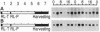Abstract
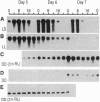
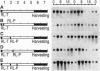
Light-induced expression of genes encoding the light-harvesting chlorophyll a/b binding proteins of photosystem II (Cab) was shown to be controlled by a circadian oscillator coupled to the red-light-absorbing plant photoreceptor phytochrome. Here we show that a red-light-insensitive oscillator is also involved in regulating the expression of the Cab genes. We provide evidence that germination leads, in a light-independent manner, to the setting and/or synchronization of endogenous oscillators and that it induces the expression of Cab genes in a circadian fashion. This circadian oscillator is not coupled to phytochrome, as it cannot be reset by red light for at least 44 h after sowing. Short red light pulses given between 12 and 44 h after sowing, however, induce new rhythms without perturbing the already free-running red-light-independent circadian oscillation. At this stage of development, the phytochrome-coupled and uncoupled circadian rhythms coexist. Both circadian rhythms are expressed and exhibit period lengths close to 24 h but are phased differently. At later stages of development (60 h or later after sowing), red light treatments synchronized these free-running rhythms and led to the appearance of a single new circadian oscillation. These data indicate that during early development the expression of single tobacco Cab genes, particularly expression of the Cab21 and Cab40 genes, is controlled in a developmentally dependent manner by two circadian oscillators.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam E., Deak M., Kay S., Chua N. H., Nagy F. Sequence of a tobacco (Nicotiana tabacum) gene coding for type A phytochrome. Plant Physiol. 1993 Apr;101(4):1407–1408. doi: 10.1104/pp.101.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borello U., Ceccarelli E., Giuliano G. Constitutive, light-responsive and circadian clock-responsive factors compete for the different l box elements in plant light-regulated promoters. Plant J. 1993 Oct;4(4):611–619. doi: 10.1046/j.1365-313x.1993.04040611.x. [DOI] [PubMed] [Google Scholar]
- Bowler C., Neuhaus G., Yamagata H., Chua N. H. Cyclic GMP and calcium mediate phytochrome phototransduction. Cell. 1994 Apr 8;77(1):73–81. doi: 10.1016/0092-8674(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Brusslan J. A., Tobin E. M. Light-independent developmental regulation of cab gene expression in Arabidopsis thaliana seedlings. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7791–7795. doi: 10.1073/pnas.89.16.7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory J., Peto C., Feinbaum R., Pratt L., Ausubel F. Arabidopsis thaliana mutant that develops as a light-grown plant in the absence of light. Cell. 1989 Sep 8;58(5):991–999. doi: 10.1016/0092-8674(89)90950-1. [DOI] [PubMed] [Google Scholar]
- Fejes E., Pay A., Kanevsky I., Szell M., Adam E., Kay S., Nagy F. A 268 bp upstream sequence mediates the circadian clock-regulated transcription of the wheat Cab-1 gene in transgenic plants. Plant Mol Biol. 1990 Dec;15(6):921–932. doi: 10.1007/BF00039431. [DOI] [PubMed] [Google Scholar]
- Gwinner E. Testosterone induces "splitting" of circadian locomotor activity rhythms in birds. Science. 1974 Jul 5;185(4145):72–74. doi: 10.1126/science.185.4145.72. [DOI] [PubMed] [Google Scholar]
- Hennessey T. L., Field C. B. Evidence of multiple circadian oscillators in bean plants. J Biol Rhythms. 1992 Summer;7(2):105–113. doi: 10.1177/074873049200700202. [DOI] [PubMed] [Google Scholar]
- Kloppstech K., Otto B., Sierralta W. Cyclic temperature treatments of dark-grown pea seedlings induce a rise in specific transcript levels of light-regulated genes related to photomorphogenesis. Mol Gen Genet. 1991 Mar;225(3):468–473. doi: 10.1007/BF00261689. [DOI] [PubMed] [Google Scholar]
- Kondo T., Strayer C. A., Kulkarni R. D., Taylor W., Ishiura M., Golden S. S., Johnson C. H. Circadian rhythms in prokaryotes: luciferase as a reporter of circadian gene expression in cyanobacteria. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5672–5676. doi: 10.1073/pnas.90.12.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar A. J., Kay S. A. Circadian Control of cab Gene Transcription and mRNA Accumulation in Arabidopsis. Plant Cell. 1991 May;3(5):541–550. doi: 10.1105/tpc.3.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar A. J., Short S. R., Chua N. H., Kay S. A. A novel circadian phenotype based on firefly luciferase expression in transgenic plants. Plant Cell. 1992 Sep;4(9):1075–1087. doi: 10.1105/tpc.4.9.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy F., Fejes E., Wehmeyer B., Dallman G., Schafer E. The circadian oscillator is regulated by a very low fluence response of phytochrome in wheat. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6290–6294. doi: 10.1073/pnas.90.13.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy F., Kay S. A., Chua N. H. Gene regulation by phytochrome. Trends Genet. 1988 Feb;4(2):37–42. doi: 10.1016/0168-9525(88)90064-9. [DOI] [PubMed] [Google Scholar]
- Neuhaus G., Bowler C., Kern R., Chua N. H. Calcium/calmodulin-dependent and -independent phytochrome signal transduction pathways. Cell. 1993 Jun 4;73(5):937–952. doi: 10.1016/0092-8674(93)90272-r. [DOI] [PubMed] [Google Scholar]
- Paulsen H., Bogorad L. Diurnal and Circadian Rhythms in the Accumulation and Synthesis of mRNA for the Light-Harvesting Chlorophyll a/b-Binding Protein in Tobacco. Plant Physiol. 1988 Dec;88(4):1104–1109. doi: 10.1104/pp.88.4.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechulla B. 'Circadian clock' directs the expression of plant genes. Plant Mol Biol. 1993 Jun;22(3):533–542. doi: 10.1007/BF00015982. [DOI] [PubMed] [Google Scholar]
- Quail P. H. Phytochrome: a light-activated molecular switch that regulates plant gene expression. Annu Rev Genet. 1991;25:389–409. doi: 10.1146/annurev.ge.25.120191.002133. [DOI] [PubMed] [Google Scholar]
- Takahashi J. S. Circadian rhythms. ICER is nicer at night (sir!). Curr Biol. 1994 Feb 1;4(2):165–168. doi: 10.1016/s0960-9822(94)00040-0. [DOI] [PubMed] [Google Scholar]
- Tavladoraki P., Kloppstech K., Argyroudi-Akoyunoglou J. Circadian Rhythm in the Expression of the mRNA Coding for the Apoprotein of the Light-Harvesting Complex of Photosystem II : Phytochrome Control and Persistent Far Red Reversibility. Plant Physiol. 1989 Jun;90(2):665–672. doi: 10.1104/pp.90.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierstra R. D. Illuminating Phytochrome Functions (There Is Light at the End of the Tunnel). Plant Physiol. 1993 Nov;103(3):679–684. doi: 10.1104/pp.103.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]