/DSS+rifaximin, had 4 and 18% with >4-mm diameter tumors, respectively (Fig. 2B). In a second cohort of PXR-humanized mice treated with AOM/DSS+rifaximin, there was a 15% tumor incidence compared with 100% in the AOM/DSS-treated PXR-humanized group, and 100% for the wild-type and Pxr-null mice treated with AOM/DSS with or without rifaximin (Supplemental Fig. 3A). In the PXR-humanized mice treated with AOM/DSS, 38% of the tumors were <4 mm in diameter (Supplemental Fig. 3B) whereas all of the tumors in the PXR-humanized mice treated with AOM/DSS+rifaximin were <4 mm in diameter (Supplemental Fig. 3C). Interestingly, in this cohort, the wild-type mice treated with AOM/DSS+rifaximin had mostly (75%) >4-mm diameter tumors with AOM/DSS+rifaximin treatment (Supplemental Fig. 3B). The reason for this difference between the first cohort of mice (Fig. 2) is not known. Tumor incidence and percentage of small and large tumors were similar in both cohorts of the Pxr-null mice treated with AOM/DSS with and without rifaximin (Fig. 2; Supplemental Fig. 3). Both cohorts revealed that rifaximin inhibited AOM/DSS-induced tumorigenesis—notably, the production of large, >4-mm diameter tumors.
Fig. 1.
Treatment with rifaximin prevents AOM/DSS-induced colon carcinogenesis in PXR-humanized mice. (A) Survival rate of PXR-humanized (hPXR), wild-type (WT), and Pxr-null mice with rifaximin treatment in the AOM/DSS-induced colon carcinogenesis model (n = 11 per group). **P < 0.01. N.S., not significant. (B) Tumor numbers in hPXR, WT, and Pxr-null mice without rifaximin treatment in AOM/DSS-induced colon carcinogenesis. (C) Tumor number in hPXR, WT, and Pxr-null mice with rifaximin treatment in AOM/DSS-induced colon cancer. **P < 0.01.
Fig. 2.
Tumor incidence in cohort 1 of PXR-humanized (hPXR), wild-type (WT), and Pxr-null mice with or without rifaximin treatment in AOM/DSS-induced colon carcinogenesis (n = 10 per group). (A) Total tumor incidence in hPXR, WT, and Pxr-null mice with or without rifaximin treatment in AOM/DSS-induced colon cancer. (B) Percentage of tumors with diameter >4 mm in hPXR, WT, and Pxr-null mice with or without rifaximin treatment. (C) Percentage of tumors with diameter <4 mm in hPXR, WT, and Pxr-null mice with or without rifaximin treatment.
Rifaximin Inhibits the NF-κB Pathway via Human PXR Activation in Colitis-Associated Colorectal Carcinogenesis.
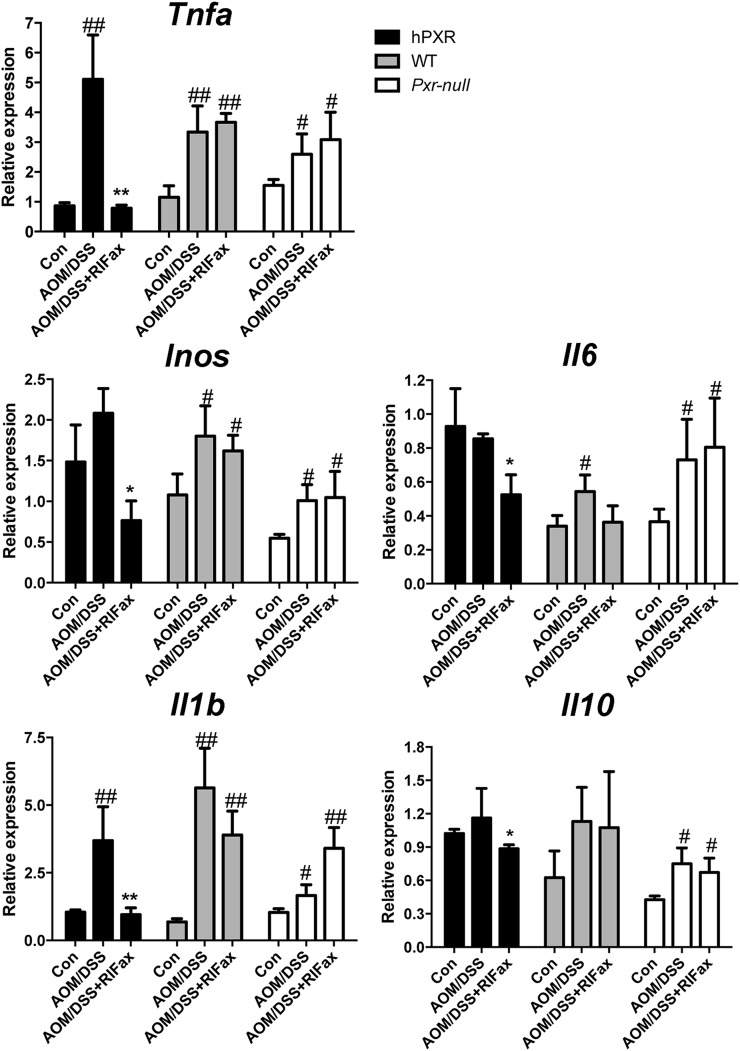
To investigate the effects of rifaximin-enriched diet on the expression of mRNAs encoding proinflammatory cytokines, qPCR was used to quantify mRNAs in tumor-adjacent tissue of the colon. Expression of tumor necrosis factor α (Tnfa) and interleukin 1β (Il-1b) mRNAs significantly increased after AOM/DSS treatment (P < 0.01); rifaximin treatment suppressed the increased expression induced by AOM/DSS in PXR-humanized mice (Fig. 3). No suppression of rifaximin toward the AOM/DSS-induced elevated expression of Tnfa and Il-1b mRNAs was found in wild-type or Pxr-null mice. In PXR-humanized mice, compared with the AOM/DSS group, expression of inducible nitric oxide synthase, Il-6, and Il-10 mRNAs was significantly reduced (P < 0.05) in the DSS/AOM+rifaximin group. However, this effect was not observed for wild-type or Pxr-null mice (Fig. 3). These results indicated that the NF-κB signaling pathway was induced in the AOM/DSS-induced colon cancer model in PXR-humanized, wild-type, and Pxr-null mice, and that the NF-κB pathway was suppressed only in PXR-humanized mice upon rifaximin treatment. Therefore, inhibition of the NF-κB signaling pathway might contribute to the prevention of AOM/DSS-induced colon cancer in PXR-humanized mice via human PXR activation by rifaximin.
Fig. 3.
Expression of human PXR and NF-κB target genes in tumor-adjacent tissue of colon in PXR-humanized (hPXR), wild-type (WT), and Pxr-null mice with or without rifaximin (RIFax) treatment in AOM/DSS-induced colon carcinogenesis [n = 5 for AOM/DSS and AOM/DSS+RIFax groups, n = 4 for the normal control (Con) group]. #P < 0.05, ##P < 0.01 compared with control (normal tissue); *P < 0.05, **P < 0.01 compared with AOM/DSS-treated group.
Effect of Rifaximin on Cell Proliferation and Apoptosis.
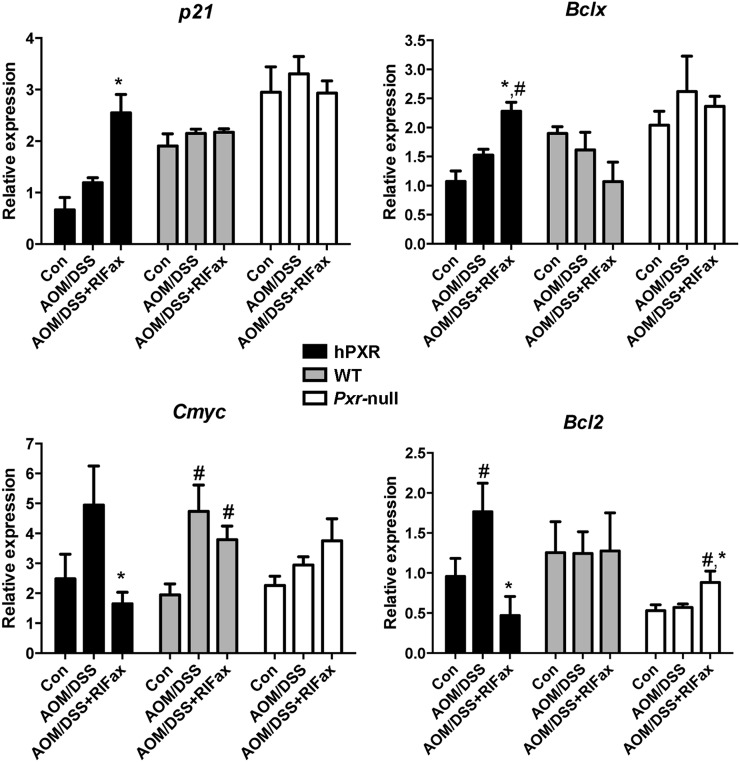
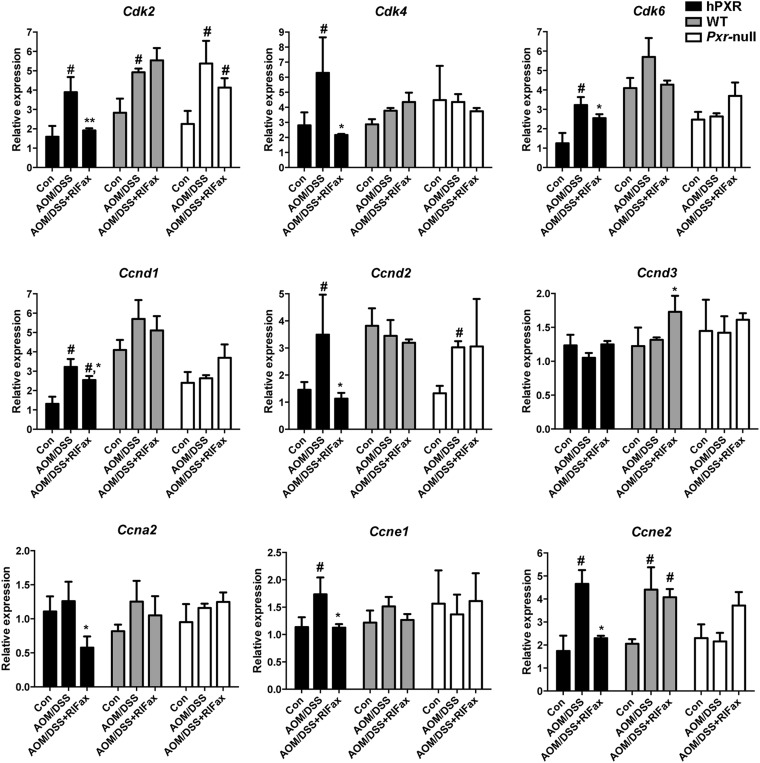
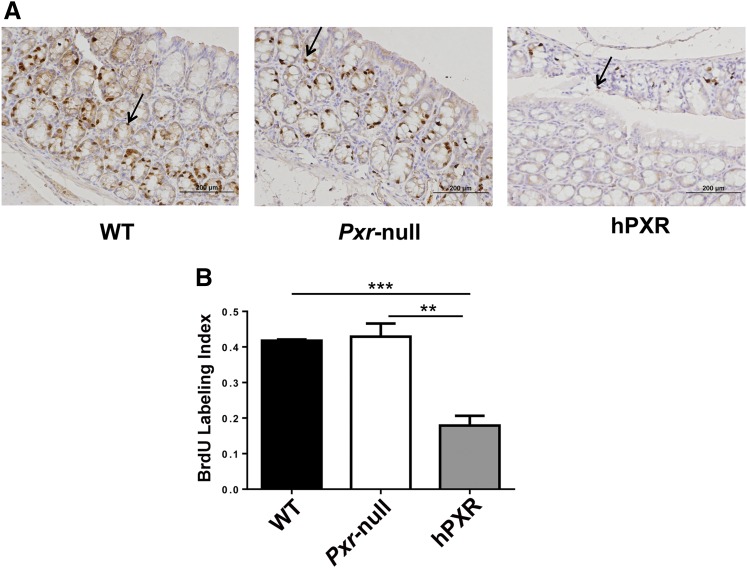
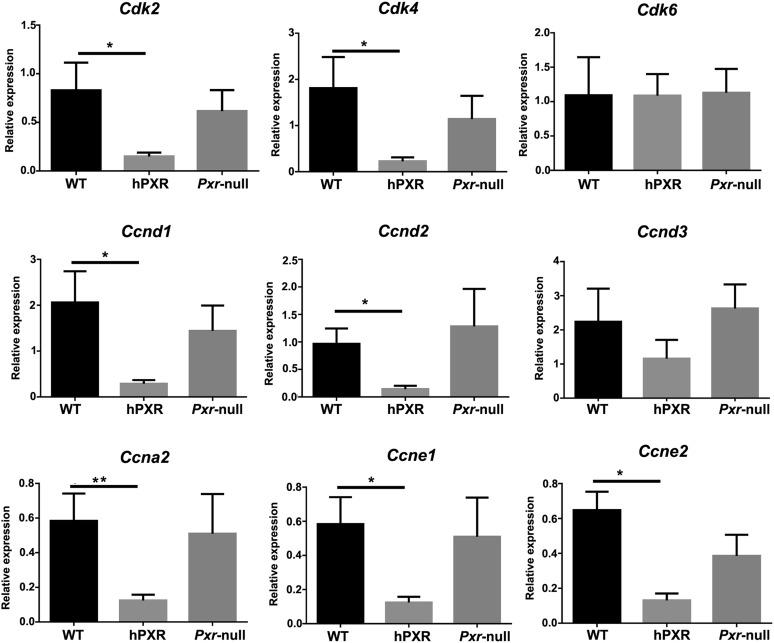
To explore the effect of rifaximin treatment on proliferation and apoptosis in the inflammation-related colon cancer mouse model, the expression of several target genes was investigated. The results revealed that p21 mRNA was significantly increased in the AOM/DSS+rifaximin group in comparison with the AOM/DSS group in PXR-humanized mice, but not in wild-type or Pxr-null mice (Fig. 4). The expression of mRNAs encoded by genes involved in apoptosis, Bcl-2 and Bclx, was significantly decreased and significantly increased, respectively, in the AOM/DSS+rifaximin group when compared with the AOM/DSS group in PXR-humanized mice, but not in wild-type or Pxr-null mice (Fig. 4). The expression of mRNA encoding C-myc, an oncoprotein expressed in proliferating and transformed cells, was also examined (Qu et al., 2014). Suppression of C-myc mRNA was only observed in AOM/DSS-treated PXR-humanized mice, and not in wild-type or Pxr-null mice upon rifaximin treatment, indicating that rifaximin efficiently decreased cell proliferation via human PXR (Fig. 4). Cyclin-dependent kinases are a family of protein kinases with important roles in regulating the cell cycle through binding to regulatory proteins called cyclins (Lim and Kaldis, 2013). Treatment with rifaximin prevented the increased expression of Cdk and Cyclin mRNAs induced by AOM/DSS in PXR-humanized mice, but not in wild-type or Pxr-null mice, including Cdk2, Cdk4, Cdk6, Ccnd1, Ccnd2, Ccna2, Ccne1, and Ccne2 (Fig. 5). To confirm the inhibitory action of rifaximin toward cell proliferation in PXR-humanized mice, BrdU staining was performed. Treatment of PXR-humanized mice with rifaximin significantly decreased incorporation of BrdU into colon epithelial cells induced by AOM/DSS treatment when compared with rifaximin-treated wild-type and Pxr-null mice (Fig. 6, A and B). Short-term treatment with rifaximin can activate human intestinal PXR, but not mouse intestinal PXR (Ma et al., 2007) (Supplemental Fig. 4), and the expression of the corresponding Cdk and Cyclin mRNAs was also significantly decreased in PXR-humanized mice in comparison with wild-type and Pxr-null mice (Fig. 7).
Fig. 4.
Expression of p21, Bcl-2, Bclx, and c-Myc mRNAs in tumor-adjacent tissue of colon in PXR-humanized (hPXR), wild-type (WT), and Pxr-null mice with or without rifaximin (RIFax) treatment in AOM/DSS-induced colon carcinogenesis [n = 5 for AOM/DSS and AOM/DSS-rifaximin group, n = 4 for normal control (Con) group]. #P < 0.05 compared with control (normal tissue); *P < 0.05 compared with AOM/DSS-treated group.
Fig. 5.
Expressions of mRNAs encoded by cyclin-dependent kinases and cyclin genes in tumor-adjacent tissue of colon in PXR-humanized (hPXR), wild-type (WT), and Pxr-null mice with or without rifaximin (RIFax) treatment in AOM/DSS-induced colon carcinogenesis mice [n = 5 for AOM/DSS and AOM/DSS+RIFax group, n = 4 for normal control (Con) group]. #P < 0.05, compared with control (normal tissue); *P < 0.05, **P < 0.01 compared with AOM/DSS-treated group.
Fig. 6.
Colon epithelial cells exhibited lower proliferation in PXR-humanized (hPXR) mice than wild-type (WT) and Pxr-null mice upon short-term treatment with rifaximin and AOM/DSS. (A) Representative BrdU staining in colon tissues obtained from hPXR, wild-type, and Pxr-null mice treated with rifaximin and AOM/DSS. Arrows indicate BrdU-positive nuclei. (B) BrdU labeling index comparison in hPXR, WT, Pxr-null mice treated with rifaximin and AOM/DSS. **P < 0.01; ***P < 0.001.
Fig. 7.
Gene expression of cyclin-dependent kinases and cyclin genes in colon tissues in PXR-humanized (hPXR), wild-type (WT), and Pxr-null mice with short-term treatment with AOM/DSS+rifaximin (n = 5 for each groups). *P < 0.05.
In Vitro Transfection of Human PXR and Rifaximin Treatment Increased Apoptosis and Decreased Proliferation in HT-29 Cells.
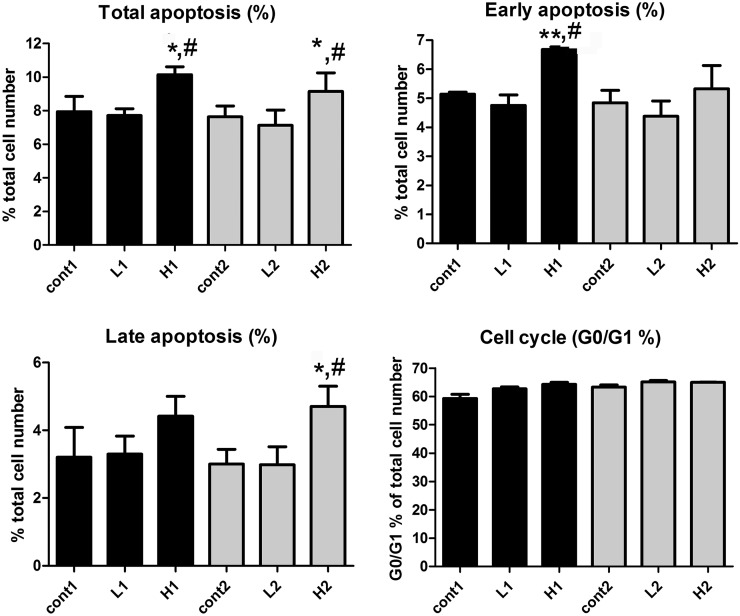
To analyze whether rifaximin can modulate apoptosis and proliferation in a human PXR–dependent manner, induction of apoptosis was evaluated in cells transfected with or without human PXR. Expression of human PXR and RXRα from the expression vector was demonstrated by analysis of human PXR mRNA and protein using qPCR and western blotting, and RXRα mRNA by qPCR (Supplemental Fig. 5). Cells with either RXRα or PXR/RXRα were simultaneously stained with PI and annexin V, and monitored by flow cytometry. Cells were treated with dimethylsulfoxide (control), low-dose rifaximin (1 μM) and high-dose rifaximin (100 μM). Cells transfected with the RXRα expression plasmid remained viable throughout the experiment, indicating that the treatment did not induce apoptosis without human PXR expression (Supplemental Fig. 6). A low dose of rifaximin produced negligible influence on apoptosis and proliferation of HT-29 cells transfected with the human PXR expression vector. However, a high dose of rifaximin significantly increased the numbers of total, early, and late apoptosis, with a slight, although not significant, elevation of cell numbers in the G0/G1 phase (Fig. 8).
Fig. 8.
Apoptosis and cell cycle in HT-29 cells transfected with human PXR with or without rifaximin treatment, including total apoptosis, early apoptosis, late apoptosis, and cell cycle. Human PXR and RXRα expression vectors were transfected to HT29 cells. These transfected cells were treated with dimethylsulfoxide or two different concentrations of rifaximin. Cont, cell treated with DMSO; H, cell treated with high dosage (100 μM) of rifaximin; L, cell treated with low dosage (1 μM) of rifaximin. Cells were treated in two batches (n = 3 per batch); therefore, cont1, L1, H1, cont2, L2, and H2 were labeled as listed. *P < 0.05, compared with the control group; **P < 0.01, compared with the control group; #P < 0.05, compared with the low dosage group.
Discussion
Inflammatory bowel disease leads to increased risk of developing colon cancer. The risk of colon cancer development is much higher in patients with inflammatory bowel disease than in the general population (Derikx et al., 2014). The tumor-promoting effect of inflammation is widely recognized to be related to the induction of gene mutations or epigenetic alterations, increased expression of factors involved in carcinogenesis (such as NF-κB and COX-2), production of reactive oxygen species, and inhibition of apoptosis and stimulation of cell proliferation (Savari et al., 2014). It is also widely accepted that chronic inflammation promotes carcinogenesis by inducing production of a variety of cytokines and chemokines that produce a localized inflammatory response by activating NF-κB, which is followed by increased expression of inducible nitric oxide synthase and proinflammatory cytokines, including tumor necrosis factorα and interleukin-6. These findings have important implications for the development of colon cancer chemoprevention strategies.
PXR regulates genes involved in the metabolism and disposition of various xenobiotics and endobiotics (Cheng et al., 2011). However, several studies reported potentially novel physiologic functions of PXR, including regulation of inflammatory pathways (Xie and Tian, 2006). By this mechanism, the role of PXR in the therapy of acute colitis and colon cancer was investigated. PXR suppressed the proliferation of colon tumor cells in culture by modulating the cell cycle at the G0/G1 cell phase by an unknown mechanism (Ouyang et al., 2010). Forced expression of human PXR in colon cancer cell lines inhibited proliferation and increased apoptosis, and the tumor size was significantly suppressed in a xenograft model (Xie et al., 2009; Ouyang et al., 2010). However, a contradictory role of PXR in colon cancer also exists. Treatment of a PXR-expressing colon cancer cell line, LS180, with the PXR agonist rifampicin inhibited apoptosis. Activation of PXR upregulated the antiapoptotic signaling molecules BAG3, BIRC2, and MCL-1, and downregulated the proapoptotic factors BAK1 and p53 (Zhou et al., 2008; Habano et al., 2011). Others showed that activation of PXR induced tumor aggressiveness in mice and humans in a fibroblast growth factor 19–dependent manner (Wang et al., 2011). Based on these studies, the role of PXR in colon cancer remains unclear, thus the need for further investigation.
Rifaximin is a gut-specific agonist of human PXR, and thus can be used to investigate the role of intestinal PXR in colon cancer. However, this can only be accomplished using PXR-humanized mice since rifaximin does not activate the mouse PXR. With this agonist, the role of human intestinal PXR in acute colitis therapy was uncovered (Cheng et al., 2010). The preventive and therapeutic role of rifaximin in experimental models of colitis was demonstrated in the PXR-humanized mouse model, where rifaximin not only prevented colitis before an inflammatory insult, as revealed by use of the DSS and 2,4,6-trinitrobenzene sulfonic acid–induced murine acute colitis models, but also decreased the symptoms after the onset of colitis (Cheng et al., 2010). These data indicated that treatment with rifaximin might have value in the long-term prevention of colon inflammation, and that activation of intestinal PXR might also be used to prevent inflammation-driven colon cancer. The present study focused on the efficient therapy of rifaximin toward ulcerative colitis–associated colorectal cancer using the AOM/DSS-induced colon cancer model. The results revealed decreased tumor numbers and total tumor incidence upon rifaximin treatment only in PXR-humanized mice, but not in wild-type or Pxr-null mice. The percentage of tumors with a diameter >4 mm was reduced only in PXR-humanized mice, and not in wild-type or Pxr-null mice. In addition, survival rates were markedly increased in PXR-humanized mice compared with wild-type and Pxr-null mice when rifaximin was administered concomitant with AOM/DSS. These results suggest that rifaximin prevents AOM/DSS-induced colon carcinogenesis in a human PXR–dependent manner. Furthermore, this study showed that the expression of cytokines significantly increased in response to DSS/AOM treatment; these are involved in the “inflammation–carcinoma sequence” (Okayasu, 2012). Treatment with rifaximin decreased cytokine expression, which was in line with data showing that oral feeding with rifaximin modulates and attenuates the colonic inflammation in experimental animal colitis (Cheng et al., 2010). These results suggested that rifaximin exerts anti-inflammatory and growth inhibitory effects on colon cancer development by reducing cytokine expression. These results further demonstrate that rifaximin has antitumor activity in PXR-humanized mice. This effect is correlated with decreased proinflammatory cytokines, attenuated colorectal inflammation, and a lack of NF-κB target gene expression in the colonic tissue at the end of the experimental model.
The effects of rifaximin on cell proliferation and apoptosis were also evaluated. Rifaximin treatment induced apoptosis and reduced proliferation activity in colon tissues of mice treated with AOM/DSS. The proliferation-inhibiting properties of rifaximin can also be demonstrated in the short-term AOM/DSS treatment model. Moreover, rifaximin displayed the ability to induce apoptosis and control proliferation in the G0/G1 phase in a sporadic colon carcinoma cell line transfected with a human PXR expression vector. These results revealed the potential effects of rifaximin toward colon cancer via regulating cell proliferation and apoptosis.
In summary, the ability of rifaximin to prevent the development of colon carcinogenesis was associated with attenuated colorectal inflammation and lower tumor incidence only in AOM/DSS-treated PXR-humanized mice, thus indicating a chemopreventive role for specific activation of intestinal human PXR toward colon cancer. The mechanism is due in part to anti-inflammation, induction of apoptosis, and inhibition of cell proliferation.
Supplementary Material
Acknowledgments
The authors thank Ronald W. Evans (Salk Institute) for the RXRα expression vector and Grace L. Guo (Rutgers University) for the human PXR expression vector. Rifaximin (Xifaxan) was provided by Salix Pharmaceuticals, Inc.
Abbreviations
- AOM
azoxymethane
- BrdU
bromodeoxyuridine
- COX
cyclooxygenase
- DSS
dextran sulfate sodium
- mAb
monoclonal antibody
- NF-κB
nuclear factor κ-light-chain-enhancer of activated B cells
- PBS
phosphate-buffered saline
- PI
propidium iodide
- PXR
pregnane X receptor
- qPCR
quantitative polymerase chain reaction
- RXRα
retinoid X receptor α
Authorship Contributions
Participated in research design: Cheng, Fang, Kimura, Gonzalez.
Conducted experiments: Cheng, Fang, Nagaoka, Okamoto, Qu, Tanaka.
Performed data analysis: Cheng, Fang, Nagaoka, Okamoto, Qu, Tanaka.
Wrote or contributed to the writing of the manuscript: Cheng, Fang, Tanaka, Kimura, Gonzalez.
Footnotes
This study was supported by the Intramural Research Program of the National Institutes of Health [National Cancer Institute].
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Cheng J, Ma X, Gonzalez FJ. (2011) Pregnane X receptor- and CYP3A4-humanized mouse models and their applications. Br J Pharmacol 163:461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Shah YM, Ma X, Pang X, Tanaka T, Kodama T, Krausz KW, Gonzalez FJ. (2010) Therapeutic role of rifaximin in inflammatory bowel disease: clinical implication of human pregnane X receptor activation. J Pharmacol Exp Ther 335:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derikx LA, Kievit W, Drenth JP, de Jong DJ, Ponsioen CY, Oldenburg B, van der Meulen-de Jong AE, Dijkstra G, Grubben MJ, van Laarhoven CJ, et al.; Dutch Initiative on Crohn and Colitis (2014) Prior colorectal neoplasia is associated with increased risk of ileoanal pouch neoplasia in patients with inflammatory bowel disease. Gastroenterology 146:119–128. [DOI] [PubMed]
- Drexler SK, Yazdi AS. (2013) Complex roles of inflammasomes in carcinogenesis. Cancer J 19:468–472. [DOI] [PubMed] [Google Scholar]
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. (2004) IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118:285–296. [DOI] [PubMed] [Google Scholar]
- Habano W, Gamo T, Terashima J, Sugai T, Otsuka K, Wakabayashi G, Ozawa S. (2011) Involvement of promoter methylation in the regulation of Pregnane X receptor in colon cancer cells. BMC Cancer 11:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno H, Suzuki R, Sugie S, Tanaka T. (2005) Beta-Catenin mutations in a mouse model of inflammation-related colon carcinogenesis induced by 1,2-dimethylhydrazine and dextran sodium sulfate. Cancer Sci 96:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Clercq CM, Sanduleanu S. (2014) Interval colorectal cancers: what and why. Curr Gastroenterol Rep 16:375. [DOI] [PubMed] [Google Scholar]
- Lim S, Kaldis P. (2013) Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development 140:3079–3093. [DOI] [PubMed] [Google Scholar]
- Ma X, Shah YM, Guo GL, Wang T, Krausz KW, Idle JR, Gonzalez FJ. (2007) Rifaximin is a gut-specific human pregnane X receptor activator. J Pharmacol Exp Ther 322:391–398. [DOI] [PubMed] [Google Scholar]
- Mladenova D, Pangon L, Currey N, Ng I, Musgrove EA, Grey ST, Kohonen-Corish MR. (2013) Sulindac activates NF-κB signaling in colon cancer cells. Cell Commun Signal 11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen KD, Sanyal AJ, Bass NM, Poordad FF, Sheikh MY, Frederick RT, Bortey E, Forbes WP. (2014) Rifaximin is safe and well tolerated for long-term maintenance of remission from overt hepatic encephalopathy. Clin Gastroenterol Hepatol 12:1390–1397. [DOI] [PubMed] [Google Scholar]
- Okayasu I. (2012) Development of ulcerative colitis and its associated colorectal neoplasia as a model of the organ-specific chronic inflammation-carcinoma sequence. Pathol Int 62:368–380. [DOI] [PubMed] [Google Scholar]
- Onizawa M, Nagaishi T, Kanai T, Nagano K, Oshima S, Nemoto Y, Yoshioka A, Totsuka T, Okamoto R, Nakamura T, et al. (2009) Signaling pathway via TNF-alpha/NF-kappaB in intestinal epithelial cells may be directly involved in colitis-associated carcinogenesis. Am J Physiol Gastrointest Liver Physiol 296:G850–G859. [DOI] [PubMed] [Google Scholar]
- Ouyang N, Ke S, Eagleton N, Xie Y, Chen G, Laffins B, Yao H, Zhou B, Tian Y. (2010) Pregnane X receptor suppresses proliferation and tumourigenicity of colon cancer cells. Br J Cancer 102:1753–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu A, Jiang C, Cai Y, Kim JH, Tanaka N, Ward JM, Shah YM, Gonzalez FJ. (2014) Role of Myc in hepatocellular proliferation and hepatocarcinogenesis. J Hepatol 60:331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savari S, Vinnakota K, Zhang Y, Sjölander A. (2014) Cysteinyl leukotrienes and their receptors: bridging inflammation and colorectal cancer. World J Gastroenterol 20:968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolfi C, De Simone V, Pallone F, Monteleone G. (2013) Mechanisms of action of non-steroidal anti-inflammatory drugs (NSAIDs) and mesalazine in the chemoprevention of colorectal cancer. Int J Mol Sci 14:17972–17985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Dashwood WM, Löhr CV, Fischer KA, Nakagama H, Williams DE, Dashwood RH. (2008) beta-catenin is strongly elevated in rat colonic epithelium following short-term intermittent treatment with 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and a high-fat diet. Cancer Sci 99:1754–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Venkatesh M, Li H, Goetz R, Mukherjee S, Biswas A, Zhu L, Kaubisch A, Wang L, Pullman J, et al. (2011) Pregnane X receptor activation induces FGF19-dependent tumor aggressiveness in humans and mice. J Clin Invest 121:3220–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Ke S, Ouyang N, He J, Xie W, Bedford MT, Tian Y. (2009) Epigenetic regulation of transcriptional activity of pregnane X receptor by protein arginine methyltransferase 1. J Biol Chem 284:9199–9205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Tian Y. (2006) Xenobiotic receptor meets NF-kappaB, a collision in the small bowel. Cell Metab 4:177–178. [DOI] [PubMed] [Google Scholar]
- Xu D, Gao J, Gillilland M, 3rd, Wu X, Song I, Kao JY, Owyang C. (2014) Rifaximin alters intestinal bacteria and prevents stress-induced gut inflammation and visceral hyperalgesia in rats. Gastroenterology 146:484–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Liu M, Zhai Y, Xie W. (2008) The antiapoptotic role of pregnane X receptor in human colon cancer cells. Mol Endocrinol 22:868–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.