Abstract
Helminthic infection has become rare in highly industrialized nations. Concurrent with the decline in helminthic infection is an increase in prevalence of inflammatory disease. Removal of helminths from our environment and their powerful effects on host immunity may have contributed to this increase. Several different helminth species can abrogate disease in murine models of inflammatory bowel disease, type 1 diabetes, multiple sclerosis and other conditions. Helminths evoke immune regulatory pathways often involving dendritic cells, Tregs and macrophages that help control disease. Cytokines such as IL4, IL10 and TGFβ have a role. Notable is helminthic modulatory effect on innate immunity, which impedes development of aberrant adaptive immunity. Investigators are identifying key helminth-derived immune modulatory molecules that may have therapeutic utility in the control of inflammatory disease.
Keywords: Helminths, dendritic cells, IBD, Treg, macrophage, autoimmunity
Introduction
Helminths are worm like animal parasites that have adapted over many millions of years to live in the gastrointestinal tract, blood, lungs or other tissues of various species. Their long-term survival requires intricate regulatory interactions between parasite and host immunity. In developed countries, the 20th Century brought unprecedented advancements in living standards associated with substantial improvements in both agricultural practices as well as water and food quality. This disrupted the life-cycle of various helminths leading to de-worming of the population. The long-standing close association between these parasites and their specific hosts has perhaps led to immune interdependency through the process of co-evolution. Epidemiologic data and animal experimentation suggest that elimination of helminths contributes to the increasing prevalence of some immune-mediated diseases in regions with ever-improving sanitation. Diseases of increasing frequency include ulcerative colitis, Crohn’s disease, Type 1 diabetes (T1D), multiple sclerosis (MS), rheumatoid arthritis (RA), asthma, food allergy and perhaps others. Studies, mostly in animal models of human disease, are providing insight into how helminths mediate protection from these conditions.
Inflammatory Bowel Disease
Inflammatory bowel disease (IBD) is the collective term for ulcerative colitis and Crohn’s disease. These diseases probably are the consequence of an inappropriately aggressive mucosal adaptive immune response to substances normally in the intestinal lumen. IBD became a significant health problem in highly developed countries in the 20th Century and presently is spreading in underdeveloped countries (1,2). Epidemiologic studies and clinic trails suggest that natural helminthic infection protects people from IBD (3).
Mechanisms of regulation
Helminths modulate intestinal inflammation through activation of interactive immune regulatory circuits involving regulatory T cells (Tregs), dendritic cells (DCs), macrophages and several cytokines (Figure 1). There are many different helminth species inhabiting different regions of their host. Some have complex life-cycles traveling through the blood stream and/or various tissues of the body, while others enter through the mouth and just stay in the lumen of gastrointestinal tract. Many only inhabit a very limited range of hosts. One may expect with wide diversity among the species, that these organisms developed distinctively creative ways to modulate host immunity. Thus, it is surprising that evolution has endowed a number of them with similar approaches to quell host immunity.
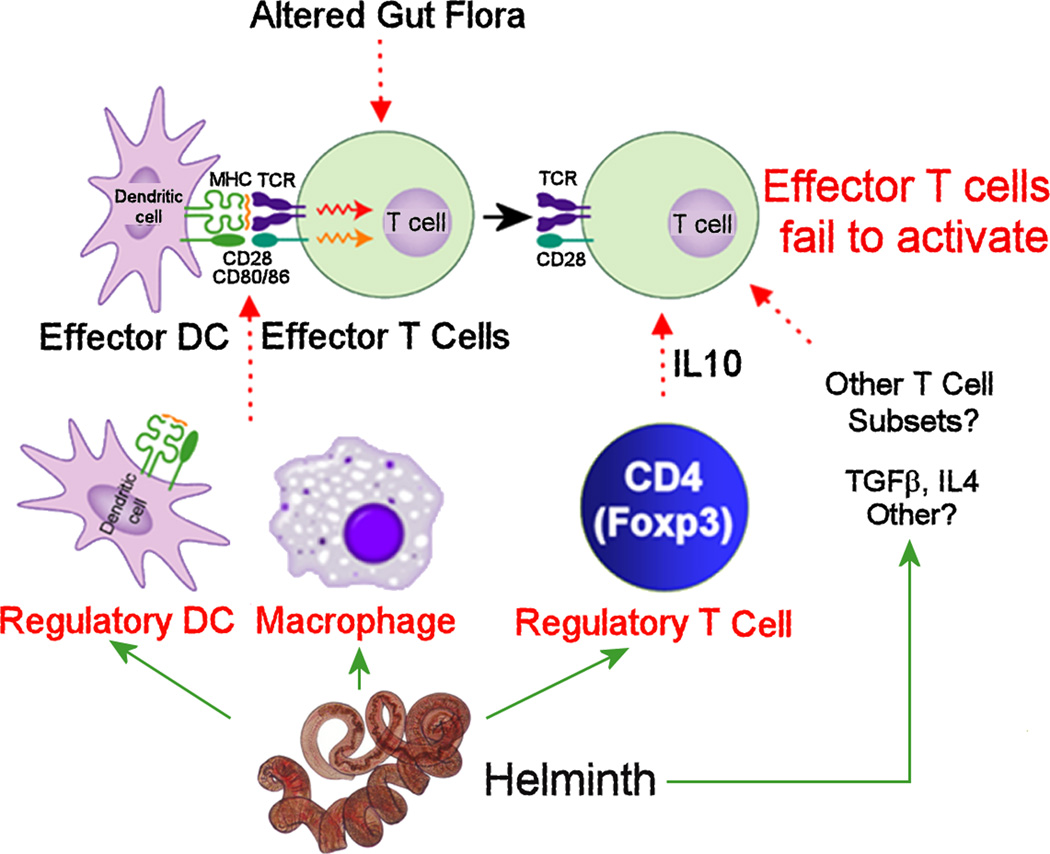
Figure 1. Helminths activate regulatory circuits that limit inflammation in IBD.
IBD results from over-responsiveness of adaptive immune pathways to normal constituents of intestinal contents. Hpb infection induces regulatory DCs and macrophages, and activates Tregs (CD4+ Foxp3+) in the gut to inhibit effector T cell responses. IL10 coming from intestinal Tregs is particularly important. TGFβ and IL4 also participate in the regulation. Intestinal helminthic infections alter the composition of gut flora. Although yet unproven, changes in intestinal flora could impact mucosal immune function leading to protection from IBD.
Regulatory T cells and cytokines
Animal models of IBD suggest that Tregs help prevent excessive intestinal inflammation (4). The murine gut harbors large numbers of Foxp3+ CD4+ Tregs. The colon and terminal ileum contain most of the intestinal flora. In the distal bowel about 25% of the lamina propria CD4+ T cells express Foxp3, and the Foxp3+ T cells are the major source of IL10 (5). These cells likely function to restrain the host immune response to the normal intestinal flora.
Heligmosomoides polygyrus bakeri (Hpb) is a luminal murine helminth that lives in the proximal small bowel with only the larval stages superficially invading the epithelial lining. This parasite expands the number of Foxp3+ T cells in the mesenteric lymph nodes (MLN) (6, 7) and intestinal lamina propria of its murine host (5). The co-stimulatory receptor ICOS assists this Treg expansion (8). This helminth also “activates” Tregs making them highly regulatory (5). Rag mice reconstituted with CD25−CD4+ T cells develop intestinal inflammation due to lack of Tregs. Foxp3+ T cells in intestines of healthy wild-type mice are not very regulatory and afford no protection from colitis when transferred into this model of IBD. However, Foxp3+ T cells isolated from the colon, terminal ileum or MLN of Hpb-infected WT mice populate the gut and MLNs of the Rag recipients more readily and prevent the disease (5).
Hpb infection induces intestinal Tregs to express several genes as revealed using microarray and rtPCR analysis, and/or ELISA. Among these include IL10 (5) and GATA3 (Weinstock, unpublished). The latter is noteworthy, since GATA3 is required for Tregs to accumulate at sites of inflammation. Moreover, it helps sustain high level Foxp3 and IL10 expression, which are needed for Tregs to protect mice from colitis (9–11).
In the CD25−CD4+ T cell transfer model of IBD, the Foxp3+IL10+ CD4+ T cell subset is essential for controlling the disease (5, 12–14). IFNγ is a driver of colitis in most IBD models. In the gut, the Hpb-activated Tregs control colitis partly through secretion of IL10, which inhibits production of IFNγ from mucosal effector T cells. Other mechanisms of action are likely as well. As with Hpb infection (15), other helminthic species like Hymenolepis diminuta (16) and Schistosoma mansoni (17) also induce IL10 secretion. Litomosoides sigmodontis suppressed B cells responses in the host through induction of IL10 and Tregs (18, 19).
Helminthic induction of IL10 synthesis is not an essential factor for controlling IBD. Helminths still prevent colitis and suppress ongoing disease in IL10−/− mice (6, 20). This suggests that helminths activate other important immune regulatory pathways that are IL10/Treg independent.
Helminths also stimulate various immune cell types to produce other cytokines that hinder the development of T cell subtypes implicated in IBD pathogenesis. Hpb infection stimulates the mucosa to make TGFβ. Transgenic mice producing T cells with disrupted TGFβ receptor signaling develop colitis. In this transgenic mouse, Hpb infection cannot dampen the mucosal Th1 response or prevent colitis (21). This shows that Hpb regulation of mucosal inflammation requires T cells that respond to TGFβ.
Helminths trigger Th2-type responses which have a role in colitis prevention. Trinitrobenzene sulfate (TNBS) mixed with alcohol and given rectally induces murine colitis. Helminths, like S. mansoni and Hpb, protect mice from TNBS colitis by limiting the colonic IFNγ and IL12 response. Helminths stimulate the expansion of Th2 cells that make IL4. Disruption of the Th2 pathway enhances Th1 cell differentiation and colitis showing the importance of Th2 cytokines for disease control in this model (17). Isolated helminth products can stimulate these pathways. For example, exposure to schistosome-derived recombinant glutathione S transferase (P28GST) decreases TNBS-colitis, inhibits T cell IFNγ and promotes IL4 and IL10 production (Monique Capron personal communication). There is a colitis model driven by Th2-type cytokines (oxazolone-induced colitis) in which infection with H. diminuta makes the inflammation worse (22). However, helminthic infections can curtail allergic reactions driven by the Th2 pathway (see below). Thus, there are mechanisms of regulation independent of Th2 cytokines.
IL17 has a role in driving colitis. Hpb blocks IL17 secretion partly through stimulating IL4 production, and to some extent IL10, which affects Th17 cell function (23). Disruption of Stat6-signaling specifically in T cells negates the ability of Hpb infection to reverse established CD25lo T cell transfer colitis and inhibit IL17 production (Elliott in preparation). Exposure to helminths also dampens MLN T cell responsiveness to IL6 through suppression of T cell IL6Ra expression and induction of SOCS3 (Elliott in preparation). Induction of Th2 circuits and suppression of IL6/Stat3 signaling constrain Th17 activity.
CD8+ T cells also may have some role in helminthic control of IBD. After Hpb infection, regulatory CD8+ T cells can reduce the severity of colitis. They inhibit T lymphocyte proliferation through direct cell contact and Class I MHC interactions without the need of IL10 or TGFβ (24). CD8+ Tregs are implicated in the control of several diseases featuring immune dysregulation (25, 26).
Regulatory dendritic cells
Using another model of colitis, it was shown that helminths also control IBD through activation of intestinal regulatory DCs. T and B cell-deficient Rag mice reconstituted with IL10−/− T cells develop colitis because their Tregs cannot make IL10. Rag mice infected with Hpb and then de-wormed with a pharmaceutical agent before IL10−/− T cell reconstitution are protected from colitis (20). Moreover, the intestinal mucosa makes less colitogenic cytokines like IFNγ and IL17 after this brief Hpb exposure even if the animals are configured to remain free from colitis. This infers that Hpb can act through cells of innate immunity to render animals resistant to disease.
The mechanism underlying this protection involves induction of regulatory DCs in the intestinal mucosa (20). Activation of these cells does not require the aid of T or B cells. Compared to DCs from uninfected animals, intestinal DCs after Hpb infection only weakly support antigen-driven IFNγ secretion. Furthermore, DCs isolated from the intestines or MLN of Hpb-infected Rag mice transferred into colitis-susceptible block colitis and mucosal antigen-induced, IFNγ and IL17 responses (27).
The mechanism through which these regulatory DCs quell colitis is partly characterized. The regulatory DCs do not prevent effector T cells from populating the gut or MLN, but they do inhibit their function. The regulatory DCs, through a cell contact-dependent mechanism, interfere with the interaction of pro-inflammatory DCs with their effector T cell counterparts. This prevents antigen-induced, IFNγ and IL17 secretion. IL4, IL10 and TGFβ as well as Tregs are not essential for this regulatory process (27).
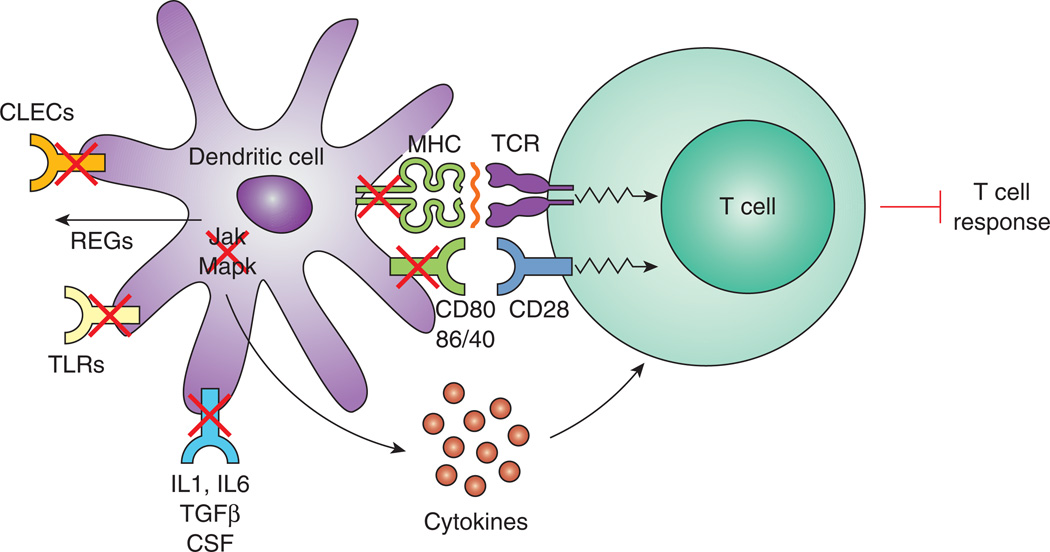
Hpb residing in the proximal bowel induces substantial changes in the activation state of DCs residing in the distal intestine (Figure 2). Microarray analysis shows substantial down-modulation of Jak1/2 and other intracellular signaling pathways (several Map kinases) important for induction of proinflammatory cytokines that drive effector T cell activation. Also, reduced are many components of the MHC antigen presenting complex (e.g. CD40, CD80, CD86, H2). In addition, the DCs display decreased expression for molecules associated with the receptor signaling pathways for IL1, CSF, IL6 and TGFβ (20), and Weinstock unpublished).
Figure 2. Effects of Hpb infection on the function of DCs.
Hpb infection blocks CLEC and TLR expression and promotes REG secretion in gut DCs. The infection also inhibits DC intracellular signaling pathways (Jak 1 and 2, and several MapK) important for proinflammatory cytokine production. There also is disruption in the signaling pathways for IL1, IL6, TGFβ and CSF. The MHC complex is down-modulated as well (CD40, CD80, CD86, MHCII). As a result of these changes, intestinal DCs are less able to activate effector T cells.
An intriguing discovery is the effect of Hpb infection on intestinal DC innate immune receptor expression. DCs are the critical link between innate and adaptive immunity (28). They sample antigens in the intestinal lumen. They then present these antigens to T cells inducing their differentiation and proliferation, or perhaps rendering them inert. DCs sense threats in the environment through germ-line encoded, pattern recognition receptors that bind motifs on bacteria, fungi, viruses or stressed hosts cells. Engagement of these receptors on or in DCs alters their function. There are four families of such receptors that include the Toll-like (Tlr) and C-type lectin receptors (CTLR). Microarray analysis revealed that the intestinal DC expressed several Tlr, and Hpb infection decreased expression of several Tlr subtypes (9 and 13). Also, there is down-modulation of LPS binding protein (LBP) and CD14, important components of the Tlr4 signaling complex (Figure 2).
Two classes of CTLR are REGs and CLECs. REG receptors are secretory proteins that act on the structure bearing the ligand without modulating the function of their cell of origin (29). Secretory REGs, like 3b and 3g, bind intestinal bacteria and other organisms leading to their demise (30, 31). REG−/− mice show the importance of some of these REGs (30). Hpb infection greatly increases expression of all REGS (REG1, 3a, 3b, 3g, 4) displayed by intestinal DCs. High REG protein secretion by intestinal DCs would help keep organisms away from the DC membrane. REG4 has anti-apoptosis properties that protect host cells from death (32, 33).
Host cells express CLECs as transmembrane proteins. When CLECs engage their ligands, the receptors trigger intracellular signaling pathways to alter cell function (34). CLEC 7A engages components of fungi and some bacteria, while CLEC 9A binds dead or dying cells (35). Some CLEC receptors, like CLEC7A and 9A, when engaged activate DCs promoting T cell activation (35). Hpb infection profoundly inhibits expression of nearly all the CLECs expressed by the intestinal DCs (4N, 7A, 9A and 12A). Therefore, decreased CLEC (e.g. 7A, 9A) expression associated with heightened REG secretion makes it less likely that organisms and necrotic cells will approach DCs and encounter membrane bound, pro-inflammatory CLEC receptors. Thus, helminthic regulation of Tlr and C-type lectin receptors (CLECs and REGs) may render intestinal DCs less likely to activate adaptive immunity and subsequently IBD.
Regulatory macrophages
Helminths also protect from IBD through induction of alternatively activated macrophages. Helminths induce the production of IL10 and Th2 cytokines like IL4 and IL5, which activate macrophages in distinct ways (36). These alternatively activated macrophages make IL10, TGFβ and other immunomodulatory factors that can modulate Th1-type inflammation (37).
Another model of IBD is dextran sodium sulfate (DSS)-induced enteritis. DSS administered orally to rodents damages the intestinal epithelial lining inducing gut inflammation.
Infection of BALB/c mice with S. mansoni protects from DSS-induced injury through induction of regulatory macrophages. The adult schistosome flukes, living in the portal vein, induce this protection. The protective process does not require Tregs or regulatory cytokines like TGFβ and IL10 (38).
A cysteine protease inhibitor (cystatin) of filarial nematodes protects mice from DSS colitis (39). Macrophages and IL10 are necessary for this protection as suggested by a lung inflammatory model. Cystatin activates intracellular signaling pathways like ERK and p38, which induce macrophages to make IL10 and IL12p40 (40).
In the IL10−/− Rag model of IBD, Hpb infection induces regulatory macrophages in the gut of the Rag mice. These cells are induced even if mice are not reconstituted with T or B cells. Thus, this induction does not require participation of adaptive immunity. These intestinal macrophages inhibit antigen-induced, IL17 and IFNγ secretion by a contact-dependent mechanism. Also, when transferred into Rag mice, they protect animals from colitis (Weinstock, et. al., unpublished).
In another study using dinitrobenzene sulfonic acid (DNBS) instead of TNBS to induce IBD, infection with H. diminuta protects mice from colitis through induction of alternatively activated macrophages in the colon. Alternatively activated macrophages transferred into mice protects the animals from DNBS-induced injury attesting to their role in the regulatory process. Extracts from H. diminuta worms injected i.p. also provides protection and suppresses macrophage function in vitro (41, 42).
Thus, macrophages, activated by helminth infection, can suffice to protect from IBD (42). This also suggests that some helminths make soluble factors that can mediate this process in lieu of live organisms. In the DNBS model, alternatively activated macrophages work through an IL10-dependent mechanism to control colitis (43).
Communication with the host and penetrating the epithelial barrier
To modulate colitis, helminths must release soluble factors or in some other fashion communicate with the host. The presence of worm-derived soluble factors is supported by experiments that use extracts from H. diminuta worms (41) or dead schistosome ova to protect mice from colitis (17). Helminths produce a number of products with immune modulatory properties (7, 44–49). For instance, helminths produce molecules that induce Tregs (7). To induce regulatory cells, intestinal helminths must breach the mucosal barrier to engage the immune system. This communication may occur through several possible mechanisms.
DCs advance dendrites across the epithelial barrier, which could permit intestinal helminths in the intestines to directly communicate with these cells. Supporting this hypothesis are data showing that Hpb and other helminths release factors that affect the state of DC activation (49, 50). This, in turn, can result in decreased antibody responses (50) and stimulation of Treg development.
Direct interactions between intestinal helminths and the gut epithelium is another possible mechanism of action. The intestinal epithelium releases regulatory molecules and sits close to immunocytes. Infection with Trichuris muris stimulates intestinal epithelial cells to make thymic stromal lymphopoietin, which can interact with the lamina propria DCs promoting a Th2 response and worm expulsion. It also limits IL12 and IFNγ production in DSS-induced colitis, reducing pathology (51).
While some intestinal helminths have no fixed association with the epithelial lining, others closely interact with the mucosal barrier (e.g. hookworm) or place holdfasts beneath the epithelial lining (e.g. whipworm). This affords further access for direct communication with T cells to induce Tregs (7), or with other cells to promote regulation (49).
Gut bacteria are important for the health of the mucosal immune system and readily interact with intestinal DC and other cells (52). Hpb infection modifies the distribution and abundance of some intestinal bacteria. There is an increase in Lactobacillaceae. Various bacterial species within this group inhibit intestinal inflammation in models of colitis (53). Rhesus monkeys develop colitis. Trichuris trichiura infection results in a milder colitis associated with reduced bacterial attachment to the epithelial surface, and changes to the composition of microbial communities attached to the intestinal mucosa (54).
Helminths also may protect via enhancement of mucosal barrier function (55). Trichuris infection stimulates IL22 production in the mucosa, which is a molecule associated with epithelial repair and enhancement of the overlying mucous layer (54, 56).
There are helminth species that suppress colitis while living in regions of the host distant from the intestines. Their mode of communication with host immunity could be different. For example, the filaria Brugia malayi resides in lymphatics and releases copious amounts of asparaginyl-tRNA synthetase, which can block IL8 signaling in human and murine leukocytes and can suppress murine T cell transfer colitis (57).
In summary, animal models suggest that helminths control colitis via induction of several distinct immune regulatory pathways. This includes promotion of Treg function through induction of Gata3, IL33R and IL10 expression, generation of regulatory DCs with a unique phenotype (Figure 2) and induction of regulatory macrophages. Also, it appears that IL4 and TGFβ, and their signaling pathways, have a role. However, these regulatory pathways are not necessary called into play simultaneously with similar importance in each distinct IBD animal model.
Helminths and Other Immune Mediated Diseases
Genome-wide association studies have demonstrated susceptibility gene overlap between IBD and other autoimmune and immune-mediated inflammatory diseases (58). Like IBD; MS, T1D, RA, and asthma have emerged in populations benefiting from advanced socioeconomic development. This suggests that those environmental factors that impact immune pathways and increased the risk for IBD, also have increased the risk for other immune-mediated illnesses. Animal models of these organ-specific inflammatory diseases, show that many of the helminth induced regulatory circuits that regulate murine colitis suppress inflammation in these diseases as well.
Animal models of MS
Mice or rats immunized with myelin-associated antigens develop autoimmune encephalitis (EAE), a model of MS (59). Mice exposed to viable S. mansoni or dead ova are protected from developing EAE (60, 61). Schistosome exposure suppresses splenocyte and CNS cell production of IL12p40, IFNγ, and TNFα while increasing TGFβ, IL10, and IL4. Infection with Hpb (62) or Fasciola hepatica (63), or treatment with soluble T. suis adult or larval Trichinella spiralis homogenate (64) also suppresses EAE disease scores with similar changes in cytokine profile. T. spiralis infection also affords protection in a rat EAE model (65). Draining popliteal lymph node cells from parasite-exposed rats produce less IFNγ and IL17 and more IL10 and IL4 in response to concanavalin-A stimulation compared to cells from helminth-naïve animals. Infection also increases the number of CD4+CD25+Foxp3+ T cells in the spleen. Adoptive transfer of splenic T cells from infected rats into helminth-naïve rats protects recipients from developing EAE (65).
As discussed above, helminths produce factors that mediate this protection. Adoptive transfer of bone marrow-derived DCs exposed to excretory/secretory products from cultured T. spiralis muscle cyst larvae also protects against EAE (66). Protection is associated with decreased DC IL12p70 and increased DC IL10 production. Splenocytes from rats that receive helminth product exposed-DCs prior to EAE challenge have more Foxp3+ T cells, make less IL17A and IFNγ, and produce more IL4, IL10 and TGFβ than splenocytes from rats that receive medium-alone exposed DC (66).
Infection with Taenia crassiceps also inhibits development of EAE (67). Inhibition is associated with suppression of TNFα and induction of alternatively activated macrophages. Factors released by Taenia crassiceps cysticerci impair LPS-stimulated bone marrow-derived IL12 and TNFα production in a cRAF dependent manner (68).
Clinical studies involving patients with MS from helminth-endemic areas show that patients with active helminthic infections have attenuated disease compared to uninfected MS patients. Treatment of helminthic infections results in worsening MS activity associated with an increase in the fraction of PBMC making IFNγ and IL12, and a decrease in the fraction producing IL10 and TGFβ (69). Helminth removal also decreases the frequency of circulating CD4+CD25+Foxp3+ T cells. Patients with MS and active helminth infections had increased frequency of spinal fluid Foxp3+ Treg cells and higher serum retinoic acid levels as compared to healthy controls or uninfected MS patients (70). Exposure of PBMC-derived DC to soluble schistosome egg antigens (SEA) induced enzymes involved in retinoic acid synthesis likely by a Tlr2-activation dependent pathway. LPS-stimulated DCs derived from PBMC of helminth-infected patients made less IL6, IL12p70, IL23, and TNFα than DCs from uninfected patients and levels were further reduced, in a SOCS3-dependent fashion, by exposure to SEA. SEA-exposed DCs co-cultured with autologous CD25− T cells reduces T cell STAT3 activation while increasing SMAD3 activation and Foxp3 expression (70).
These studies show that helminths can suppress other organ-specific inflammatory diseases beyond colitis. In addition, they show that Infection with diverse helminths (nematodes, trematodes, and cestodes) can suppress a specific disease. These different classes of helminths evolved independently and may utilize different products to influence immune regulatory pathways. It will be interesting to determine how divergent or convergent are the mechanisms employed by differing helminths.
Animal models of T1D
T1D develops spontaneously in autoimmune prone nonobese diabetic (NOD) mice or can be elicited after serial injection of low dose streptozotocin (STZ, an islet β-cell toxin) in other strains (71). Infection with S. mansoni, T. spiralis, Hpb, or L. sigmodontis protects NOD mice from insulitis (72–75). However, the mechanisms of protection may differ between species.
Young NOD mice exposed to schistosome ova alone or to SEA are protected from developing diabetes. SEA-induced protection is associated with increased pancreatic mononuclear cell expression of TGFβ, IL4 and IL10 mRNA (76). SEA treatment increases the number of CD4+CD25+Foxp3+ T cells in the pancreas and spleen. Splenocytes from SEA-treated NOD mice do not produce disease when transferred into NOD.scid recipients (76). Depletion of Tregs from these splenocytes restores their pathogenicity showing that this is a critical pathway for protection.
Intraperitoneal injection of excretory/secretory antigens from F. hepatica also provides protection from insulitis in NOD mice (77). Disease prevention is associated with the induction of IL10-secreting B cells and transcripts indicative of M2 macrophage induction in pancreatic lymph node cell populations.
Infection with L. sigmodontis delays diabetes in IL4-deficient NOD mice and is associated with increased numbers of splenic CD4+CD25+Foxp3+ Tregs. Like splenocytes from SEA-treated NOD mice, splenocytes from L. sigmodontis-infected mice do not produce diabetes when transferred into NOD.scid recipients. However unlike the SEA model, depletion of Tregs does not restore pathogenicity suggesting that this is not a critical pathway for L. sigmodontis-mediated protection. Instead, blockade of TGFβ function abrogates protection indicating that this pathway is critical for this helminth and disease model (75).
Infection with Hpb also protects IL4-deficient NOD mice from developing diabetes (78). Protection is associated with induction of IL10 production by CD127hiFoxp3neg T cells present in pancreatic lymph nodes. Blockade of IL10 function in vivo in IL4-deficient (but not IL4-sufficient) NOD mice abrogates protection (78). This suggests that helminth amplified IL4 and IL10 circuits can independently provide protection.
Infection with T. crassiceps decreases insulitis and protects Balb/C and C57BL/6 mice from STZ diabetes. T. crassiceps induced protection is associated with an increase in IL4 and alternatively activated macrophages but not with induction of Tregs (79).
Infection with Hpb also affords protection from STZ-induced diabetes in C57BL/6 mice (80). Hpb induced protection remains intact in STAT6- or IL10-deficient mice suggesting that IL4 and IL10 are individually not required for protection in this model.
These studies show that helminths residing in different host tissues can suppress the same organ-specific inflammation. Critical mechanisms for protection from T1D appear to vary among various helminthic species. Also, they employ mechanisms that differ from those that protect from colitis or EAE. This may reflect the varying importance of specific regulatory pathways for each model system.
Animal models of RA
Collagen-induced arthritis (CIA) is a murine model of RA that develops in mice immunized with type II collagen in complete Freund’s adjuvant (CFA) (81). Infection with S. mansoni before collagen sensitization protects mice from developing polyarticular arthritis (82). This protection is associated with reduced IFNγ, TNFα and IL17, but increased IL4 and IL10 production by splenocytes as compared to collagen-sensitized helminth-naïve mice (82). S. japonicum, also protects mice from collagen-induced arthritis. The infection results in suppressed IFNγ but augmented IL4 and IL10 secretion by mitogen-stimulated splenocytes (83, 84).
Another rodent arthritis model is mono-articular inflammation provoked by injection of CFA (without collagen) into a knee joint. Treatment with a 16 Kd recombinant protein derived from S. japonicum (rSj16) protects rats from CFA-induced joint inflammation. Protection is associated with reduction in serum TNFα, NO and IL1β and restoration of IL10 levels as compared to untreated arthritic and control rats (85).
A 62Kd phosphocholine-containing glycoprotein (ES-62) isolated from Acanthocheilonema viteae prevents and treats established CIA (86). Collagen-stimulated draining lymph node cells from ES-62 treated mice make less IFNγ and TNFα, and more IL10 than do cells from untreated mice. Treatment of mice with ES-62 before CIA induction results in reduced serum IL17 levels and fewer Th17 cells in the draining lymph node and affected joints (87). Bone marrow-derived DCs stimulated with LPS and ES-62 make less TNFα, IL6 and IL23, and have reduced ability to induce IL17 production by OT-II T cells in vitro. ES-62 also works directly on polarized Th17 cells to reduce IL17 production and Myd88 expression (87). A synthetic small molecule modeled after ES-62 (N-(2-[(4-bromobenzyl)sulfonyl]ethyl)-N,N-dimethylamine, 11a) inhibits development of CIA and suppresses release of IL12p40 and IL6 from LPS-stimulated macrophages (88).
Culturing CpG-stimulated bone marrow-derived DCs with F. hepatica total extract increases IL10 and TGFβ production and suppresses IL12p70, IL23, IL6 and TNFα production compared to DCs stimulated without extract (89). F. hepatica extract-treated DC pulsed with collagen and then given to mice suppress CIA and inhibit IL17 and IFNγ but augment IL4, IL10, and TGFβ production by collagen-stimulated draining lymph node cells. Extract-treated DCs increase the frequency of CD25+Foxp3+ Treg cells in draining lymph nodes and transfer of these T cells suppressed CIA in recipients (89).
H. diminuta infection before intra-articular CFA challenge reduces joint swelling and speeds the resolution of inflammation in mice (90). H. diminuta exposure suppresses CFA-induced TNFα mRNA expression in challenged joints. Infection increases splenocyte IL4 and IL10 production, and H. diminuta infection does not protect IL10-deficient mice from CFA arthritis (90) suggesting that IL10 is important for this protection.
The same group found that H. diminuta worsens joint inflammation in another arthritis model where disease results from injecting Balb/c mice with serum from arthritogenic K/BxN mice that contains antibodies against autologous glucose-6-phosphate isomerase (91). Mast cells are required for joint inflammation in the K/BxN model, and worsening of arthritis is likely due to a helminth-induced mast cell activation (91).
These studies again confirm the broad nature of helminth-associated immune regulation. In addition, they demonstrate the central protective roles for IL10 and TGFβ production and suppression of IFNγ and IL17 circuitry by helminths in control of arthritis.
Animal models of allergy/asthma
Animal models and clinical studies indicate that dysregulated Th1/Th17 responses underlay IBD, MS, T1D, and rheumatoid arthritis. Because helminth infection directly suppresses those cytokine pathways, helminth-associated suppression of these diseases appears somewhat straightforward.
On the other hand, allergy and asthma appear to result from excessive Th2-type inflammation. Because helminth infections usually stimulate strong Th2 responses, it is counterintuitive that helminthic exposure would lessen allergic inflammation. However, studies in people comparing groups treated to remove helminths to untreated controls suggest that helminth infection decreases the prevalence of atopy, at least as measured by skin prick test positivity (92).
Animal models indicate that helminths induce regulatory pathways that can suppress atopic disease. A major murine model of allergic inflammation is airway hyper-responsiveness (AHR) induced by respiratory exposure to an antigen previously used to sensitize the animals (93). This sensitization uses antigen mixed with alum adjuvant.
Infection with Hpb during or prior to ovalbumin (OVA) sensitization inhibits subsequent airway reactivity (94) and inflammation (94, 95) upon aerosol challenge. Transfer of MLN cells or splenocytes from infected mice into helminth naïve animals inhibits airway inflammation, showing activation of regulatory cells. Hpb exposure increases the percentage of CD4+ CD25+ Foxp3+ T cells in the mesenteric and thoracic lymph nodes (94, 95). In addition, Hpb colonization induces a CD19+CD23+ regulatory B cell population that can adoptively transfer suppression of airway inflammation independent of IL10 production (62). Excretory/secretory products from cultured adult Hpb worms also inhibit OVA-stimulated airway inflammation and hyper-reactivity when given at the time of OVA-alum sensitization (96). This protection is associated with reduced IL4, IL5, IL13 and IFNγ levels in bronchoalveolar lavage fluid, suppression of the OVA-induced increase in alternatively activated macrophage markers, and reduced Teffector/Treg ratios in lung tissue (96).
Exposure to other helminths such as S. mansoni (97–99), S. japonicum (100) or T. spiralis (101) also affords protection from allergic airway reactivity and inflammation. Helminth exposure is associated with decreased OVA-stimulated IL4 and IL5, but increased IL10 and TGFβ production as measured in either bronchoalveolar lavage fluid or supernatants from cultured pulmonary draining lymph node cells or splenocytes. Splenic CD11c+ DCs isolated from S. japonicum-infected mice transfer protection to helminth-naïve mice (100). Transfer of splenic T cells from T. spiralis-infected mice, which contain more than 2-fold higher percentage of CD4+CD25+Foxp3+ cells, provides partial protection against OVA-induced airway inflammation (102). S. mansoni infection also induces CD4+CD25+Foxp3+ Tregs, and targeted in vivo depletion of Foxp3-expressing cells negates the protective influence of infection supporting the importance of Tregs for this protection (99). In addition, like Hpb, exposure to S. mansoni induces CD19+CD23+ regulatory B cells that can transfer protection from AHR (103). These regulatory B cells express CD1d, require intact IL10 production, and act in part by increasing the number of pulmonary CD4+CD25+Foxp3+ regulatory T cells in the lungs (103).
Treatment of mice with ES-62 from A. viteae also protects mice from OVA-AHR and pulmonary inflammation (46, 104) in association with reduced mast cell degranulation, lower OVA-stimulated IL4, IL5 and IL13 production by draining lymph node cells, and decreased Th17 cells compared to ES-62 naïve mice. Treatment with anti-IFNγ abrogates protection from airway inflammation/reactivity and reverses changes in cytokine profile and Th17 frequency (46) indicating that ES-62 induces counter-regulatory Th1 circuitry in this model.
Conclusion
Helminth infections exerted a strong selective pressure on our genome (105). Many host factors that confer risk for immune-mediated disease evolved under the selection pressure of helminths (106). Thus, it is plausible that eradication of helminthic infections and the loss of their immune modulatory effects have promoted development of some of the immunological diseases.
There are numerous animal models representing a diverse range of diseases for which helminths prevent and/or abrogate inflammation in various organs. Many helminth species mediate protection evoking similar immune regulatory mechanisms. Common themes include modulation of DC function, activation of Tregs, alteration of macrophage activity and enhancement of regulatory cytokine synthesis. Several of these mechanisms appear to function concurrently and independently of each other. Thus, the loss of any one regulatory pathway will not necessarily abrogate protection from disease. A complex array of different gene interactions, environmental factors and aberrant host immune responses drive immunological diseases. Thus, the mechanisms of protect should not be expected to be the same for all diseases, mouse strains and humans. The vast array of independent regulatory circuits that helminths engage may explain why they affect many disease states.
A number of investigations are underway to identify the helminth-derived molecular signals that mediate host immune modulation. This could lead to new pharmaceutical agents that target unique immune regulatory pathways, which will allow safe control or prevent of some immune-mediated illnesses.
Acknowledgments
Supported by DK38327, DK058755, VAMC, Schneider family, Gilman family
Abbreviations
- AHR
Airway hyper-responsiveness
- CLEC
C-type lectin-like domain-containing
- CTLR
C-type lectin receptors
- CIA
Collagen-induced arthritis
- CFA
Complete Freund’s adjuvant
- DC
Dendritic cells
- DSS
Dextran sodium sulfate
- DNBS
Dinitrobenzene sulfonic acid
- EAE
Experimental autoimmune encephalitis
- Hpb
Heligmosomoides polygyrus bakeri
- IBD
Inflammatory bowel disease
- LPMC
Lamina propria mononuclear cells
- MLN
Mesenteric lymph nodes
- MS
Multiple sclerosis
- NOD
Nonobese diabetic
- OVA
Ovalbumin
- REG
Regenerating islet-derived
- RA
Rheumatoid arthritis
- Tregs
Regulatory T cells
- SEA
Soluble egg antigen
- STZ
Streptozotocin
- Tlr
Toll-like receptors
- TNBS
Trinitrobenzene sulfate
- T1D
Type 1 diabetes
REFERENCES
- 1.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Ng SC, Bernstein CN, Vatn MH, Lakatos PL, Loftus EV, Jr, Tysk C, O'Morain C, Moum B, Colombel JF Epidemiology, and D. Natural History Task Force of the International Organization of Inflammatory Bowel. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut. 2013;62:630–649. doi: 10.1136/gutjnl-2012-303661. [DOI] [PubMed] [Google Scholar]
- 3.Weinstock JV, Elliott DE. Translatability of helminth therapy in inflammatory bowel diseases. Int J Parasitol. 2013;43:245–251. doi: 10.1016/j.ijpara.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacDonald TT, Monteleone I, Fantini MC, Monteleone G. Regulation of homeostasis and inflammation in the intestine. Gastroenterology. 2011;140:1768–1775. doi: 10.1053/j.gastro.2011.02.047. [DOI] [PubMed] [Google Scholar]
- 5.Hang L, Blum AM, Setiawan T, Urban JP, Jr, Stoyanoff KM, Weinstock JV. Heligmosomoides polygyrus bakeri infection activates colonic Foxp3+ T cells enhancing their capacity to prevent colitis. J Immunol. 2013;191:1927–1934. doi: 10.4049/jimmunol.1201457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott DE, Setiawan T, Metwali A, Blum A, Urban JF, Jr, Weinstock JV. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur J Immunol. 2004;34:2690–2698. doi: 10.1002/eji.200324833. [DOI] [PubMed] [Google Scholar]
- 7.Grainger JR, Smith KA, Hewitson JP, McSorley HJ, Harcus Y, Filbey KJ, Finney CA, Greenwood EJ, Knox DP, Wilson MS, Belkaid Y, Rudensky AY, Maizels RM. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-beta pathway. J Exp Med. 2010;207:2331–2341. doi: 10.1084/jem.20101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redpath SA, van der Werf N, Cervera AM, MacDonald AS, Gray D, Maizels RM, Taylor MD. ICOS controls Foxp3(+) regulatory T-cell expansion, maintenance and IL-10 production during helminth infection. Eur J Immunol. 2013;43:705–715. doi: 10.1002/eji.201242794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wohlfert EA, Grainger JR, Bouladoux N, Konkel JE, Oldenhove G, Ribeiro CH, Hall JA, Yagi R, Naik S, Bhairavabhotla R, Paul WE, Bosselut R, Wei G, Zhao K, Oukka M, Zhu J, Belkaid Y. GATA3 controls Foxp3(+) regulatory T cell fate during inflammation in mice. J Clin Invest. 2011;121:4503–4515. doi: 10.1172/JCI57456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Su MA, Wan YY. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity. 2011;35:337–348. doi: 10.1016/j.immuni.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shoemaker J, Saraiva M, O'Garra A. GATA-3 directly remodels the IL-10 locus independently of IL-4 in CD4+ T cells. J Immunol. 2006;176:3470–3479. doi: 10.4049/jimmunol.176.6.3470. [DOI] [PubMed] [Google Scholar]
- 12.Schmitt EG, Haribhai D, Williams JB, Aggarwal P, Jia S, Charbonnier LM, Yan K, Lorier R, Turner A, Ziegelbauer J, Georgiev P, Simpson P, Salzman NH, Hessner MJ, Broeckel U, Chatila TA, Williams CB. IL-10 produced by induced regulatory T cells (iTregs) controls colitis and pathogenic ex-iTregs during immunotherapy. J Immunol. 2012;189:5638–5648. doi: 10.4049/jimmunol.1200936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, Benoist C, Rudensky AY. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uhlig HH, Coombes J, Mottet C, Izcue A, Thompson C, Fanger A, Tannapfel A, Fontenot JD, Ramsdell F, Powrie F. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Setiawan T, Metwali A, Blum AM, Ince MN, Urban JF, Jr, Elliott DE, Weinstock JV. Heligmosomoides polygyrus promotes regulatory T cell cytokine production in normal distal murine intestine. Infect Immun. 2007;75:4655–4663. doi: 10.1128/IAI.00358-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunter MM, Wang A, Hirota CL, McKay DM. Neutralizing anti- IL-10 antibody blocks the protective effect of tapeworm infection in a murine model of chemically induced colitis. J Immunol. 2005;174(11):7368–7375. doi: 10.4049/jimmunol.174.11.7368. [DOI] [PubMed] [Google Scholar]
- 17.Elliott D, Li J, Blum A, Metwali A, Qadir K, Urban JF, Jr, JV W. Exposure to schistosome eggs protects mice from TNBS-induced colitis. Am J Physiol. 2003;284:G385–G391. doi: 10.1152/ajpgi.00049.2002. [DOI] [PubMed] [Google Scholar]
- 18.Hartmann W, Haben I, Fleischer B, Breloer M. Pathogenic nematodes suppress humoral responses to third-party antigens in vivo by IL-10-mediated interference with Th cell function. J Immunol. 2011;187:4088–4099. doi: 10.4049/jimmunol.1004136. [DOI] [PubMed] [Google Scholar]
- 19.Haben I, Hartmann W, Specht S, Hoerauf A, Roers A, Muller W, Breloer M. T-cell-derived, but not B-cell-derived, IL-10 suppresses antigen-specific T-cell responses in Litomosoides sigmodontis-infected mice. Eur J Immunol. 2013;43:1799–1805. doi: 10.1002/eji.201242929. [DOI] [PubMed] [Google Scholar]
- 20.Hang L, Setiawan T, Blum AM, Urban J, Stoyanoff K, Arihiro S, Reinecker HC, Weinstock JV. Heligmosomoides polygyrus infection can inhibit colitis through direct interaction with innate immunity. J Immunol. 2010;185:3184–3189. doi: 10.4049/jimmunol.1000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ince MN, Elliott DE, Setiawan T, Blum A, Metwali A, Wang Y, Urban JF, Jr, Weinstock JV. Heligmosomoides polygyrus induces TLR4 on murine mucosal T cells that produce TGFbeta after lipopolysaccharide stimulation. J Immunol. 2006;176(2):726–729. doi: 10.4049/jimmunol.176.2.726. [DOI] [PubMed] [Google Scholar]
- 22.Wang A, Fernando M, Leung G, Phan V, Smyth D, McKay DM. Exacerbation of oxazolone colitis by infection with the helminth Hymenolepis diminuta: involvement of IL-5 and eosinophils. Am J Pathol. 2010;177:2850–2859. doi: 10.2353/ajpath.2010.100537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elliott DE, Metwali A, Leung J, Setiawan T, Blum AM, Ince MN, Bazzone LE, Stadecker MJ, Urban JF, Jr, Weinstock JV. Colonization with Heligmosomoides polygyrus suppresses mucosal IL-17 production. J Immunol. 2008;181:2414–2419. doi: 10.4049/jimmunol.181.4.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metwali A, Setiawan T, Blum AM, Urban J, Elliott DE, Hang L, Weinstock JV. Induction of CD8+ regulatory T cells in the intestine by Heligmosomoides polygyrus infection. Am J Physiol Gastrointest Liver Physiol. 2006;291:G253–G259. doi: 10.1152/ajpgi.00409.2005. [DOI] [PubMed] [Google Scholar]
- 25.Leavenworth JW, Tang X, Kim HJ, Wang X, Cantor H. Amelioration of arthritis through mobilization of peptide-specific CD8+ regulatory T cells. J Clin Invest. 2013;123:1382–1389. doi: 10.1172/JCI66938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim HJ, Cantor H. Regulation of self-tolerance by Qa-1-restricted CD8(+) regulatory T cells. Seminars in Immunol. 2011;23:446–452. doi: 10.1016/j.smim.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blum AM, Hang L, Setiawan T, Urban JP, Jr, Stoyanoff KM, Leung J, Weinstock JV. Heligmosomoides polygyrus bakeri induces tolerogenic dendritic cells that block colitis and prevent antigen-specific gut T cell responses. J Immunol. 2012;189:2512–2520. doi: 10.4049/jimmunol.1102892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Idoyaga J, Fiorese C, Zbytnuik L, Lubkin A, Miller J, Malissen B, Mucida D, Merad M, Steinman RM. Specialized role of migratory dendritic cells in peripheral tolerance induction. J Clin Invest. 2013;123:844–854. doi: 10.1172/JCI65260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao J, Wang J, Wang H, Lai M. Reg proteins and their roles in inflammation and cancer of the human digestive system. Adv Clin Chem. 2013;61:153–173. doi: 10.1016/b978-0-12-407680-8.00006-3. [DOI] [PubMed] [Google Scholar]
- 30.Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, Hooper LV. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Ampting MT, Loonen LM, Schonewille AJ, Konings I, Vink C, Iovanna J, Chamaillard M, Dekker J, van der Meer R, Wells JM, Bovee-Oudenhoven IM. Intestinally secreted C-type lectin Reg3b attenuates salmonellosis but not listeriosis in mice. Infect Immun. 2012;80:1115–1120. doi: 10.1128/IAI.06165-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bishnupuri KS, Luo Q, Sainathan SK, Kikuchi K, Sureban SM, Sabarinathan M, Gross JH, Aden K, May R, Houchen CW, Anant S, Dieckgraefe BK. Reg IV regulates normal intestinal and colorectal cancer cell susceptibility to radiation-induced apoptosis. Gastroenterology. 2010;138:616–626. 626 e611–626 e612. doi: 10.1053/j.gastro.2009.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sancho D, Reis e Sousa C. Sensing of cell death by myeloid C-type lectin receptors. Curr Opin Immunol. 2013;25:46–52. doi: 10.1016/j.coi.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dragicevic A, Dzopalic T, Vasilijic S, Vucevic D, Tomic S, Bozic B, Colic M. Signaling through Toll-like receptor 3 and Dectin-1 potentiates the capability of human monocyte-derived dendritic cells to promote T-helper 1 and T-helper 17 immune responses. Cytotherapy. 2012;14:598–607. doi: 10.3109/14653249.2012.667873. [DOI] [PubMed] [Google Scholar]
- 35.Plato A, Willment JA, Brown GD. C-type lectin-like receptors of the dectin-1 cluster: ligands and signaling pathways. Int Rev Immunol. 2013;32:134–156. doi: 10.3109/08830185.2013.777065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kreider T, Anthony RM, Urban JF, Jr, Gause WC. Alternatively activated macrophages in helminth infections. Curr Opin Immunol. 2007;19:448–453. doi: 10.1016/j.coi.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reyes JL, Terrazas LI. The divergent roles of alternatively activated macrophages in helminthic infections. Parasite Immunol. 2007;29:609–619. doi: 10.1111/j.1365-3024.2007.00973.x. [DOI] [PubMed] [Google Scholar]
- 38.Smith P, Mangan NE, Walsh CM, Fallon RE, McKenzie AN, van RN, Fallon PG. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J Immunol. 2007;178:4557–4566. doi: 10.4049/jimmunol.178.7.4557. [DOI] [PubMed] [Google Scholar]
- 39.Schnoeller C, Rausch S, Pillai S, Avagyan A, Wittig BM, Loddenkemper C, Hamann A, Hamelmann E, Lucius R, Hartmann S. A Helminth Immunomodulator Reduces Allergic and Inflammatory Responses by Induction of IL-10-Producing Macrophages. J Immunol. 2008;180:4265–4272. doi: 10.4049/jimmunol.180.6.4265. [DOI] [PubMed] [Google Scholar]
- 40.Klotz C, Ziegler T, Figueiredo AS, Rausch S, Hepworth MR, Obsivac N, Sers C, Lang R, Hammerstein P, Lucius R, Hartmann S. A helminth immunomodulator exploits host signaling events to regulate cytokine production in macrophages. PLoS pathog. 2011;7:e1001248. doi: 10.1371/journal.ppat.1001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnston MJ, Wang A, Catarino ME, Ball L, Phan VC, MacDonald JA, McKay DM. Extracts of the rat tapeworm, Hymenolepis diminuta, suppress macrophage activation in vitro and alleviate chemically induced colitis in mice. Infect Immun. 2010;78:1364–1375. doi: 10.1128/IAI.01349-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hunter MM, Wang A, Parhar KS, Johnston MJ, van RN, Beck PL, McKay DM. In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology. 2010;138:1395–1405. doi: 10.1053/j.gastro.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 43.Leung G, Wang A, Fernando M, Phan VC, McKay DM. Bone marrow-derived alternatively activated macrophages reduce colitis without promoting fibrosis: participation of IL-10. Am J Physiol Gastrointestinal and Liver Physiol. 2013;304:G781–G792. doi: 10.1152/ajpgi.00055.2013. [DOI] [PubMed] [Google Scholar]
- 44.Klaver EJ, Kuijk LM, Laan LC, Kringel H, van Vliet SJ, Bouma G, Cummings RD, Kraal G, van Die I. Trichuris suis-induced modulation of human dendritic cell function is glycan-mediated. Int J Parasitol. 2013;43:191–200. doi: 10.1016/j.ijpara.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 45.Danilowicz-Luebert E, Steinfelder S, Kuhl AA, Drozdenko G, Lucius R, Worm M, Hamelmann E, Hartmann S. A nematode immunomodulator suppresses grass pollen-specific allergic responses by controlling excessive Th2 inflammation. Int J Parasitol. 2013;43:201–210. doi: 10.1016/j.ijpara.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 46.Rzepecka J, Siebeke I, Coltherd JC, Kean DE, Steiger CN, Al-Riyami L, McSharry C, Harnett MM, Harnett W. The helminth product, ES-62, protects against airway inflammation by resetting the Th cell phenotype. Int J Parasitol. 2013;43:211–223. doi: 10.1016/j.ijpara.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hartmann W, Brenz Y, Kingsley MT, Ajonina-Ekoti I, Brattig NW, Liebau E, Breloer M. Nematode-derived proteins suppress proliferation and cytokine production of antigen-specific T cells via induction of cell death. PloS one. 2013;8:e68380. doi: 10.1371/journal.pone.0068380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adisakwattana P, Saunders SP, Nel HJ, Fallon PG. Helminth-derived immunomodulatory molecules. Adv Exp Med Biol. 2009;666:95–107. doi: 10.1007/978-1-4419-1601-3_8. [DOI] [PubMed] [Google Scholar]
- 49.Hewitson JP, Grainger JR, Maizels RM. Helminth immunoregulation: the role of parasite secreted proteins in modulating host immunity. Mol Biochem Parasitol. 2009;167:1–11. doi: 10.1016/j.molbiopara.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Segura M, Su Z, Piccirillo C, Stevenson MM. Impairment of dendritic cell function by excretory-secretory products: a potential mechanism for nematode-induced immunosuppression. Eur J Immunol. 2007;37:1887–1904. doi: 10.1002/eji.200636553. [DOI] [PubMed] [Google Scholar]
- 51.Taylor BC, Zaph C, Troy AE, Du Y, Guild KJ, Comeau MR, Artis D. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med. 2009;206:655–667. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strober W. The multifaceted influence of the mucosal microflora on mucosal dendritic cell responses. Immunity. 2009;31:377–388. doi: 10.1016/j.immuni.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Walk ST, Blum AM, Ewing SA, Weinstock JV, Young VB. Alteration of the murine gut microbiota during infection with the parasitic helminth Heligmosomoides polygyrus. Inflamm Bowel Dis. 2010;16:1841–1849. doi: 10.1002/ibd.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Broadhurst MJ, Ardeshir A, Kanwar B, Mirpuri J, Gundra UM, Leung JM, Wiens KE, Vujkovic-Cvijin I, Kim CC, Yarovinsky F, Lerche NW, McCune JM, Loke P. Therapeutic helminth infection of macaques with idiopathic chronic diarrhea alters the inflammatory signature and mucosal microbiota of the colon. PLoS pathogens. 2012;8:e1003000. doi: 10.1371/journal.ppat.1003000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leung JM, Loke P. A role for IL-22 in the relationship between intestinal helminths, gut microbiota and mucosal immunity. Int J Parasitol. 2013;43:253–257. doi: 10.1016/j.ijpara.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Broadhurst MJ, Leung JM, Kashyap V, McCune JM, Mahadevan U, McKerrow JH, Loke P. IL-22+ CD4+ T cells are associated with therapeutic trichuris trichiura infection in an ulcerative colitis patient. Sci Transl Med. 2010;2:60ra88. doi: 10.1126/scitranslmed.3001500. [DOI] [PubMed] [Google Scholar]
- 57.Kron MA, Metwali A, Vodanovic-Jankovic S, Elliott D. Nematode asparaginyl-tRNA synthetase resolves intestinal inflammation in mice with T-cell transfer colitis. Clin Vaccine Immunol. 2013;20:276–281. doi: 10.1128/CVI.00594-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Knight JC. Genomic modulators of the immune response. Trends in Genetics. 2013;29:74–83. doi: 10.1016/j.tig.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kurschus FC, Wortge S, Waisman A. Modeling a complex disease: multiple sclerosis. Adv Immunol. 2011;110:111–137. doi: 10.1016/B978-0-12-387663-8.00001-6. [DOI] [PubMed] [Google Scholar]
- 60.Sewell D, Qing Z, Reinke E, Elliott D, Weinstock J, Sandor M, Fabry Z. Immunomodulation of experimental autoimmune encephalomyelitis by helminth ova immunization. International Immunol. 2003;15:59–69. doi: 10.1093/intimm/dxg012. [DOI] [PubMed] [Google Scholar]
- 61.La Flamme AC, Ruddenklau K, Backstrom BT. Schistosomiasis decreases central nervous system inflammation and alters the progression of experimental autoimmune encephalomyelitis. Infect Immun. 2003;71:4996–5004. doi: 10.1128/IAI.71.9.4996-5004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilson MS, Taylor MD, O'Gorman MT, Balic A, Barr TA, Filbey K, Anderton SM, Maizels RM. Helminth-induced CD19+CD23hi B cells modulate experimental allergic and autoimmune inflammation. Eur J Immunol. 2010;40:1682–1696. doi: 10.1002/eji.200939721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walsh KP, Brady MT, Finlay CM, Boon L, Mills KH. Infection with a helminth parasite attenuates autoimmunity through TGF-beta-mediated suppression of Th17 and Th1 responses. J Immunol. 2009;183:1577–1586. doi: 10.4049/jimmunol.0803803. [DOI] [PubMed] [Google Scholar]
- 64.Kuijk LM, Klaver EJ, Kooij G, van der Pol SM, Heijnen P, Bruijns SC, Kringel H, Pinelli E, Kraal G, de Vries HE, Dijkstra CD, Bouma G, van Die I. Soluble helminth products suppress clinical signs in murine experimental autoimmune encephalomyelitis and differentially modulate human dendritic cell activation. Mol Immunol. 2012;51:210–218. doi: 10.1016/j.molimm.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 65.Gruden-Movsesijan A, Ilic N, Mostarica-Stojkovic M, Stosic-Grujicic S, Milic M, Sofronic-Milosavljevic L. Mechanisms of modulation of experimental autoimmune encephalomyelitis by chronic Trichinella spiralis infection in Dark Agouti rats. Parasite Immunol. 2010;32:450–459. doi: 10.1111/j.1365-3024.2010.01207.x. [DOI] [PubMed] [Google Scholar]
- 66.Sofronic-Milosavljevic LJ, Radovic I, Ilic N, Majstorovic I, Cvetkovic J, Gruden-Movsesijan A. Application of dendritic cells stimulated with Trichinella spiralis excretory-secretory antigens alleviates experimental autoimmune encephalomyelitis. Med Microbiol Immunol. 2013;202:239–249. doi: 10.1007/s00430-012-0286-6. [DOI] [PubMed] [Google Scholar]
- 67.Reyes JL, Espinoza-Jimenez AF, Gonzalez MI, Verdin L, Terrazas LI. Taenia crassiceps infection abrogates experimental autoimmune encephalomyelitis. Cell Immunol. 2011;267:77–87. doi: 10.1016/j.cellimm.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 68.Terrazas CA, Alcantara-Hernandez M, Bonifaz L, Terrazas LI, Satoskar AR. Helminth-excreted/secreted products are recognized by multiple receptors on DCs to block the TLR response and bias Th2 polarization in a cRAF dependent pathway. FASEB J. 2013;27:4547–4560. doi: 10.1096/fj.13-228932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Correale J, Farez MF. The impact of parasite infections on the course of multiple sclerosis. J Neuroimmunol. 2011;233:6–11. doi: 10.1016/j.jneuroim.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 70.Correale J, Farez MF. Parasite infections in multiple sclerosis modulate immune responses through a retinoic acid-dependent pathway. J Immunol. 2013;191:3827–3837. doi: 10.4049/jimmunol.1301110. [DOI] [PubMed] [Google Scholar]
- 71.Leiter EH, Schile A. Genetic and Pharmacologic Models for Type 1 Diabetes. Current Protocols in Mouse Biology. 2013;3:9–19. doi: 10.1002/9780470942390.mo120154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cooke A, Tonks P, Jones FM, O'Shea H, Hutchings P, Fulford AJ, Dunne DW. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 1999;21:169–176. doi: 10.1046/j.1365-3024.1999.00213.x. [DOI] [PubMed] [Google Scholar]
- 73.Liu Q, Sundar K, Mishra PK, Mousavi G, Liu Z, Gaydo A, Alem F, Lagunoff D, Bleich D, Gause WC. Helminth infection can reduce insulitis and type 1 diabetes through CD25− and IL-10-independent mechanisms. Infect Immun. 2009;77:5347–5358. doi: 10.1128/IAI.01170-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saunders KA, Raine T, Cooke A, Lawrence CE. Inhibition of autoimmune type 1 diabetes by gastrointestinal helminth infection. Infect Immun. 2007;75:397–407. doi: 10.1128/IAI.00664-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hubner MP, Shi Y, Torrero MN, Mueller E, Larson D, Soloviova K, Gondorf F, Hoerauf A, Killoran KE, Stocker JT, Davies SJ, Tarbell KV, Mitre E. Helminth protection against autoimmune diabetes in nonobese diabetic mice is independent of a type 2 immune shift and requires TGF-beta. J Immunol. 2012;188:559–568. doi: 10.4049/jimmunol.1100335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zaccone P, Burton O, Miller N, Jones FM, Dunne DW, Cooke A. Schistosoma mansoni egg antigens induce Treg that participate in diabetes prevention in NOD mice. Eur J Immunol. 2009;39:1098–1107. doi: 10.1002/eji.200838871. [DOI] [PubMed] [Google Scholar]
- 77.Lund ME, O'Brien BA, Hutchinson AT, Robinson MW, Simpson AM, Dalton JP, Donnelly S. Secreted proteins from the helminth Fasciola hepatica inhibit the initiation of autoreactive T cell responses and prevent diabetes in the NOD mouse. PloS one. 2014;9:e86289. doi: 10.1371/journal.pone.0086289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mishra PK, Patel N, Wu W, Bleich D, Gause WC. Prevention of type 1 diabetes through infection with an intestinal nematode parasite requires IL-10 in the absence of a Th2-type response. Mucosal immunol. 2013;6:297–308. doi: 10.1038/mi.2012.71. [DOI] [PubMed] [Google Scholar]
- 79.Espinoza-Jimenez A, Rivera-Montoya I, Cardenas-Arreola R, Moran L, Terrazas LI. Taenia crassiceps infection attenuates multiple low-dose streptozotocin-induced diabetes. J Biomed Biotechnol. 2010;2010:850541. doi: 10.1155/2010/850541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Osada Y, Yamada S, Nabeshima A, Yamagishi Y, Ishiwata K, Nakae S, Sudo K, Kanazawa T. Heligmosomoides polygyrus infection reduces severity of type 1 diabetes induced by multiple low-dose streptozotocin in mice via STAT6- and IL-10-independent mechanisms. Exp Parasitol. 2013;135:388–396. doi: 10.1016/j.exppara.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 81.Brand DD, Kang AH, Rosloniec EF. Immunopathogenesis of collagen arthritis. Springer Semin Immunopathol. 2003;25:3–18. doi: 10.1007/s00281-003-0127-1. [DOI] [PubMed] [Google Scholar]
- 82.Osada Y, Shimizu S, Kumagai T, Yamada S, Kanazawa T. Schistosoma mansoni infection reduces severity of collagen-induced arthritis via down-regulation of pro-inflammatory mediators. Int J Parasitol. 2009;39:457–464. doi: 10.1016/j.ijpara.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 83.He Y, Li J, Zhuang W, Yin L, Chen C, Li J, Chi F, Bai Y, Chen XP. The inhibitory effect against collagen-induced arthritis by Schistosoma japonicum infection is infection stage-dependent. BMC Immunol. 2010;11:28. doi: 10.1186/1471-2172-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Song X, Shen J, Wen H, Zhong Z, Luo Q, Chu D, Qi Y, Xu Y, Wei W. Impact of Schistosoma japonicum infection on collagen-induced arthritis in DBA/1 mice: a murine model of human rheumatoid arthritis. PLoS one. 2011;6:e23453. doi: 10.1371/journal.pone.0023453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sun X, Liu YH, Lv ZY, Yang LL, Hu SM, Zheng HQ, Hu W, Cao JP, Fung MQ, Wu ZD. rSj16, a recombinant protein of Schistosoma japonicum-derived molecule, reduces severity of the complete Freund's adjuvant-induced adjuvant arthritis in rats' model. Parasite Immunol. 2010;32:739–748. doi: 10.1111/j.1365-3024.2010.01240.x. [DOI] [PubMed] [Google Scholar]
- 86.McInnes IB, Leung BP, Harnett M, Gracie JA, Liew FY, Harnett W. A novel therapeutic approach targeting articular inflammation using the filarial nematode-derived phosphorylcholine-containing glycoprotein ES-62. J Immunol. 2003;171:2127–2133. doi: 10.4049/jimmunol.171.4.2127. [DOI] [PubMed] [Google Scholar]
- 87.Pineda MA, McGrath MA, Smith PC, Al-Riyami L, Rzepecka J, Gracie JA, Harnett W, Harnett MM. The parasitic helminth product ES-62 suppresses pathogenesis in collagen-induced arthritis by targeting the interleukin-17-producing cellular network at multiple sites. Arthritis and Rheumatism. 2012;64:3168–3178. doi: 10.1002/art.34581. [DOI] [PubMed] [Google Scholar]
- 88.Al-Riyami L, Pineda MA, Rzepecka J, Huggan JK, Khalaf AI, Suckling CJ, Scott FJ, Rodgers DT, Harnett MM, Harnett W. Designing anti-inflammatory drugs from parasitic worms: a synthetic small molecule analogue of the Acanthocheilonema viteae product ES-62 prevents development of collagen-induced arthritis. J Med Chem. 2013;56:9982–10002. doi: 10.1021/jm401251p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carranza F, Falcon CR, Nunez N, Knubel C, Correa SG, Bianco I, Maccioni M, Fretes R, Triquell MF, Motran CC, Cervi L. Helminth antigens enable CpG-activated dendritic cells to inhibit the symptoms of collagen-induced arthritis through Foxp3+ regulatory T cells. PloS one. 2012;7:e40356. doi: 10.1371/journal.pone.0040356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shi M, Wang A, Prescott D, Waterhouse CC, Zhang S, McDougall JJ, Sharkey KA, McKay DM. Infection with an intestinal helminth parasite reduces Freund's complete adjuvant-induced monoarthritis in mice. Arthritis Rheum. 2011;63:434–444. doi: 10.1002/art.30098. [DOI] [PubMed] [Google Scholar]
- 91.Graepel R, Leung G, Wang A, Villemaire M, Jirik FR, Sharkey KA, McDougall JJ, McKay DM. Murine autoimmune arthritis is exaggerated by infection with the rat tapeworm, Hymenolepis diminuta. Int J Parasitol. 2013;43:593–601. doi: 10.1016/j.ijpara.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 92.Amoah AS, Boakye DA, van Ree R, Yazdanbakhsh M. Parasitic worms and allergies in childhood: Insights from population studies 2008–2013. Pediatr Allergy Immunol. 2014;25:208–217. doi: 10.1111/pai.12174. [DOI] [PubMed] [Google Scholar]
- 93.Zosky GR, Amoah R, Sly PD. Animal models of asthma. Clin Exp Allergy. 2007;37:973–988. doi: 10.1111/j.1365-2222.2007.02740.x. [DOI] [PubMed] [Google Scholar]
- 94.Kitagaki K, Businga TR, Racila D, Elliott DE, Weinstock JV, Kline JN. Intestinal helminths protect in a murine model of asthma. J Immunol. 2006;177:1628–1635. doi: 10.4049/jimmunol.177.3.1628. [DOI] [PubMed] [Google Scholar]
- 95.Wilson MS, Taylor MD, Balic A, Finney CA, Lamb JR, Maizels RM. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med. 2005;202:1199–1212. doi: 10.1084/jem.20042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McSorley HJ, O'Gorman MT, Blair N, Sutherland TE, Filbey KJ, Maizels RM. Suppression of type 2 immunity and allergic airway inflammation by secreted products of the helminth Heligmosomoides polygyrus. Eur J Immunol. 2012;42:2667–2682. doi: 10.1002/eji.201142161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Smits HH, Hammad H, van Nimwegen M, Soullie T, Willart MA, Lievers E, Kadouch J, Kool M, Kos-van Oosterhoud J, Deelder AM, Lambrecht BN, Yazdanbakhsh M. Protective effect of Schistosoma mansoni infection on allergic airway inflammation depends on the intensity and chronicity of infection. J Allergy Clin Immunol. 2007;120:932–940. doi: 10.1016/j.jaci.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 98.Pacifico LG, Marinho FA, Fonseca CT, Barsante MM, Pinho V, Sales-Junior PA, Cardoso LS, Araujo MI, Carvalho EM, Cassali GD, Teixeira MM, Oliveira SC. Schistosoma mansoni antigens modulate experimental allergic asthma in a murine model: a major role for CD4+ CD25+ Foxp3+ T cells independent of interleukin-10. Infect Immun. 2009;77:98–107. doi: 10.1128/IAI.00783-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Layland LE, Straubinger K, Ritter M, Loffredo-Verde E, Garn H, Sparwasser T, Prazeres da Costa C. Schistosoma mansoni-mediated suppression of allergic airway inflammation requires patency and Foxp3+ Treg cells. PLoS Neglected Tropical Diseases. 2013;7:e2379. doi: 10.1371/journal.pntd.0002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu P, Li J, Yang X, Shen Y, Zhu Y, Wang S, Wu Z, Liu X, An G, Ji W, Gao W, Yang X. Helminth infection inhibits airway allergic reaction and dendritic cells are involved in the modulation process. Parasite Immunol. 2010;32:57–66. doi: 10.1111/j.1365-3024.2009.01161.x. [DOI] [PubMed] [Google Scholar]
- 101.Park HK, Cho MK, Choi SH, Kim YS, Yu HS. Trichinella spiralis: infection reduces airway allergic inflammation in mice. Exp Parasitol. 2011;127:539–544. doi: 10.1016/j.exppara.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 102.Aranzamendi C, de Bruin A, Kuiper R, Boog CJ, van Eden W, Rutten V, Pinelli E. Protection against allergic airway inflammation during the chronic and acute phases of Trichinella spiralis infection. Clin Exp Allergy. 2013;43:103–115. doi: 10.1111/cea.12042. [DOI] [PubMed] [Google Scholar]
- 103.Amu S, Saunders SP, Kronenberg M, Mangan NE, Atzberger A, Fallon PG. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J Allergy Clin Immunol. 2010;125:1114–1124. doi: 10.1016/j.jaci.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 104.Melendez AJ, Harnett MM, Pushparaj PN, Wong WS, Tay HK, McSharry CP, Harnett W. Inhibition of Fc epsilon RI-mediated mast cell responses by ES-62, a product of parasitic filarial nematodes. Nat Med. 2007;13:1375–1381. doi: 10.1038/nm1654. [DOI] [PubMed] [Google Scholar]
- 105.Fumagalli M, Pozzoli U, Cagliani R, Comi GP, Riva S, Clerici M, Bresolin N, Sironi M. Parasites represent a major selective force for interleukin genes and shape the genetic predisposition to autoimmune conditions. J Exp Med. 2009;206:1395–1408. doi: 10.1084/jem.20082779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fumagalli M, Pozzoli U, Cagliani R, Comi GP, Bresolin N, Clerici M, Sironi M. The landscape of human genes involved in the immune response to parasitic worms. BMC Evol Biol. 2010;10:e264. doi: 10.1186/1471-2148-10-264. [DOI] [PMC free article] [PubMed] [Google Scholar]