Abstract
Background
The long-term consequences of adolescent alcohol abuse that persist into adulthood are poorly understood and have not been widely investigated. We have shown that intermittent exposure to alcohol during adolescence decreased the amplitude of GABAA receptor-mediated tonic currents in hippocampal dentate granule cells in adulthood. The aim of the present study was to investigate the enduring effects of chronic intermittent alcohol exposure during adolescence or adulthood on the expression of hippocampal GABAA receptors (GABAARs).
Methods
We used a previously characterized tissue fractionation method to isolate detergent resistant membranes and soluble fractions, followed by western blots to measure GABAAR protein expression. We also measured mRNA levels of GABAAR subunits using quantitative real-time PCR.
Results
Although the protein levels of α1-, α4- and δ-GABAAR subunits remained stable between postnatal day (PD) 30 (early adolescence) and PD71 (adulthood), the α5-GABAAR subunit was reduced across that period. In rats that were subjected to adolescent intermittent ethanol (AIE) exposure between PD30–46, there was a significant reduction in the protein levels of the δ-GABAAR, in the absence of any changes in mRNA levels, at 48 hours and 26 days after the last ethanol exposure. Protein levels of the α4-GABAAR subunit were significantly reduced, but mRNA levels were increased, 26 days (but not 48 hours) after the last AIE exposure. Protein levels of α5-GABAAR were not changed by AIE, but mRNA levels were reduced at 48hrs but normalized 26 days after AIE. In contrast to the effects of AIE, chronic intermittent exposure to ethanol during adulthood (CIE) had no effect on expression of any of the GABAAR subunits examined.
Conclusions
AIE produced both short- and long-term alterations of GABAAR subunits mRNA and protein expression in the hippocampus, whereas CIE produced no long lasting effects on those measures. The observed reduction of protein levels of the δ-GABAAR, specifically, is consistent with previously reported altered hippocampal GABAAR-mediated electrophysiological responses after AIE. The absence of effects of CIE underscores the emerging view of adolescence as a time of distinctive vulnerability to the enduring effects of repeated ethanol exposure.
Introduction
Alcohol abuse and alcohol related problems represent a major health and social problem worldwide. The probability of developing alcohol dependence is strongly correlated with the age at which experimentation with alcohol consumption begins. According to a nationwide survey (NIAAA 2004), binge drinking --- defined by 5 or more drinks per occasion for males, four or more for females --- increases markedly across adolescence from 1.6% at ages 12–13, 17% at ages 16–17, and 34.7% at ages 18–20. This prevalence of binge drinking occurs at a developmental period when the brain is undergoing rapid changes in structure and function that make it vulnerable to negative consequences of alcohol exposure (Monti et al. 2005). Although it is clear that alcohol abuse at any age can have enduring effects on brain function (see Brown et al 2009), the question of whether adolescence is a period of distinctive vulnerability associated with long-term effects on brain and behavior has only recently begun to be more rigorously investigated.
Several lines of evidence indicate that adolescent rats are more sensitive than adult rats to the memory impairing effects of acute alcohol exposure (for reviews, see Land and Spear 2004; White and Swartzwelder 2004). For example, adolescent rats show greater alcohol-induced memory deficits in the Morris water maze and in a discrimination task than do adult rats (Land and Spear 2004; Markwiese et al. 1998). Moreover, in humans, individuals in their early 20’s are more sensitive to alcohol’s effects on a verbal and figural memory tasks than those in their late 20’s (Acheson et al. 1998). These findings are underscored by studies indicating that acute alcohol impairs the induction of long-term potentiation (LTP) (Swartzwelder et al, 1995a), and diminishes n-methyl-d-aspartate (NMDA) receptor-mediated neurotransmission (Swartzwelder et al, 1995b) more potently in hippocampal slices from adolescent rats compared to those from adults. The convergence of these findings indicates that both memory formation and memory-related hippocampal function are more sensitive to the effects of acute ethanol during adolescence than during adulthood, and supports the hypothesis that the adolescent hippocampal formation may also be particularly vulnerable to long-term impairment after repeated ethanol exposure.
Early studies of chronic ethanol exposure in adulthood revealed long lasting (presumably permanent) decreases in hippocampal dendritic spines (Riley and Walker, 1978) and neurons (Walker et al, 1980), and in the capacity for the induction of LTP (Durand and Carlen, 1984). Subsequently, chronic ethanol exposure during prenatal development was shown to reduce the capacity for LTP induction (Swartzwelder et al, 1988), and diminish NMDA receptor-mediated neurotransmission (Morrisett et al, 1989) in the hippocampal formation in adulthood. However, despite the long-standing awareness that human adolescents and young adults consume high amounts of ethanol, studies of the long-term consequences of chronic ethanol exposure during adolescent development on hippocampal function have only now begun to emerge. For example, we have recently shown that adolescent intermittent ethanol (AIE) reduces tonic γ-aminobutyric acidA receptor (GABAAR)-mediated inhibition in the hippocampal formation in adulthood (Fleming et al, 2012; 2013), and reduces the density of the A-type K+ current (IA) in GABAergic hippocampal interneurons (Li et al, 2013). Importantly, such long-term alterations were not observed after comparable CIE exposure during adulthood (CIE), suggesting that adolescence is a distinctively vulnerable period for such effects.
It is well known that currents carried by GABAARs modulate memory-related hippocampal synaptic plasticity and learning (Collinson et al. 2002; Crestani et al. 2002; Fritschy and Brunig 2003). Furthermore, drugs that promote GABAAR function shift the balance of excitation and inhibition in hippocampal circuits toward inhibition, thus making it less likely that incoming excitatory synaptic drive will be sufficient to induce LTP (Semyanov et al, 2004), and have been shown to inhibit hippocampally-mediated memory formation (Farr et al, 2000). Thus, it may be that repeated ethanol exposure during adolescence results in alterations of hippocampal GABAAR function that could compromise the excitability of memory-related circuits within that structure and compromise learning in adulthood. Therefore, the present study was designed to uncover the GABAAR subunit alterations that may underlie recently reported deficits in tonic GABAA currents following AIE exposure, and to determine if such changes are also observed after CIE in adulthood.
Methods
Animals and intermittent ethanol exposure
The animal research reported in this study was conducted according to the protocols that were approved by the Durham VAMC and Duke University Institutional Animal Care and Use Committees. Male Sprague-Dawley rats were obtained from Charles River (Raleigh, NC). The rats arrived at the Durham VAMC vivarium 5 or 6 days before the beginning of EtOH exposure and were acclimated to handling for 3 days. They were housed 2 animals per cage with a 12-hour on 12-hour off reversed light/dark cycle and provided with ad libitum access to food and water for the duration of the study. In the first experiment, hippocampus was extracted from EtOH naïve rats and the samples were prepared for western blotting at PD30, PD46, and PD71 to characterize the developmental expression patterns of the GABAA receptor subunits. For adolescent and adult alcohol exposure, two age groups of rats were exposed to an intermittent ethanol exposure regimen: adolescent rats that began the regimen at 30 days of age, and adult rats that began the regimen at 70 days of age. The intermittent ethanol exposure regimen consisted of 10 doses of 5 g/kg EtOH (35% v/v in 0.9% saline) by intragastric gavage in a 2-day on, 1-day off, 2 days on, 2 days off sequence such that the animals received single gavage exposures on days 1 and 2, 4 and 5, 8 and 9, 11 and 12, and 15 and 16 of the regimen (Acheson et al., 2013). Control rats received 18.12 ml/kg saline by intragastric gavage. After the last EtOH exposure, the adolescent rats were housed in the vivarium for either 2 or 26 days until the brain was extracted and the tissue was prepared for western blotting. The hippocampi of the adult rats exposed to intermittent ethanol were extracted 26 days following the last EtOH exposure. The EtOH naïve rats from which tissue was extracted on PD46 also served as saline controls in the subsequent study of adolescent ethanol exposure. A recent study from this laboratory using identical EtOH exposure protocols have generated average BECs (in mg/dl ± sem) of 199.7 ± 19.9 60 min after the first dose, and 172.8 ± 13.3 60min after the last dose (Risher et al, 2013a). Those blood ethanol concentrations are consistent with BECs achieved in our previous studies (Acheson et al., 2013), and by adolescent humans during binge drinking episodes serving as part of the binge drinking criteria (NIAAA, 2004).
Animals were sacrificed and hippocampal tissue harvested at 48 hours or 26 days after the last ethanol exposure. The 48-hour interval was chosen to reflect short-term effects of the ethanol exposure, but after any acute withdrawal signs would presumably have passed. The 26-day interval was chosen to reflect long-term (possibly permanent) effects of the ethanol exposure. Based on the initial findings 26 days after CIE exposure during adulthood, effects at the 48-hour interval were only assessed after AIE during adolescence.
Western Blotting
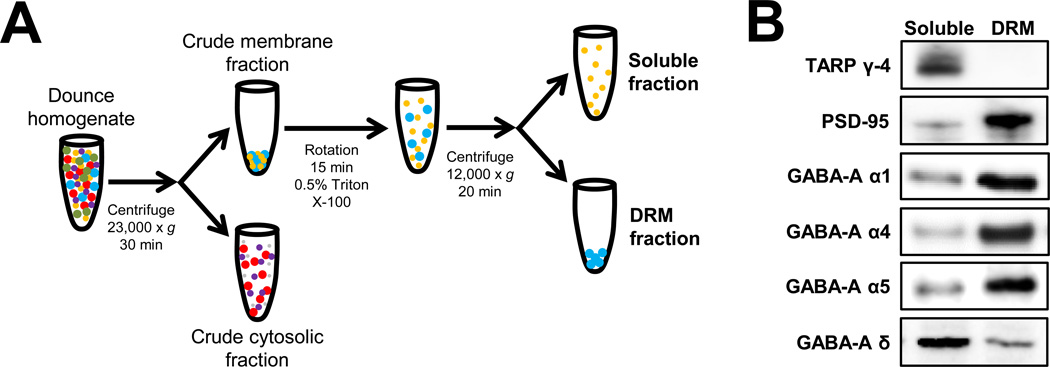
Following previously reported methods (Mulholland et al., 2011; Kroener et al., 2012), Triton X-100 detergent resistant membranes (DRM) and soluble fractions were prepared from hippocampus extracted from rats at the time points described in the previous section. As shown in Figure 1A, a Dounce homogenate was prepared from hippocampus and centrifuged at 23,000 × g for 30 min. The supernatant (crude cytosolic fraction) was removed and the pellet (crude membrane fraction) was gently re-suspended in lysis buffer containing 0.5% Triton X-100 and was rotated at 4°C for 15 min. This fraction was then centrifuged at 12,000 × g for 20 min to yield DRM and soluble fractions. The DRM fraction was then solubilized into 2% LDS and probe sonicated for ~ 5 sec. An aliquot of each fraction was taken for determination of protein concentration by the bicinchoninic acid assay (Pierce Biotechnology, Inc., Rockford, IL). The remaining samples were stored at −80°C until immunoblot analysis. An aliquot of each sample was diluted with NuPAGE 4× LDS sample loading buffer (Invitrogen Corp., Carlsbad, CA; pH 8.5) containing 50 mM dithiothreitol, and samples were denatured for 10 min at 70° C. Five µg of each sample was separated using the Bis-Tris (375 mM resolving buffer and 125 mM stacking buffer, pH 6.4; 7.5% acrylamide) discontinuous buffer system with MOPS electrophoresis buffer (50 mM MOPS, 50 mM Tris, 0.1% SDS, 1 mM EDTA, pH 7.7). Protein was then transferred to Immobilon-P PVDF membranes (Millipore, Bedford, MA) using a semi-dry transfer apparatus (Bio-Rad Laboratories, Hercules, CA). After transfer, blots were washed with phosphate-buffered saline containing 0.1% Tween 20 (PBST) and then blocked with PBST containing 5% nonfat dried milk (NFDM) for 1 hr at room temperature with agitation. The membranes were then incubated overnight at 4°C with primary antibodies diluted in PBST containing 0.5% NFDM and washed in PBST prior to 1-hr incubation at room temperature with horseradish peroxidase conjugated secondary antibodies diluted 1:2000 in PBST. Membranes received a final wash in PBST and the antigen-antibody complex was detected by enhanced chemiluminescence using a ChemiDoc MP Imaging system (Bio-Rad Laboratories, Hercules, CA). The bands were quantified by mean optical density using computer-assisted densitometry with ImageJ v1.41 (National Institutes of Health, USA). PSD-95 (1:5000) was purchased from NeuroMab (Antibodies, Inc. & UC Davis, Davis, CA; Catalog # 75-028). Transmembrane AMPA receptor regulatory protein (TARP) γ-4 was purchased from Millipore Corporation (Billerica, MA; Catalog # AB5795) Antibodies targeting the GABA-A receptor subunits that were used in these studies were α1 (1:2000; NeuroMab, Antibodies, Inc. & UC Davis, Davis, CA; Catalog # 75–136), α4 (1:1000; Millipore Corp., Billerica, MA; Catalog # AB5457), α5 (1:1000; PhosphoSolutions, Aurora, CO; Catalog # 846-GA5C), and δ (1:1000; PhosphoSolutions, Aurora, CO; Catalog # 868-GDN).
Figure 1.
Characterization of GABAAR subunit expression in Triton X-100 soluble membrane and detergent resistant membrane (DRM) fractions. (A) Schematic of the subcellular fraction protocol coupled with 0.5% Triton X-100 detergent extraction of insoluble membranes. Expanded details on the extraction method and detergent extraction are listed in the Methods section. (B) Characterization of synaptic and extrasynaptic proteins and GABAAR subunits in soluble membrane and DRM fractions prepared from hippocampus of rats. Representative western blots of equal amounts of protein from each fraction using antibodies targeting the postsynaptic density protein PSD-95, the extrasynaptic membrane-associated protein TARP γ-4, and the GABAAR α1, α4, α5, and δ subunits.
Real-Time quantitative Polymerase Chain Reaction
Total RNA was extracted from hippocampal tissue from control or ethanol exposed animals using the Qiagen RNAeasy Mini protocol & DNase Kit according to the manufacturer's instructions. Briefly, frozen tissue was homogenized on ice using phenol, guanidine isothiocyanate and chloroform and then treated with RNase-free DNase. RNA pellets were then washed with a series of ethanol rinses and dissolved in 50 µL of RNAase free water. Sixty micrograms of total RNA per sample was reverse transcribed in duplicate at 37°C for two hours using the High capacity cDNA RT kit (Life Technologies). The reaction was stopped by a 5-minute incubation at 85°C.
GABAA receptor subunit expression was quantified using cDNA aliquots from reverse transcribed sample preparations and amplified in triplicate by Real-time PCR using Maxima SYBR Green (ThermoScientific). The starting concentration of cDNA to be amplified for each gene was determined by plotting a standard curve to test the primer efficiency at five different template cDNA concentrations. Hypoxanthine phosphoribosyltransferase 1 (Hprt1) was used as the internal control and PCR conditions for all primers were: 3min at 95°C followed by 30s at 95°C, 30s at 58°C and 30s 72°C for 40 cycles. The sequences for each primer have been provided in Table 1. Quantitative PCR was performed using the Mx3000P qPCR system (Agilent Technologies) and analyzed with MxPro software. The c(t) value of the gene of interest was corrected with the c(t) value of the respective Hprt1 internal standard gene. The ΔΔc(t) values were calculated for each sample by subtracting the mean of Δc(t) values of control groups and the respective fold changes were calculated as 2−ΔΔc(t) (Schmittgen & Livak, 2008). Data is presented as average fold change per experimental condition.
Table 1.
Primers used in the study of mRNA expression of GABAAR Subunits in the hippocampus
| δ-GABAAR Forward: | ATC ACC AGT TAC CGC TTC ACC |
| δ-GABAAR Reverse: | AGT GTA AGC TGA GTC GAG GGA |
| α4-GABAAR Forward: | ACC AAA GGC CCT GAG AAG TC |
| α4-GABAAR Reverse: | CCA TCT TCC GTC TGA GGT GA |
| α5-GABAAR Forward: | ACT GGG AAT GGA CAA TGG AA |
| α5-GABAAR Reverse: | CGT CCA AGA TCC TGG TGA AT |
| Hprt1 Forward: | TCC TCA GAC CGC TTT TCC CGC |
| Hprt1 Reverse: | TCA TCA TCA CTA ATC ACG ACG CTG G |
Statistical analysis
Western blot data obtained as raw arbitrary optical units were analyzed by two-way analysis of variance (ANOVA) with Student-Newman-Keuls post-hoc tests when appropriate. The mRNA data were analyzed with Student’s t-tests. All data are reported as mean ± SEM and statistical significance was established with p < 0.05.
Results
Characterization of the GABAA Receptor Subunit Membrane Localization in the Hippocampus
Using a modified subcellular fractionation protocol (Figure 1A), we examined the subcellular localization of several functionally critical GABAAR subunits in the hippocampus. As expected, the postsynaptic density protein PSD-95 was enriched in the DRM fraction with minor PSD-95 expression levels observed in the soluble membrane fraction (Mulholland et al., 2011, 2012; El-Husseini et al., 2000). In contrast, TARP γ-4 was highly enriched in the soluble fraction, consistent with its known abundance in extrasynaptic membranes (Ferrario et al., 2011a,b). We identified α1-, α4- and α5-GABAAR as being primarily expressed in the DRM fraction with a small amount of protein expression in the soluble fraction. In contrast, δ-GABAAR protein was more highly expressed in the soluble fraction while a small proportion of the protein was observed in the DRM fraction (Figure 1B). This pattern of expression remained evident throughout all of the experiments presented in this study. The main subunit of interest was the δ-GABAAR protein because of its association with extrasynaptic GABA receptor function and our previous electrophysiological findings related to the effects of AIE on GABAA receptor-mediated tonic current in adulthood (Fleming et al, 2012; 2013). We chose to assess the α4 and α5 GABAAR proteins because they are associated with tonic GABAAR-mediated neuronal inhibition in the hippocampal formation (see Belelli et al, 2009). The α1 GABAAR protein was included for comparison, as a synaptic subunit not associated with the generation of tonic current.
Developmental Changes in Protein Expression of Hippocampal GABAA Receptor Subunits
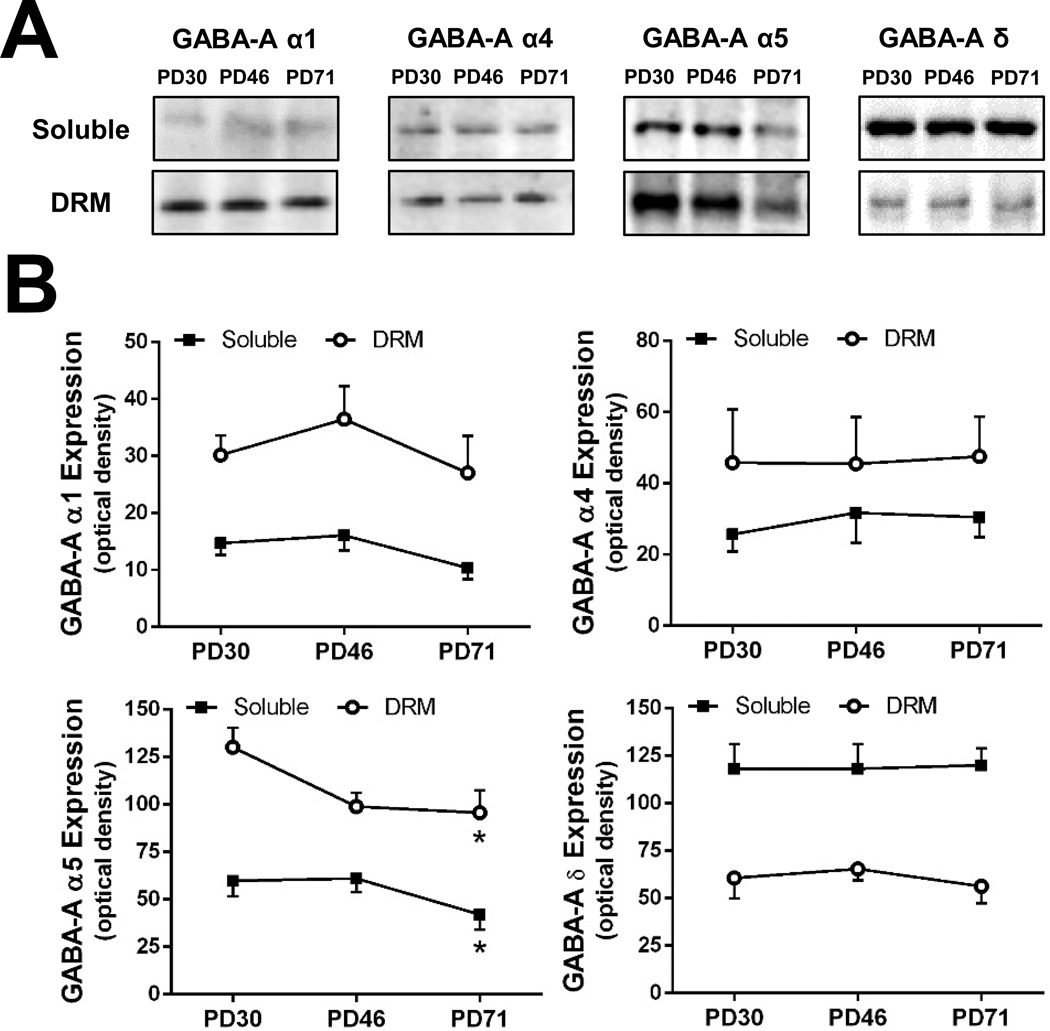
As a follow-up to the recent reports that AIE exposure attenuates tonic GABAAR-mediated currents in adulthood (Fleming et al; 2012; 2013), we examined the effect of AIE or CIE on developmental changes in the expression of GABAAR subunits that are linked to mediation of tonic and synaptic currents. However, before assessing the effects of AIE or CIE, we measured the expression of α1-, α4-, α5- and δ-GABAAR in the DRM and membrane soluble fraction isolated from the hippocampus of control (i.e. ethanol-naïve) rats at PD30 (early adolescence), PD46 (late adolescence) and PD71 (adult). As shown in Figure 2, there were no significant changes in the relative protein levels of α1-, α4- and δ-GABAAR subunits measured across the three developmental periods. In contrast, α5-GABAAR protein expression exhibited a significant age-dependent reduction during the transition from adolescence to adulthood that was observed in both the soluble and the DRM fractions [F(1, 35) = 4.343, p=0.022]. Also as shown in Figure 2 there were no changes in the relative distribution of any of the subunits between the DRM and soluble membrane fractions across the developmental time frame assessed.
Figure 2.
Expression patterns of α1-, α4-, α5- and δ-containing GABAAR in the DRM and soluble membrane fraction isolated from the hippocampus of control rats at PD30 (early adolescence), PD46 (late adolescence), and PD71 (adult). (A) Representative western blots of GABAAR α1, α4, α5, and δ subunits in the soluble and DRM fractions. (B) Quantitation of western blots of GABAAR α1, α4, α5, and δ subunits at PD30 to PD71 in both fractions (n = 5–6/fraction/time point; *p = 0.022).
Effect of AIE Exposure on Protein Levels of Hippocampal GABAAR subunits
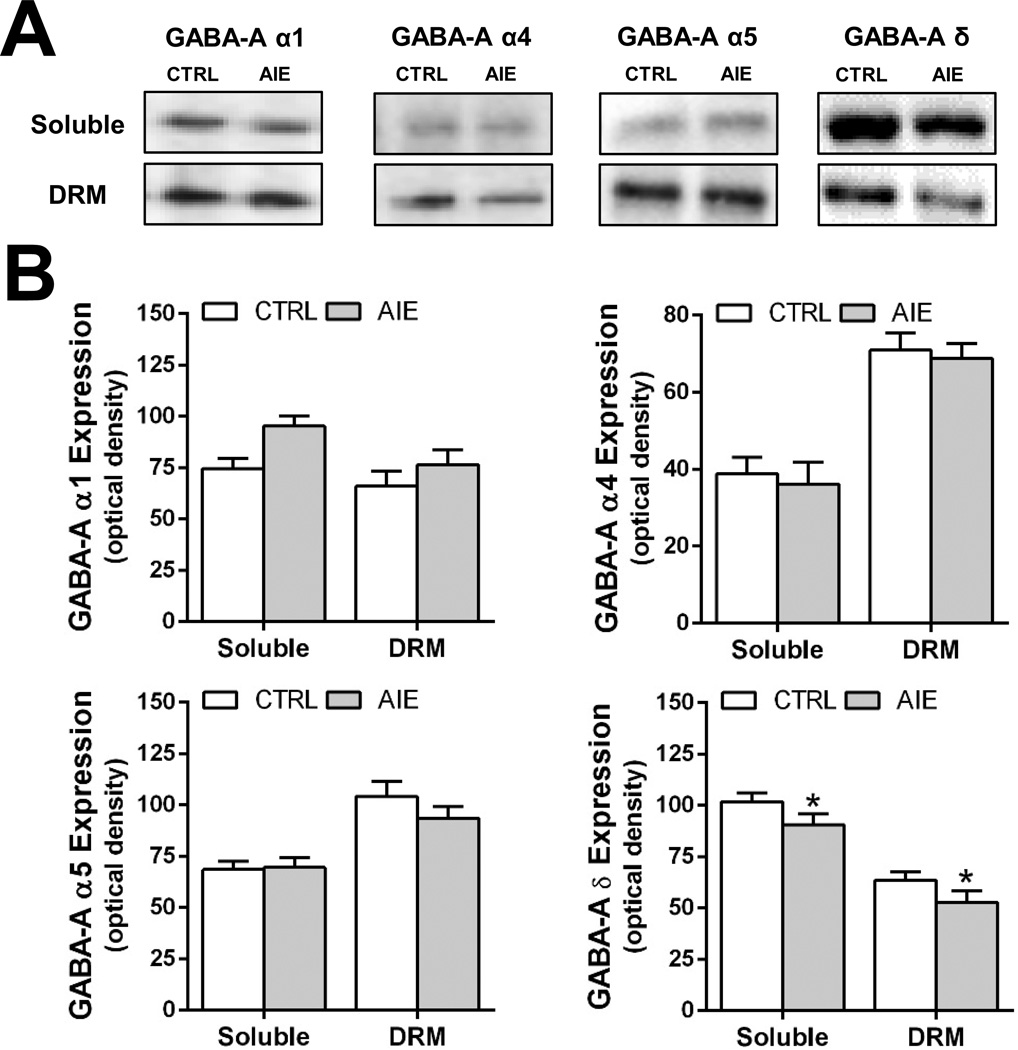
The next experiment examined the effect of AIE exposure on GABAAR protein expression in the hippocampus at 48 hours after the last ethanol exposure and 26 days after the last day of ethanol exposure (i.e. in adulthood). As Figure 3 illustrates, western blot analysis revealed that protein levels of δ-GABAAR in both the DRM and soluble fractions in the adolescent hippocampus was significantly reduced relative to controls 48 hours after the last ethanol dose [F(1,35)=5.186; p=0.03]. In contrast, AIE had no effect on the protein expression of α1-, α4- and α5-GABAAR in either the DRM or soluble fraction measured 48 hours after the last AIE exposure.
Figure 3.
Intermittent ethanol exposure during adolescence reduces expression of δ-containing GABAAR in the DRM and soluble membrane fractions prepared from the hippocampus of rats 48 hours after the last ethanol dose. (A) Representative western blots of GABAAR α1, α4, α5, and δ subunits in the soluble and DRM fractions in control (CRTL) and adolescent intermittent ethanol (AIE) exposed rats. (B) Quantitation of western blots of GABAAR α1, α4, α5, and δ subunits in the soluble and DRM fractions from control (open bars) and alcohol exposed (shaded bars) rats (n = 9–10/fraction/group; *p = 0.03).
The next experiment examined protein levels of GABAAR subunits in adulthood, 26 days after the last AIE-exposure. As shown in Figure 4, expression of the δ-GABAAR subunit remained significantly reduced in the membrane soluble fraction [F(1,28)=4.649; p=0.041] but not in the DRM fraction. In contrast to a lack of effect of AIE on expression of the α4-GABAAR subunit 48 hours after AIE, it was significantly reduced in the DRM fraction in adulthood [F(1,26)=7.145; p=0.014]. Neither α1- nor α5-GABAAR protein levels were altered in adulthood by AIE exposure, in either the DRM fraction or the soluble membrane fraction.
Figure 4.
Intermittent ethanol exposure during adolescence reduces expression of α4- and δ-containing GABAAR in the DRM and soluble membrane fractions prepared from the hippocampus of rats 26 days after the last alcohol dose. (A) Representative western blots of GABAAR α1, α4, α5, and δ subunits in the soluble and DRM fractions in control (CRTL) and alcohol exposed (AIE) rats. (B) Quantitation of western blots of GABAAR α1, α4, α5, and δ subunits in the soluble and DRM fractions from control (open bars) and alcohol exposed (shaded bars) rats (n = 6–8/fraction/group; *p = 0.014).
Effect of AIE Exposure on mRNA Levels of Hippocampal GABAAR subunits
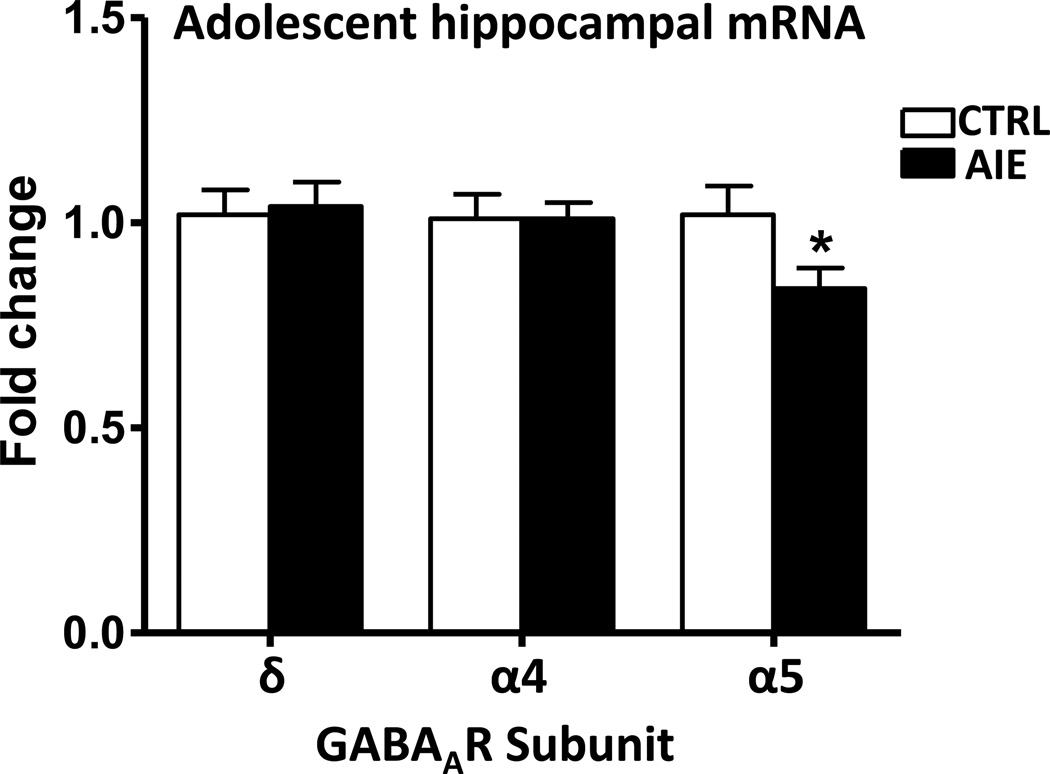
We examined whether changes in GABAAR subunit protein levels are related to alterations in respective subunit mRNA expression within the hippocampus after AIE. For this purpose, the effect of AIE exposure on GABAAR subunit mRNA levels in the hippocampus was investigated at 48 hours and 26 days after the last ethanol exposure. As shown in Figure 5, mRNA levels of the α5-GABAAR in the adolescent hippocampus was significantly (p=0.044) decreased as compared with controls 48 hours after the last ethanol dose. On the other hand, AIE had no significant effect on the expression of α4- and δ-GABAAR in the hippocampus measured 48 hours after the last AIE exposure.
Figure 5.
The effects of adolescent intermittent ethanol exposure (48 hours after last dose of ethanol) on the mRNA expression of α4, α5 and δ-GABAAR subunits in the hippocampus of adolescent rats. Values are the mean SEM of 10 rats in each group. *Significantly different from the control group (p=0.044; F=18; t=2.16)
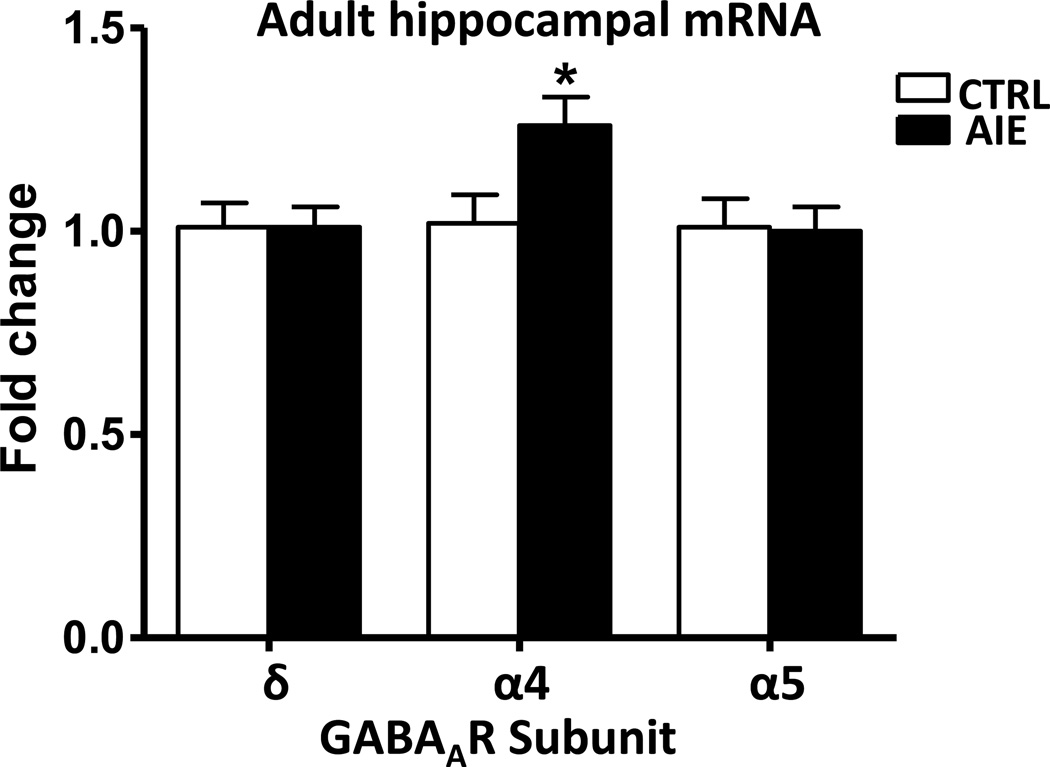
The mRNA levels of GABAAR subunits in adulthood (26 days after the last AIE-exposure) were also investigated. As shown in Figure 6, mRNA levels of the δ-GABAAR subunit remained unchanged in the hippocampus of adult AIE rats compared with controls. In contrast to a lack of effect of AIE on the mRNA levels of α4-GABAAR subunit 48 hours after AIE, it was significantly (p=0.026) increased in the hippocampus of adult rats (26 days after the last AIE exposure). Interestingly, α5-GABAAR mRNA levels in the hippocampus, which were decreased in adolescent AIE, were normalized in adulthood.
Figure 6.
The effects of adolescent intermittent ethanol exposure (26 days after last dose of ethanol) on the mRNA expression of α4, α5 and δ-GABAAR subunits in the hippocampus of adult rats. Values are the mean SEM of 7 rats in each group. *Significantly different from the control group (p=0.026; F=12; t= 2.54)
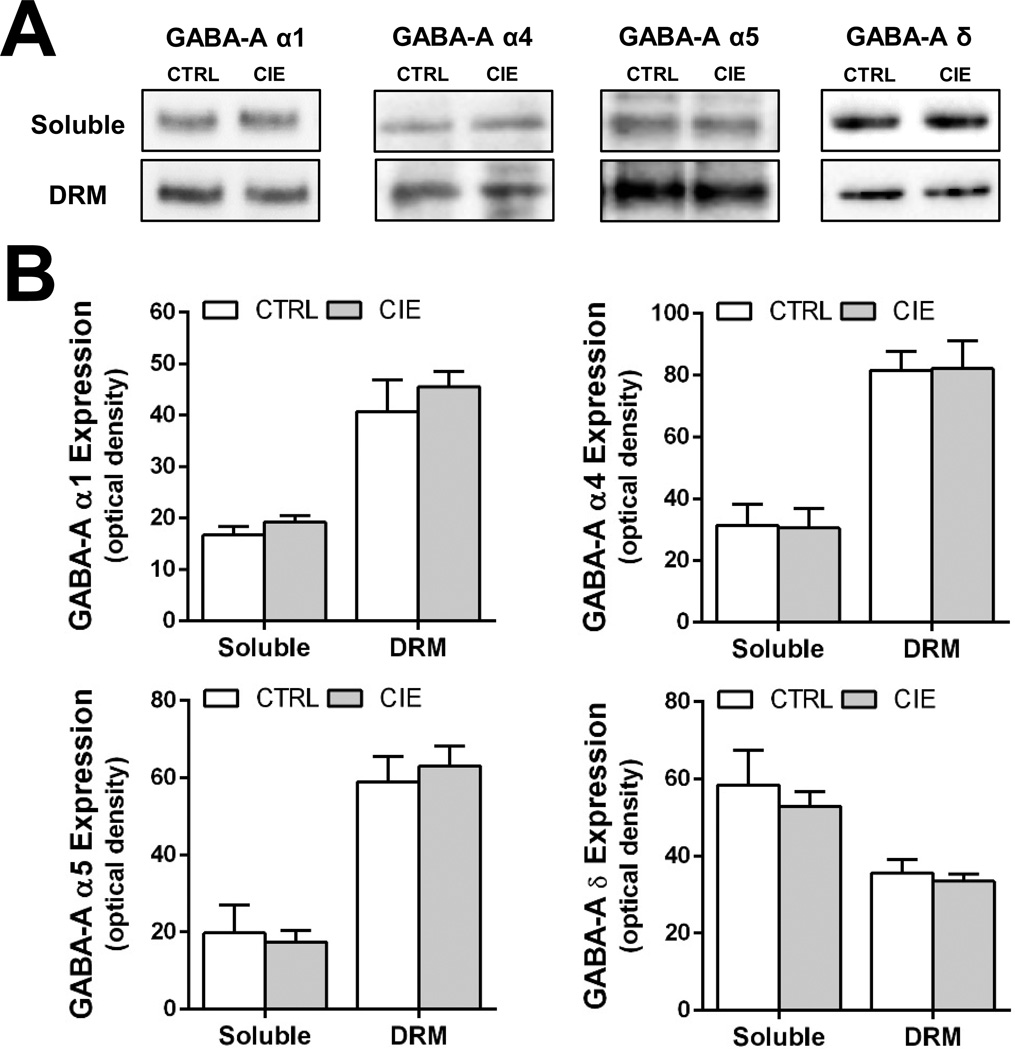
Effect of CIE Exposure during Adulthood on Protein Expression of Hippocampal GABAAR subunits
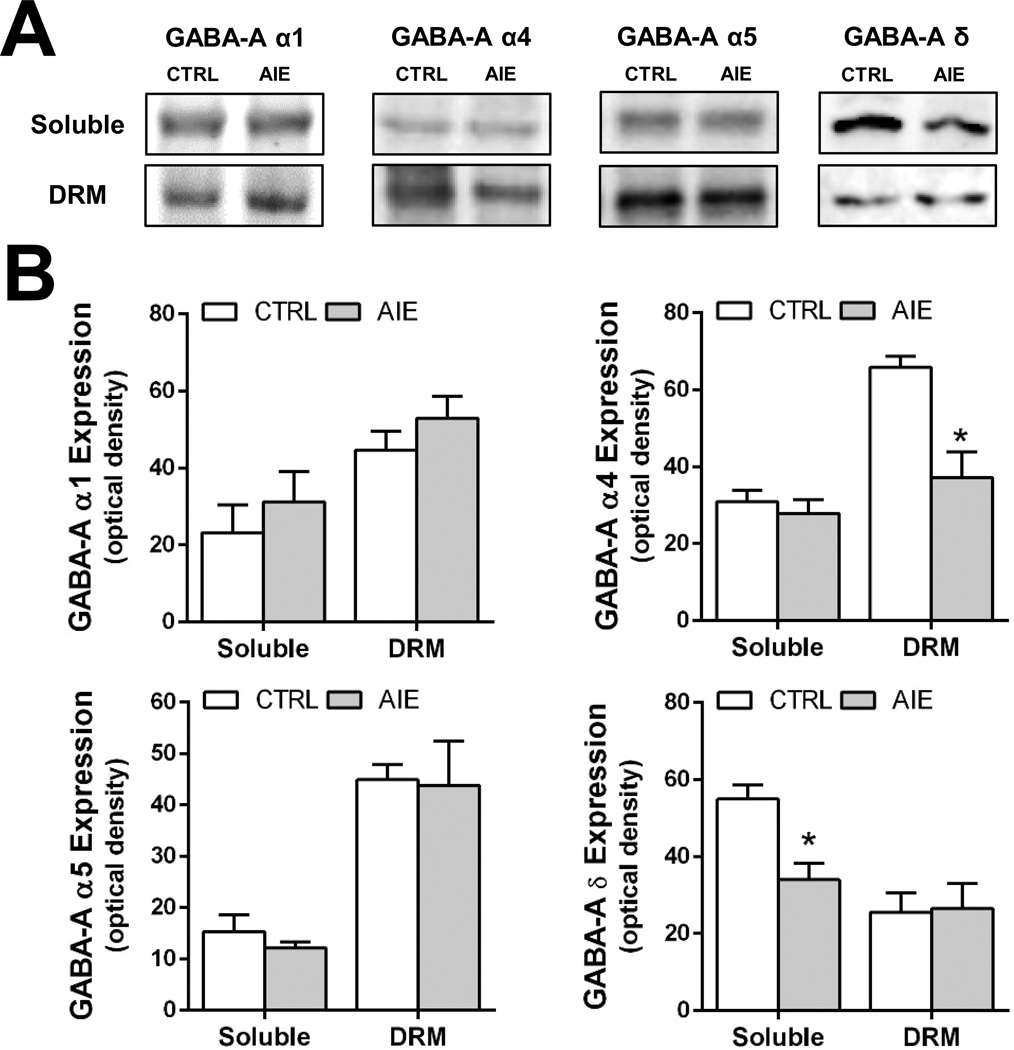
Using the alcohol exposure paradigm employed in the current study, it has been demonstrated that, in contrast to AIE, CIE in adulthood does not alter GABAAR mediated tonic currents recorded from dentate granule cells in the acute hippocampal slice preparation (Fleming et al 2012; 2013). We therefore examined the effect of CIE exposure during adulthood on the expression of GABAAR subunits in the hippocampus. For these studies, CIE exposure was initiated on PD70 and hippocampal tissue extracts were obtained 26 days after the last ethanol exposure. As shown in Figure 7, CIE exposure did not alter the expression of any of the GABAAR subunits examined, in either the soluble membrane or DRM fractions.
Figure 7.
Intermittent ethanol exposure during adulthood (CIE) does not alter expression of GABAAR α1, α4, α5, and δ subunits in the soluble and DRM fractions. (A) Representative western blots of GABAAR α1, α4, α5, and δ subunits in control (CRTL) and CIE exposed (CIE) rats. (B) Quantitation of western blots of GABAAR α1, α4, α5, and δ subunits in the soluble and DRM fractions from control (open bars) and alcohol exposed (shaded bars) rats (n = 6–8/fraction/group).
Discussion
The main finding of this study is that intermittent ethanol exposure during adolescence resulted in decreased levels of hippocampal δ-GABAAR subunit protein, without altering mRNA levels, when examined 48 hours and 26 days after the last ethanol dose. In addition, α4-GABAAR subunit protein levels were decreased, whereas mRNA levels were increased, 26 days after the last ethanol dose. Interestingly, protein levels of the α5-GABAAR in the hippocampus were not changed, although mRNA levels were decreased 48 hours after last ethanol dose. Importantly, intermittent ethanol exposure during adulthood did not alter the protein levels of any of the various GABAAR subtypes. The discrepancies between changes in the protein levels and mRNA levels of GABAAR subtypes in the hippocampus after AIE may be related to post-translational modifications involving changes in signaling mechanism and membrane trafficking (Kumar et al., 2012). Nonetheless, AIE-induced reduction in protein levels of δ- and α4-GABAAR subtypes that have been associated with tonic GABAA receptor-mediated inhibition in hippocampal neurons, is consistent with previous reports showing that AIE reduces inhibitory tonic current in hippocampal neurons (Fleming et al, 2012; 2013), and may reflect at the receptor level events that underlie tonic inhibition. It is important to note that the previous electrophysiological studies (Fleming et al, 2012; 2013) involved recording from single granule cells in the dentate gyrus, whereas the present data were collected using tissue from whole hippocampus. Thus, the parallels between those studies and the present study should be considered cautiously. Still, the fact that both the previous electrophysiological findings and the present subunit protein expression results occurred after AIE, but not after CIE, strongly suggests that adolescence is a developmental period during which hippocampal GABAergic systems are especially vulnerable to long-term disruption by repeated ethanol exposure.
It is notable that AIE produced changes in the mRNA levels of α5-GABAAR subtypes in the hippocampus 48 hours after the last dose (while the animals were still adolescents), and α4-GABAAR subtypes mRNA levels 26 days after the last dose (when the animals were adults). These results suggest that AIE produced effects at a transcriptional level on some of the GABAAR subtypes in the hippocampus. However, δ-GABAAR subtype mRNA levels were not changed by AIE although protein levels of this subunit were decreased for both 48 hours and 26 days after the termination of AIE exposure. Several previous studies have examined changes in the mRNA levels of various subunits in the hippocampus after ethanol exposure in adults. For example, it has been shown that mRNA levels of δ-GABAAR subunits in hippocampal neurons are increased during ethanol exposure but normalized during withdrawal (Follesa et al., 2005). No change was observed in the mRNA levels of δ-GABAAR subunit, but increased α4-GABAAR in the hippocampus of human alcoholics (Jin et al., 2012). It has also been shown that withdrawal after chronic ethanol exposure in adult animals leads to an increase in α4-GABAAR mRNA without any change in α5-GABAAR mRNA in the hippocampus (Mahmoudi et al., 1997). These observations are consistent with our findings that hippocampal mRNA expression of α5- and δ-GABAAR are unchanged, whereas α4-GABAAR mRNA is increased after AIE. The changes that we observed in the protein levels of α4-GABAAR and δ−GABAAR subunits in the hippocampus after AIE suggest that AIE causes changes in protein levels of GABAAR subunits that are physiologically abnormal based on electrophysiological data (Fleming et al., 2012; 2013), underscoring the importance of complex mechanisms related to transcription, translation, and post-translational modifications of GABAAR subunits.
An enduring (perhaps permanent) down-regulation of GABAergic subunit expression and tonic current in the hippocampal formation during adulthood after AIE exposure could have untoward effects on hippocampal circuit function, synaptic plasticity, and possibly learning and memory. While a decrease in inhibitory hippocampal function might seem likely to enhance memory-related hippocampal function, an increase in the capacity for synaptic plasticity is not necessarily beneficial (see Zorumski and Izumi 2012). In fact, the biasing of neural circuits toward excitatory synaptic plasticity has been associated with neurotoxic liability (Olney, 1969), neuronal death (Cull-Candy et al 2001), and the occlusion of memory-related function (Moser et al, 1998). Consistent with this, our recent findings indicate a long-lasting up-regulation in the capacity for synaptic plasticity after AIE that is accompanied by compromises in spatial learning capacity (Risher et al, 2013b), at the same long post-exposure interval at which the present decreases in GABA receptor subunit protein expression was observed (Fig. 4).
GABAA receptors containing the α4, α5, and δ subunits are predominantly expressed in perisynaptic and/or extrasynaptic membrane, whereas the α1 subunit is enriched in postsynaptic GABAergic synapses (for review see Luscher et al., 2011). While our findings demonstrate that α1 and δ subunits are predominantly found in the fractions enriched in synaptic (e.g., PSD-95) and extrasynaptic (e.g., TARP γ-4) proteins, respectively, the α4 and α5 subunits were found predominately in the synaptic fraction. GABAA receptors have been reported in non-synaptic lipid raft fractions (Li et al., 2007; Dalskov et al., 2005; Nothdurfter et al., 2013), and, importantly, many proteins expressed in non-synaptic lipid rafts are also insoluble in Triton X-100 and thus are recovered in the DRM fraction. Therefore, our findings do not necessarily reflect a synaptic localization of α4 and α5 subunits. Importantly, the AIE-induced reduction in α4 subunit expression in the DRM fraction does not appear to correspond with a functional change in phasic GABAergic synaptic currents (Fleming et al., 2012, 2013). One possibility is that changes in synaptic expression of α4 subunits do not translate into reduced current perhaps due to a possible compensation from other subunits.
It is notable that all of the changes observed after AIE occurred in the absence of any significant changes in baseline receptor subtype protein expression across the developmental trajectory from adolescence to adulthood (Fig. 2). The only receptor subtype that changed across adolescent development, the α5-GABAAR subtype, showed no sensitivity to the effects of AIE. However, since the AIE-induced changes in the δ and α4 subunits were decreases, it is possible that a natural developmental decrease in α5-GABAAR protein could have masked an AIE-induced decrease. The present study was not designed to assess that possibility. The fact that the observed AIE-induced decreases in the δ and α4 subunits occurred against the backdrop of a stable developmental baseline raises the possibility that the changes could be the result of an alteration of the normal developmental trajectory or a cytotoxic effect that may have resulted in fewer of the types of cells that express those subtypes. This is actually a core question related to the effects of AIE. That is, do the effects of exposure reflect an effect on development, a neurotoxic effect, or both? Since the expression of the affected subtypes was stable across adolescence in control animals, it seems unlikely that the present effects reflect a change in developmental trajectory. Similarly, it seems unlikely that the decreased protein expression we have observed results from a decrease in the number of hippocampal neurons that express them, because the GABA receptor subunits that mediate tonic and phasic currents are expressed in the same neurons (see Belleli et al, 2009) and because we have observed no effect of AIE on phasic GABAAR-mediated currents (Fleming et al, 2012; 2013). In addition, our previous observations that tonic current (Fleming et al, 2013) or tonic noise (Fleming et al, 2012) decreases after AIE in hippocampal neurons indicates that the AIE-induced changes are the result of a cellular process rather than a direct cytotoxic one.
In contrast to the observations in present study that CIE exposure in the adult rat did not alter the expression of any of the GABAAR subunits examined, a number of previous studies have shown that CIE exposure is associated with differential alteration in the expression of GABAAR subunits. For example, older studies involving measurement of total protein expression reported that chronic ethanol exposure decreased levels of α5-GABAAR in the cerebral cortex (Charlton et al 1997), α1-GABAAR and α4-GABAAR in the amygdala (Papadeas et al 2001), as well as increased levels of γ1-GABAAR in the hippocampus (Devaud et al 1997). More recent studies assessing changes in membrane trafficking found that CIE exposure decreased the surface expression of both α1-GABAAR and δ-GABAAR in the nucleus accumbens (Liang et al, 2014), hippocampus (Liang et al, 2007) and amygdala (Lindemeyer et al, 2014), as well as increased expression of γ3-GABAAR, α4-GABAAR and α5-GABAAR in these same studies. Although the reason for the discrepancy of these observations with those in the present study are not clear, there are a number of methodological differences that are likely to be critically important. For example, while our studies focused on examination of the subcellular distribution of subunits by isolating a soluble and DRM fraction, we did not distinguish surface from non-surface expression as was the case in the above studies. Another important factor may relate to differences in the CIE models that were used. We utilized an exposure model that involves 10 intragastric doses of ethanol or saline in a 2-day on, 1-day off, 2 days on, 2 days off sequence for both adolescent and adult exposure in order to allow direct comparisons of the effects on the two age groups of rats. In contrast, studies that observed alterations in GABAAR subunits following chronic alcohol have typically examined exposure over much longer periods of time that are associated with the induction of physical dependence. Thus, it is likely that changes in GABAAR in response to chronic ethanol exposure are a function of not only the pattern of exposure (e.g., intermittent versus continuous), but also the length both of the exposure and withdrawal periods. With respect to the gavage exposure paradigm, it is important to note that we did not include a naïve control group in the present study. That is, all animals received gavage treatments. This leaves open the possibility that the stress associated with the gavage procedure could have influenced the study outcomes; and, therefore, interpretations of the present data must be made with the possibility of stress effects in mind. Because all animals in the present study received equal numbers of gavage treatments, it is likely that any stress related to those treatments would have been experienced by all animals, though we cannot completely rule out an interaction between age and/or ethanol exposure condition and possible gavage-related stress.
The present findings support and expand the emerging view that adolescence is a developmental period during which the brain is distinctively vulnerable to long lasting negative consequences associated with repeated ethanol exposure. More specifically, the results indicate that the long-term expression of specific GABA receptor subunits in the hippocampal formation is altered by AIE, but not by CIE, and this may help to explain why AIE, but not CIE, alters subsequent vulnerability to the effects of acute ethanol on spatial learning (White et al, 2000; Risher et al, 2013a) and why AIE alters the induction of hippocampal LTP and spatial learning in adulthood (Risher et al, 2013b). The specific focus on GABA receptor subunits regulation at mRNA and protein levels in this report adds a mechanistic focus to the important and growing literature on the long-term effects of alcohol exposure during adolescence, and the importance of this developmental period for subsequent hippocampal function and related phenotypes.
Acknowledgments
Grant Support
NIH U01AA019925 to HSS
VA Senior Research Career Scientist Award to HSS
NIH U01AA019971 to SCP
VA Merit Review and Research Career Scientist Award to SCP
NIH U01AA019967, P50AA010761, R01AA010983 to LJC
NIH U01AA020930 to PJM
Veteran’s Affairs CDA 2-010-10S and BX-002128-01 to RLF
Veteran’s Affairs CDA IK2BX001267 to SKA
NIH F31AA022843 to SWC
References
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Acheson SK, Stein RM, Swartzwelder HS. Impairment of semantic and figural memory by acute ethanol: age-dependent effects. Alcohol Clin Exp Res. 1998;22:1437–1442. doi: 10.1111/j.1530-0277.1998.tb03932.x. [DOI] [PubMed] [Google Scholar]
- Acheson SK, Bearson C, Risher M-L, Abdelwahab SH, Wilson WA, Swartzwelder HS. Effects of acute or chronic ethanol exposure during adolescence on behavioral inhibition and efficiency in a modified water maze task. PLOS ONE. 2013;8(10):e77768 1–e77768 15. doi: 10.1371/journal.pone.0077768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J. Neurosci. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, McGue M, Maggs J, Schulenberg J, Hingson R, Swartzwelder HS, Martin C, Chung T, Tapert S, Sher K, Winters K, Lowman C, Murphy S. Underage alcohol use: Summary of developmental processes and mechanisms, ages 16–20. Alcohol Research and Health. 2009;32:41–52. [PMC free article] [PubMed] [Google Scholar]
- Brun VH, Ytterbo K, Morris RG, Moser MB, Moser EI. Retrograde amnesia for spatial memory induced by NMDA receptor-mediated long-term potentiation. J. Neurosci. 2001;21:356–362. doi: 10.1523/JNEUROSCI.21-01-00356.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton ME, Sweetnam PM, Fitzgerald LW, Terwilliger RZ, Nestler EJ, Duman RS. Chronic ethanol administration regulates the expression of GABA-A receptor alpha-1 and alpha-5 subunits in the ventral tegmental area and hippocampus. J. Neurochem. 1997;68:121–127. doi: 10.1046/j.1471-4159.1997.68010121.x. [DOI] [PubMed] [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Otu FM, Howell O, Atack JR, McKernan RM, Seabrook GR, Dawson GR, Whiting PJ, Rosahl TW. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABAA receptor. J Neurosci. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Blüthmann H, Möhler H, Rudolph U. Trace fear conditioning involves hippocampal α5 GABAA receptors. Proc Natl Acad Sci U S A. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr. Opin. Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Dalskov SM, Immerdal L, Niels-Christiansen L, Hansen GH, Schousboe A, Danielsen EM. Lipid raft localization of GABA A receptor and Na+, K+-ATPase in discrete microdomain clusters in rat cerebellar granule cells. Neurochem. Int. 2005;46:489–499. doi: 10.1016/j.neuint.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Fritschy J-M, Sieghart W, Morrow AL. Bidirectional alterations of GABA-A receptor subunit peptide levels in rat cortex during chronic ethanol consumption and withdrawal. J Neurochem. 1997;69:126–130. doi: 10.1046/j.1471-4159.1997.69010126.x. [DOI] [PubMed] [Google Scholar]
- Durand D, Carlen PL. Impairment of long-term potentiation in rat hippocampus following chronic ethanol treatment. Brain Research. 1984;308:325–332. doi: 10.1016/0006-8993(84)91072-2. [DOI] [PubMed] [Google Scholar]
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- Farr SA, Flood JF, Morley JE. The effect of cholinergic, GABAergic, serotonergic, and glutamatergic receptor modulation on post-trial memory processing in the hippocampus. Neurobiol Learn Mem. 2000;73(2):150–167. doi: 10.1006/nlme.1999.3927. (2000). [DOI] [PubMed] [Google Scholar]
- Ferrario C, Loweth JA, Milovanovic M, Wang X, Wolf ME. Distribution of AMPA receptor subunits and TARPs in synaptic and extrasynaptic membranes of the adult rat nucleus accumbens. Neurosci. Lett. 2011a;490:180–184. doi: 10.1016/j.neulet.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Loweth JA, Milovanovic M, Ford KA, Galiñanes GL, Heng LJ, Tseng KY, Wolf ME. Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca2+-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology. 2011b;61(7):1141–1151. doi: 10.1016/j.neuropharm.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming RL, Acheson SK, Moore SD, Wilson WA, Swartzwelder HS. In the rat, chronic intermittent ethanol exposure during adolescence alters the ethanol sensitivity of tonic inhibition in adulthood. Alcohol Clin Exp Res. 2012;36(2):279–285. doi: 10.1111/j.1530-0277.2011.01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming RL, Li Q, Risher ML, Sexton HG, Moore SD, Wilson WA, Acheson SK, Swartzwelder HS. Binge-pattern ethanol exposure during adolescence, but not adulthood, causes persistent changes in GABAA receptor-mediated tonic inhibition in dentate granule cells. Alcohol Clin Exp Res. 2013;37(7):1154–1160. doi: 10.1111/acer.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follesa P, Mostallino MC, Biggio F, Gorini G, Caria S, Busonero F, Murru L, Mura ML, Sanna E, Biggio G. Distinct patterns of expression and regulation of GABA receptors containing the delta subunit in cerebellar granule and hippocampal neurons. J Neurochem. 2005;94(3):659–671. doi: 10.1111/j.1471-4159.2005.03303.x. (2005) [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Brunig I. Formation and plasticity of GABAergic synapses: physiological mechanisms and pathophysiological implications. Pharmacol Ther. 2003;98:299–323. doi: 10.1016/s0163-7258(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Jin Z, Bazov I, Kononenko O, Korpi ER, Bakalkin G, Birnir B. Selective Changes of GABA(A) Channel Subunit mRNAs in the Hippocampus and Orbitofrontal Cortex but not in Prefrontal Cortex of Human Alcoholics. Front Cell Neurosci. 2012;3:5–30. doi: 10.3389/fncel.2011.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroener S, Mulholland PJ, New NN, Gass JT, Becker HC, Chandler LJ. Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS One. 2012;7:e37541. doi: 10.1371/journal.pone.0037541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Ren Q, Beckley JH, O'Buckley TK, Gigante ED, Santerre JL, Werner DF, Morrow AL. Ethanol Activation of Protein Kinase A Regulates GABA(A) Receptor Subunit Expression in the Cerebral Cortex and Contributes to Ethanol-Induced Hypnosis. Front Neurosci. 2012;6:44. doi: 10.3389/fnins.2012.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land C, Spear NE. Ethanol impairs memory of a simple discrimination in adolescent rats at doses that leave adult memory unaffected. Neurobiol Learn Mem. 2004;81:75–81. doi: 10.1016/j.nlm.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Li Q, Fleming RL, Acheson SK, Madison RD, Moore SD, Risher M-L, Wilson WA, Swartzwelder HS. Long-term modulation of A-type K+ conductances in hippocampal CA1 interneurons in rats after chronic intermittent ethanol exposure during adolescence or adulthood. Alcoholism: Clin. Exp. Res. 2013;37(12):2074–2085. doi: 10.1111/acer.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Serwanski DR, Miralles CP, Bahr BA, De Blas AL. Two pools of Triton X-100-insoluble GABAA receptors are present in the brain, one associated to lipid rafts and another one to the post-synaptic GABAergic complex. J Neurochem. 2007;102:1329–1345. doi: 10.1111/j.1471-4159.2007.04635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Suryanarayanan A, Abriam A, Snyder B, Olsen RW, Spigelman I. Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication. J Neurosci. 2007;27:12367–12377. doi: 10.1523/JNEUROSCI.2786-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Marty VN, Mulpuri Y, Olsen RW, Spigelman I. Selective modulation of GABAergic tonic current by dopamine in the nucleus accumbens of alcohol-dependent rats. J Neurophysiol. 2014;112(1):51–60. doi: 10.1152/jn.00564.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemeyer AK, Liang J, Marty VN, Meyer EM, Suryanarayanan A, Olsen RW, Spigelman I. Ethanol-induced plasticity of GABAA receptors in the basolateral amygdala. Neurochem Res. 2014;39(6):1162–1170. doi: 10.1007/s11064-014-1297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Fuchs T, Kilpatrick C. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011;12:385–409. doi: 10.1016/j.neuron.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi M, Kang MH, Tillakaratne N, Tobin AJ, Olsen RW. Chronic intermittent ethanol treatment in rats increases GABA(A) receptor alpha4-subunit expression: possible relevance to alcohol dependence. J Neurochem. 1997;68(6):2485–2492. doi: 10.1046/j.1471-4159.1997.68062485.x. [DOI] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–421. [PubMed] [Google Scholar]
- Monti PM, Miranda R, Jr, Nixon K, Sher KJ, Swartzwelder HS, Tapert SF, White A, Crews FT. Adolescence: booze, brains, and behavior. Alcohol Clin Exp Res. 2005;29:207–220. doi: 10.1097/01.alc.0000153551.11000.f3. [DOI] [PubMed] [Google Scholar]
- Morrisett RA, Martin D, Wilson WA, Savage DD, Swartzwelder HS. Prenatal exposure to ethanol decreases the sensitivity of the adult hippocampus to N Methyl D Aspartate. Alcohol. 1989;6:1–6. doi: 10.1016/0741-8329(89)90013-x. [DOI] [PubMed] [Google Scholar]
- Moser EI, Krobert KA, Moser MB, Morris RG. Impaired spatial learning after saturation of long-term potentiation. Science. 1998;281:2038–2042. doi: 10.1126/science.281.5385.2038. [DOI] [PubMed] [Google Scholar]
- Mulholland PJ, Becker HC, Woodward JJ, Chandler LJ. Small conductance calcium-activated potassium type 2 channels regulate alcohol-associated plasticity of glutamatergic synapses. Biol Psychiatry. 2011;69(7):625–632. doi: 10.1016/j.biopsych.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland PJ, Jordan BA, Chandler LJ. Chronic ethanol up-regulates the synaptic expression of the nuclear translational regulatory protein AIDA-1 in primary hippocampal neurons. Alcohol. 2012;46(6):569–576. doi: 10.1016/j.alcohol.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA Council Approves Definition of Binge Drinking. NIAAA Newsletter. 2004 [Google Scholar]
- Nothdurfter C, Tanasic S, Di Benedetto B, Uhr M, Wagner EM, Gilling KE, Parsons CG, Rein T, Holsboer F, Rupprecht R, Rammes G. Lipid raft integrity affects GABA(A) receptor, but not NMDA receptor modulation by psychopharmacological compounds. International Journal of Neuropsychopharmacology. 2013;16(6):1361–1371. doi: 10.1017/S146114571200140X. [DOI] [PubMed] [Google Scholar]
- Olney J. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969;164:719–721. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- Papadeas S, Grobin AC, Morrow AL. Chronic ethanol consumption differentially alters GABA(A) receptor alpha1 and alpha4 subunit peptide expression and GABA(A) receptor-mediated 36 Cl uptake in mesocorticolimbic regions of rat brain. Alcohol Clin Exp Res. 2001;25:1270–1275. [PubMed] [Google Scholar]
- Riley JN, Walker DW. Morphological alterations in hippocampus after long-term alcohol consumption in mice. Science. 1978;201:646–648. doi: 10.1126/science.566953. [DOI] [PubMed] [Google Scholar]
- Risher M-L, Fleming RL, Boutros N, Semenova S, Wilson WA, Levin ED, Markou A, Swartzwelder HS, Acheson SK. Long-term effects of chronic intermittent ethanol exposure in adolescent and adult rats: radial-arm maze performance and operant food reinforced responding. PLOS ONE. 2013a;8(5):e62940 1–e62940.14. doi: 10.1371/journal.pone.0062940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risher M-L, Morin D, Fleming RL, Wilson WA, Acheson SK, Swartzwelder HS. Chronic intermittent ethanol exposure during adolescence results in long-term structural and functional changes in the adult hippocampus. Society for Neuroscience, San Diego, Nanosymposium, Alcohol and Adolescence (212.04) 2013b [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. (2004). [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Age-dependent inhibition of long-term potentiation by ethanol in immature versus mature hippocampus. Alcoholism: Clin. Exp. Res. 1995a;19:1480–1485. doi: 10.1111/j.1530-0277.1995.tb01011.x. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Differential sensitivity of NMDA receptor-mediated synaptic potentials to ethanol in immature vs. mature hippocampus. Alcoholism: Clin. Exp. Res. 1995b;19:320–323. doi: 10.1111/j.1530-0277.1995.tb01509.x. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Farr K, Wilson WA, Savage DD. Prenatal exposure to ethanol decreases physiological plasticity in the hippocampus of the adult rat. Alcohol. 1988;5:121–124. doi: 10.1016/0741-8329(88)90008-0. [DOI] [PubMed] [Google Scholar]
- Walker DW, Barnes DE, Zornetzer SF, Hunter BE, Kubanis P. Neuronal loss in hippocampus induced by prolonged ethanol consumption in rats. Science. 1980;209:711–713. doi: 10.1126/science.7394532. [DOI] [PubMed] [Google Scholar]
- White AM, Ghia AJ, Levin ED, Swartzwelder HS. Binge pattern ethanol exposure in adolescent and adult rats: differential impact on subsequent responsiveness to ethanol. Alcohol Clin Exp Res. 2000;24:1251–1256. [PubMed] [Google Scholar]
- White AM, Swartzwelder HS. Hippocampal function during adolescence: a unique target of ethanol effects. Ann N Y Acad Sci. 2004;1021:206–220. doi: 10.1196/annals.1308.026. [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Izumi Y. NMDA receptors and metaplasticity: mechanisms and possible roles in neuropsychiatric disorders. Neurosci. Biobehav. Rev. 2012;36:989–1000. doi: 10.1016/j.neubiorev.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]