Abstract
The tight interaction between genomic DNA and histones, which normally represses gene transcription, can be relaxed by histone acetylation. This loosening of the DNA-histone complex is important for selective gene activation during stem cell differentiation. Histone acetylation may be increased through the application of histone deacetylase inhibitors at the early stages of differentiation to modulate lineage commitment. We examined the effects of the histone deacetylase inhibitor valproic acid on the differentiation of pluripotent stem cells into skeletal myocytes. Our data demonstrated that valproic acid can act in concert with retinoic acid to enhance the commitment of stem cells into the skeletal myocyte lineage reinforcing the notion that histone acetylation is important for skeletal myogenesis. Thus, using a combination of small molecules to exploit different signaling pathways pertaining to specific gene programs will allow for modulation of lineage specification and stem cell differentiation in potential cell-based therapies.
Normal skeletal myogenesis and muscle repair require the coordination of a diverse set of cellular events to initiate myogenic differentiation. Many of the transcriptional events that occur during the process of skeletal myogenesis have been established1. They are largely regulated by a group of transcription factors known as myogenic regulatory factors (MRFs), which include Myf5, MyoD and myogenin. While Myf5 and MyoD are involved in the commitment of stem cells to the myogenic fate, myogenin is involved in the terminal differentiation of skeletal myocytes2.
Chromatin organization is also a key regulatory mechanism of stem cell differentiation. Histone modifications alter the accessibility of DNA to the binding of transcription factors such as MRFs3. One such example is histone acetylation, which involves the transfer of an acetyl group to positively charged lysine residues in the histone tails. Many studies have shown that histone acetyltransferase (HAT) activity renders the chromatin more accessible for downstream transcriptional events4,5. Genetic data has also shown that HATs are critical for skeletal myogenesis6, particularly through locus-specific histone acetylation7,8. As such, increased histone acetylation and subsequent activation of gene transcription may contribute to the modulation of stem cell fate decisions.
While HATs effectively relax the chromatin complex, histone deacetylases (HDACs) condense the structure9,10. As a result, elevated levels of histone acetylation may be achieved by using an approach targeting HDAC activity with an HDAC inhibitor, leading to the accumulation of histones in hyper-acetylated states11. The differentiation of pluripotent stem cells into skeletal myocytes occurs at a low frequency and requires developmental cues to stimulate the process12,13. Since histone acetylation is important for myogenic differentiation7,8, enhancing histone acetylation should therefore promote the development of skeletal myocytes.
In this report, we provide evidence supporting this hypothesis by using an HDAC inhibitor approach. We show that using small molecules to exploit signaling pathways underpinning the regulation of gene transcription will allow for control of cell fate decisions.
Results
Effects of small molecules on stem cell differentiation
P19 pluripotent stem cells have been used extensively to study the effects of small molecules on myogenic differentiation. They form embryoid bodies (EBs) readily, but require external signals to induce their differentiation into skeletal myocytes. While retinoic acid (RA) signaling is important, myogenic conversion also requires additional small molecules to reach a high frequency of skeletal myocyte development14,15,16,17. As previously reported, treatment of the EBs with DMSO or RA alone during EB formation produced about 5% skeletal myocytes by day 9 of differentiation, whereas treatment of the EBs with a combination of RA and DMSO increased the rate of myogenic conversion to about 20% (Fig. 1A and B). We previously observed a significant increase in the level of global H3 acetylation in the EBs7. Elevated levels of histone acetylation may be achieved by inhibiting HDAC activity, which results in the accumulation of histones in a hyper-acetylated state. This approach has been used for cardiomyogenesis wherein HDAC activity appears to be integral to cardiac differentiation18. Therefore, increasing the levels of histone acetylation through HDAC inhibition presents an interesting avenue to enhance the differentiation of pluripotent stem cells into skeletal myocyte lineage.
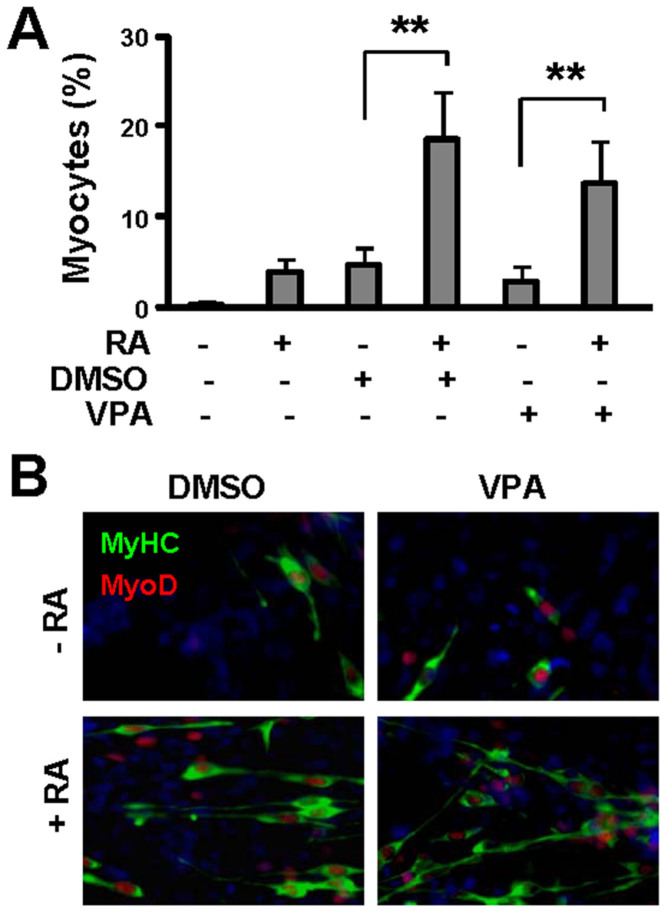
Figure 1. Effects of valproic acid on myogenic differentiation.
(A) Pluripotent P19 cells were grown as EBs for 4 days and treated with DMSO (1%), RA (10 nM) or valproic acid (VPA, 0.5 mM). The cells were cultured for an additional 5 days without treatment and stained for myosin heavy chain and nuclei on day 9 of differentiation before microscopic analysis. Quantification is presented as the percentage of cells differentiated into skeletal myocytes. Error bars are the standard deviations of four independent experiments. Statistical significance is denoted by ** (p< 0.01). (B) Representative microscopic images of myosin heavy chain (MyHC, green), MyoD (red) and nuclei (blue) co-staining.
Valproic acid enhances myogenic conversion
Valproic acid has been used to treat epilepsy and bipolar disorders for decades19,20. It is now classified as an HDAC inhibitor and has been used to generate pluripotent stem cells from primary human fibroblasts21,22,23. We thus examined the effects of valproic acid on myogenic conversion during stem cell fate decisions. When used alone, valproic acid was similar to DMSO and produced about 5% skeletal myocytes (Fig. 1A). However, it achieved a significantly higher efficacy when used in combination with RA, with a rate of myogenic conversion around 15% (Fig. 1A). Also similar to DMSO and RA-mediated myogenic conversion, MyoD, a skeletal muscle regulatory factor, co-stained with myosin heavy chain to the elongated bipolar skeletal myocytes (Fig. 1 B).
Additionally, myogenin, another important muscle regulatory factor, was detected by immunofluorescence microscopy by day 9 of differentiation following co-treatment of the EBs with valproic acid and RA (Fig. 2A). This result was similar to that observed following co-treatment with DMSO and RA (Fig. 2A). The extent of myogenin expression correlated with that of myosin heavy chain as shown by quantitative microscopy (Table 1). Consistent with our previous report16, Western blotting detected myogenin protein by day 9 of differentiation following co-treatment of the EBs with DMSO and RA (Fig. 2B). While the presence of myogenin was also evident following co-treatment with valproic acid and RA, myogenin was not readily detected in cells treated with DMSO or valproic acid alone, likely limited by the sensitivity of the reagents and exposure time (Fig. 2B). Nonetheless, the detection of myogenin protein with Western blotting as a measure of myogenic conversion provides objective means for validating the presence of skeletal myocytes, since the activation of myogenin expression is integral to the terminal differentiation of myoblasts.
Figure 2. Myogenic expression and histone acetylation.
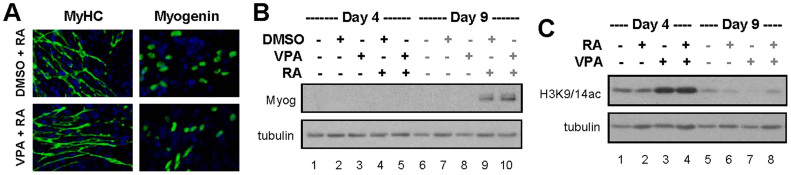
(A) Cells were grown as EBs for 4 days and treated with DMSO (1%), RA (10 nM) or valproic acid (VPA, 0.5 mM). The cells were then cultured for 5 days without treatment, and stained for myosin heavy chain and myogenin in parallel before microscopic analysis. Shown are representative images of myosin heavy chain (MyHC, green), myogenin (green) and nuclei (blue) co-staining. (B) Myogenin protein expression was examined by Western blotting on day 4 and day 9 of differentiation. β-tubulin was used as a loading control. Shown are the cropped blot images representing indicated proteins. Full-length blots are presented in the Supplementary Figure S1A. (C) The levels of H3K9/14 acetylation (H3K9/14ac) were examined by Western blotting. Shown are the cropped blot images representing indicated proteins. Full-length blots are presented in the Supplementary Figure S1B.
Table 1. Microscopic analysis of myogenin and myosin heavy chain expression. P19 pluripotent stem cells were grown as EBs for 4 days and treated with DMSO, valproic acid (VPA, 0.5 mM), or in combination with RA (10 nM). The cells were then cultured for 5 days without treatment, and stained for myosin heavy chain (MyHC) and myogenin in parallel before microscopic analysis. Untreated cells (Control) were also included.
| Myogenic conversion indicated by MyHC stain (% ± SD) | Myogenic conversion indicated by myogenin stain (% ± SD) | |
|---|---|---|
| Control | 0.66 ± 0.48 | 0.15 ± 0.02 |
| DMSO + RA | 24.51 ± 1.61 | 20.59 ± 2.23 |
| DMSO | 4.02 ± 2.48 | 3.27 ± 1.48 |
| RA | 2.84 ± 0.73 | 3.56 ± 2.05 |
| VPA | 2.88 ± 2.25 | 2.26 ± 2.23 |
| VPA + RA | 17.85 ± 1.07 | 15.83 ± 0.33 |
Notably, valproic acid treatment increased global H3K9/14 acetylation in the EBs (Fig. 2 C). Thus, valproic acid, just like DMSO, can act in concert with RA to enhance myogenic conversion at the early stages of lineage specification, further highlighting the importance of histone acetylation in the specification of skeletal muscle lineage.
Discussion
We examined the effects of a histone deacetylase inhibitor on the differentiation of pluripotent stem cells into skeletal myocytes. Co-treatment of stem cells with a small molecule that inhibits HDAC activity, such as valproic acid, leads to enhancement of myogenic conversion reinforcing the notion that histone acetylation is important for skeletal myogenesis.
Interestingly, when the HDAC inhibitor valproic acid was applied in combination with RA, a known regulatory signal of the skeletal muscle lineage16,17, the rate of myogenic differentiation was enhanced to a greater extent than the sum of each treatment alone (Fig. 1–2). This is possibly due to the ability of valproic acid to increase histone acetylation and consequently facilitate RA responsive receptors to dissociate from co-repressors and recruit co-activators24,25 such as p300, which is important for activating the expression of muscle regulatory factors including Myf5 and MyoD6,7,8.
While valproic acid may facilitate myogenic conversion by enhancing histone acetylation, we cannot exclude the possibility that an increase in the acetylation of non-histone proteins or other targets of valproic acid may also contribute to the positive effect of valproic acid on specification of the skeletal muscle lineage. Nevertheless, the results of our study suggest that HDAC inhibition may provide a useful avenue to develop safe non-toxic protocols that enhance the development of skeletal myocytes, based on fundamental insights into myogenic differentiation. Notably, the HDAC inhibitor used in our study has track records for its safe therapeutic applications.
The mechanism by which DMSO enhances myogenic differentiation is not well understood, but DMSO treatment increases intracellular stores of calcium in a variety of cell types including P19 pluripotent stem cells26. In addition, DMSO treatment induce genome wide epigenetic changes by altering DNA methylation27. It has been established for years that RA signaling is associated with chromatin change and lineage commitment, but little is known as to how RA signaling mediates chromatin changes during lineage specification and stem cell differentiation. Apparently, histone deacetylase inhibitors can act in concert with RA signals to direct lineage commitment in a chromatin environment. Understanding the molecular mechanisms of these interactions will help us develop the best strategy to reprogram differentiated cells or to direct the differentiation of pluripotent stem cells.
Methods
Cell culture and reagents
The P19 pluripotent stem cells (ATCC) were maintained in Dulbecco's Modified Eagle Medium containing 5% fetal bovine serum and 5% bovine calf serum at 37°C and 5% CO2. To allow differentiation to occur, the cells were first grown in Petri dishes to form EBs for 4 days, at which time the EBs were plated onto coverslips or tissue culture dishes for an additional 5 days without treatment7. All-trans retinoic acid (RA) was dissolved in ethanol and stored at −20°C. Valproic acid was dissolved in water and stored at 4°C. Addition of valproic acid from a 0.3 M stock solution to the culture medium to obtain a treatment concentration of 0.5 mM did not affect the pH of the medium. Dimethyl sulfoxide (DMSO) was stored at room temperature. All chemicals were from Sigma-Aldrich.
Immunofluorescence microscopy
Following culture on coverslips, the cells were washed with phosphate buffered saline (PBS), fixed in methanol, rehydrated with PBS and incubated with antibodies against myosin heavy chain, MyoD or myogenin at 4°C overnight. The cells were then washed with PBS and incubated with secondary antibodies Alexa Flor®488 goat anti-rabbit and Alexa Flor®594 donkey anti-mouse (Invitrogen, A-11008 and A-21203) for 45 minutes in darkness at room temperature. The cells were also incubated with Hoechst (0.5 mg/ml, Molecular Probes) for 5 minutes in darkness to stain the DNA. The coverslips were then mounted on slides in 50% glycerol28. Cell images were captured with the Axiovert 200M microscope (Zeiss), AxioCam HRM camera (Zeiss) and AxioVision Rel 4.6 software (Zeiss) through different filters29. For each coverslip, about 100 fields of view were analyzed and the extent of differentiation was estimated based on the percentage of cells that stained positively for myogenic markers such as myosin heavy chain or myogenin relative to the total cell population as determined by Hoechst staining16. Student t-tests were used for statistical analysis. The mouse myosin heavy chain and myogenin monoclonal antibodies were produced respectively by MF20 and F5D hybridomas grown in the lab16. The MyoD rabbit polyclonal antibody was from Santa Cruz Biotechnology (SC-760).
Whole-cell extract and Western analysis
After 4 or 9 days of differentiation, cells were washed with PBS, harvested and centrifuged at 3,000 g for 3 minutes at 4°C. The cell pellets were suspended and incubated at 4°C for 30 minutes in whole-cell extract buffer containing 50 mM Tris-HCl (pH 7.6), 400 mM NaCl, 10% glycerol, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, and 1% nonidet P-40 (NP-40). Following incubation, the cells were centrifuged at 14,000 g for 20 minutes at 4°C. Protein concentrations of the supernatants were determined using the Bradford assay (Bio-Rad). Western blotting with the indicated antibodies were visualized using Western Lightning™ Chemiluminescence reagents8. The H3K9/14ac rabbit polyclonal antibody was from Santa Cruz Biotechnology (SC-8655). β-tubulin antibody was described previously16.
Author Contributions
Q.L. and J.C. designed the research, interpreted the data and prepared the manuscript. M.F. executed microscopy and Western analyses. J.C. participated in the microscopy and Western analysis. All authors reviewed the manuscript.
Supplementary Material
Supplementary Figure
Acknowledgments
This work was sponsored by an operating grant from Natural Sciences and Engineering Research Council of Canada (to Q.L.).
References
- Tapscott S. J. The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development 132, 2685–95 (2005). [DOI] [PubMed] [Google Scholar]
- Francetic T. & Li Q. Skeletal myogenesis and Myf5 activation. Transcription 2, 109–14 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polesskaya A. et al. CBP/p300 and muscle differentiation: no HAT, no muscle. EMBO J 20, 6816–25 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl B. D. & Allis C. D. The language of covalent histone modifications. Nature 403, 41–5 (2000). [DOI] [PubMed] [Google Scholar]
- Visel A. et al. ChIP-seq accurately predicts tissue-specific activity of enhancers. Nature 457, 854–8 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. F. et al. Differential role of p300 and CBP acetyltransferase during myogenesis: p300 acts upstream of MyoD and Myf5. EMBO J 22, 5186–96 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francetic T. et al. Regulation of Myf5 early enhancer by histone acetyltransferase p300 during stem cell differentiation. Mol Biol 1, 103. 10.4172/2168-9547.1000103 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamed M., Khilji S., Chen J. & Li Q. Stepwise acetyltransferase association and histone acetylation at the Myod1 locus during myogenic differentiation. Sci Rep 3, 2390 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. & Li Q. Life and death of transcriptional co-activator p300. Epigenetics 6, 957–61 (2011). [DOI] [PubMed] [Google Scholar]
- Xu L., Glass C. K. & Rosenfeld M. G. Coactivator and corepressor complexes in nuclear receptor function. Curr Opin Genet Dev 9, 140–7 (1999). [DOI] [PubMed] [Google Scholar]
- Chen J., Ghazawi F. M., Bakkar W. & Li Q. Valproic acid and butyrate induce apoptosis in human cancer cells through inhibition of gene expression of Akt/protein kinase B. Mol Cancer 5, 71 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. & Li Q. Enhancing myogenic differentiation of pluripotent stem cells with small molecule inducers. Cell Biosci 3, 40 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. & Li Q. Enhancing myogenic differentiation of pluripotent stem cells with small molecule inducers. Cell Biosci 3, 40 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. K., Harris J. F. & McBurney M. W. Induced muscle differentiation in an embryonal carcinoma cell line. Mol Cell Biol 3, 2280–6 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobus A. M. et al. Retinoic acid accelerates embryonic stem cell-derived cardiac differentiation and enhances development of ventricular cardiomyocytes. J Mol Cell Cardiol 29, 1525–39 (1997). [DOI] [PubMed] [Google Scholar]
- Le May M. et al. Contribution of Retinoid X Receptor Signaling to the Specification of Skeletal Muscle Lineage. J Biol Chem 286, 26806–12 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Le May M., Lacroix N. & Chen J. Induction of Pax3 gene expression impedes cardiac differentiation. Sci Rep 3, 2498 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamboulas C. et al. HDAC activity regulates entry of mesoderm cells into the cardiac muscle lineage. J Cell Sci 119, 4305–14 (2006). [DOI] [PubMed] [Google Scholar]
- Loscher W. Valproate: a reappraisal of its pharmacodynamic properties and mechanisms of action. Prog Neurobiol 58, 31–59 (1999). [DOI] [PubMed] [Google Scholar]
- Johannessen C. U. & Johannessen S. I. Valproate: past, present, and future. CNS Drug Rev 9, 199–216 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D. et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol 26, 1269–75 (2008). [DOI] [PubMed] [Google Scholar]
- Phiel C. J. et al. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem 276, 36734–41 (2001). [DOI] [PubMed] [Google Scholar]
- Kramer O. H. et al. The histone deacetylase inhibitor valproic acid selectively induces proteasomal degradation of HDAC2. EMBO J 22, 3411–20 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higazi A., Abed M., Chen J. & Li Q. Promoter context determines the role of proteasome in ligand-dependent occupancy of retinoic acid responsive elements. Epigenetics 6, 202–211 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth F. J., Seaver K. J., Fishburn A. L., Htet S. L. & Tapscott S. J. In vitro transcription system delineates the distinct roles of the coactivators pCAF and p300 during MyoD/E47-dependent transactivation. Proc Natl Acad Sci U S A 101, 11593–8 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley P. & Whitfield J. F. The differentiation inducer, dimethyl sulfoxide, transiently increases the intracellular calcium ion concentration in various cell types. J Cell Physiol 156, 219–25 (1993). [DOI] [PubMed] [Google Scholar]
- Iwatani M. et al. Dimethyl sulfoxide has an impact on epigenetic profile in mouse embryoid body. Stem Cells 24, 2549–56 (2006). [DOI] [PubMed] [Google Scholar]
- Chen J., Halappanavar S. S., St-Germain J. R., Tsang B. K. & Li Q. Role of Akt/protein kinase B in the activity of transcriptional coactivator p300. Cell Mol Life Sci 61, 1675–83 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Germain J. R., Chen J. & Li Q. Involvement of PML nuclear bodies in CBP degradation through the ubiquitin-proteasome pathway. Epigenetics 3, 342–9 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure