Abstract
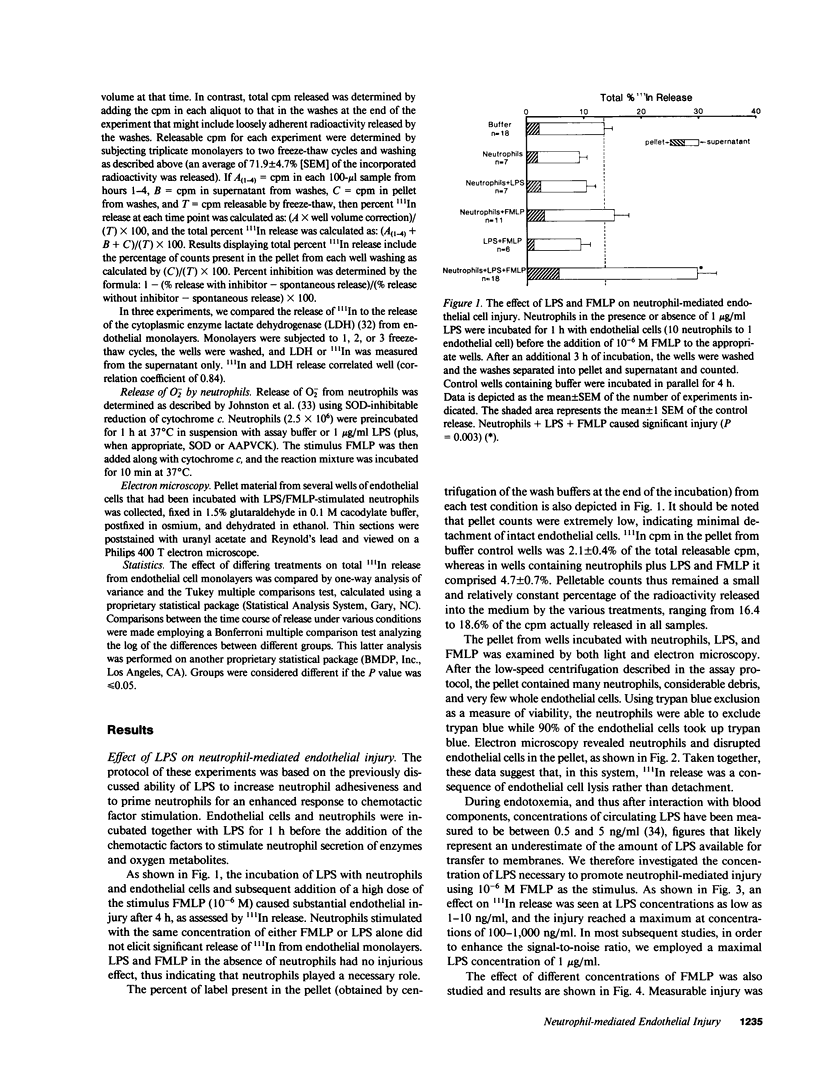
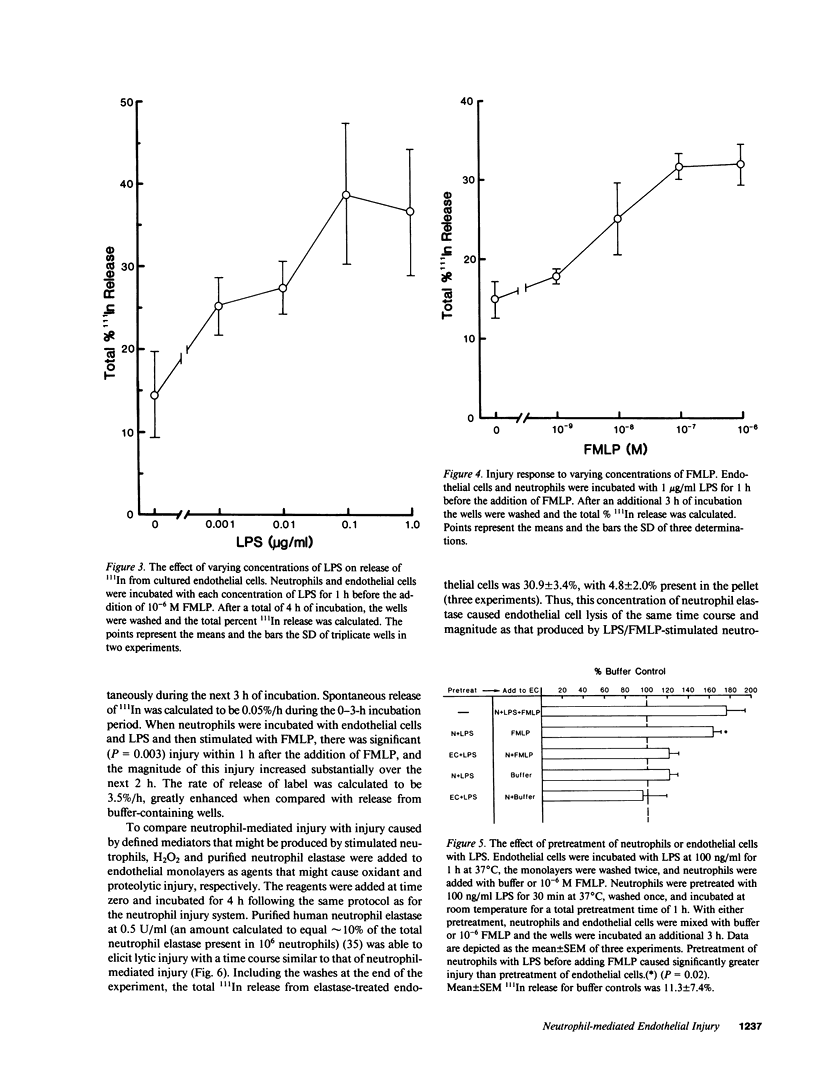
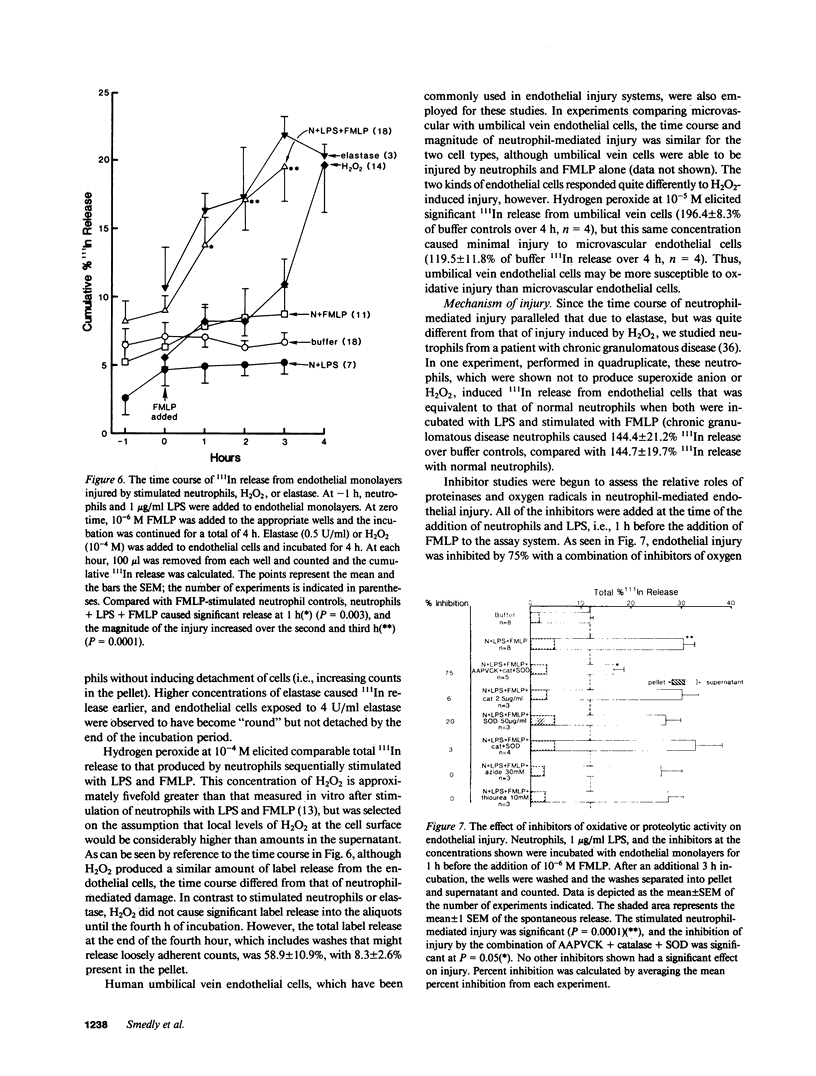
The neutrophil has been implicated as an important mediator of vascular injury, especially after endotoxemia. This study examines neutrophil-mediated injury to human microvascular endothelial cells in vitro. We found that neutrophils stimulated by formyl-methionyl-leucyl-phenylalanine (FMLP), the complement fragment C5a, or lipopolysaccharide (LPS) (1-1,000 ng/ml) alone produced minimal endothelial injury over a 4-h assay. In contrast, neutrophils incubated with endothelial cells in the presence of low concentrations of LPS (1-10 ng/ml) could then be stimulated by FMLP or C5a to produce marked endothelial injury. Injury was maximal at concentrations of 100 ng/ml LPS and 10(-7) M FMLP. Pretreatment of neutrophils with LPS resulted in a similar degree of injury, suggesting that LPS effects were largely on the neutrophil. Endothelial cell injury produced by LPS-exposed, FMLP-stimulated neutrophils had a time course similar to that induced by the addition of purified human neutrophil elastase, and different from that induced by hydrogen peroxide (H2O2). Further, neutrophil-mediated injury was not inhibited by scavengers of a variety of oxygen radical species, and occurred with neutrophils from a patient with chronic granulomatous disease, which produced no H2O2. In contrast, the specific serine elastase inhibitor methoxy-succinyl-alanyl-alanyl-prolyl-valyl-chloromethyl ketone inhibited 63% of the neutrophil-mediated injury and 64% of the neutrophil elastase-induced injury. However, neutrophil-mediated injury was not inhibited significantly by 50% serum, 50% plasma, or purified alpha 1 proteinase inhibitor. These results suggest that, in this system, chemotactic factor-stimulated human neutrophil injury of microvascular endothelial cells is enhanced by small amounts of LPS and may be mediated in large part by the action of neutrophil elastase.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREAE W. A. A sensitive method for the estimation of hydrogen peroxide in biological materials. Nature. 1955 May 14;175(4463):859–860. doi: 10.1038/175859a0. [DOI] [PubMed] [Google Scholar]
- Ambruso D. R., Johnston R. B., Jr Lactoferrin enhances hydroxyl radical production by human neutrophils, neutrophil particulate fractions, and an enzymatic generating system. J Clin Invest. 1981 Feb;67(2):352–360. doi: 10.1172/JCI110042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachofen M., Weibel E. R. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med. 1982 Jan;3(1):35–56. [PubMed] [Google Scholar]
- Banda M. J., Clark E. J., Werb Z. Regulation of alpha 1 proteinase inhibitor function by rabbit alveolar macrophages. Evidence for proteolytic rather than oxidative inactivation. J Clin Invest. 1985 Jun;75(6):1758–1762. doi: 10.1172/JCI111887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh R. J., Travis J. Human leukocyte granule elastase: rapid isolation and characterization. Biochemistry. 1976 Feb 24;15(4):836–841. doi: 10.1021/bi00649a017. [DOI] [PubMed] [Google Scholar]
- Brigham K. L., Meyrick B. Granulocyte-dependent injury of pulmonary endothelium: a case of miscommunication? Tissue Cell. 1984;16(2):137–155. doi: 10.1016/0040-8166(84)90039-9. [DOI] [PubMed] [Google Scholar]
- Brigham K. L., Woolverton W. C., Blake L. H., Staub N. C. Increased sheep lung vascular permeability caused by pseudomonas bacteremia. J Clin Invest. 1974 Oct;54(4):792–804. doi: 10.1172/JCI107819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell E. J., Senior R. M., McDonald J. A., Cox D. L. Proteolysis by neutrophils. Relative importance of cell-substrate contact and oxidative inactivation of proteinase inhibitors in vitro. J Clin Invest. 1982 Oct;70(4):845–852. doi: 10.1172/JCI110681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp H., Janoff A. Potential mediator of inflammation. Phagocyte-derived oxidants suppress the elastase-inhibitory capacity of alpha 1-proteinase inhibitor in vitro. J Clin Invest. 1980 Nov;66(5):987–995. doi: 10.1172/JCI109968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahinden C., Galanos C., Fehr J. Granulocyte activation by endotoxin. I. Correlation between adherence and other granulocyte functions, and role of endotoxin structure on biologic activity. J Immunol. 1983 Feb;130(2):857–862. [PubMed] [Google Scholar]
- Fanestil D. D., Barrows C. H., Jr Aging in the rotifer. J Gerontol. 1965 Oct;20(4):462–469. [PubMed] [Google Scholar]
- Fernandez H. N., Hugli T. E. Partial characterization of human C5a anaphylatoxin. I. Chemical description of the carbohydrate and polypeptide prtions of human C5a. J Immunol. 1976 Nov;117(5 Pt 1):1688–1694. [PubMed] [Google Scholar]
- Fowler A. A., Hamman R. F., Good J. T., Benson K. N., Baird M., Eberle D. J., Petty T. L., Hyers T. M. Adult respiratory distress syndrome: risk with common predispositions. Ann Intern Med. 1983 May;98(5 Pt 1):593–597. doi: 10.7326/0003-4819-98-5-593. [DOI] [PubMed] [Google Scholar]
- Gallin J. I. Neutrophil specific granules: a fuse that ignites the inflammatory response. Clin Res. 1984 Sep;32(3):320–328. [PubMed] [Google Scholar]
- Guthrie L. A., McPhail L. C., Henson P. M., Johnston R. B., Jr Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J Exp Med. 1984 Dec 1;160(6):1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M., Harker L. A., Reidy M. A., Gajdusek C. M., Schwartz S. M., Striker G. E. Lipopolysaccharide-mediated bovine endothelial cell injury in vitro. Lab Invest. 1983 Mar;48(3):269–274. [PubMed] [Google Scholar]
- Harlan J. M., Killen P. D., Harker L. A., Striker G. E., Wright D. G. Neutrophil-mediated endothelial injury in vitro mechanisms of cell detachment. J Clin Invest. 1981 Dec;68(6):1394–1403. doi: 10.1172/JCI110390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M., Schwartz B. R., Reidy M. A., Schwartz S. M., Ochs H. D., Harker L. A. Activated neutrophils disrupt endothelial monolayer integrity by an oxygen radical-independent mechanism. Lab Invest. 1985 Feb;52(2):141–150. [PubMed] [Google Scholar]
- Hart D. H. Polymorphonuclear leukocyte elastase activity is increased by bacterial lipopolysaccharide: a response inhibited by glucocorticoids. Blood. 1984 Feb;63(2):421–426. [PubMed] [Google Scholar]
- Haslett C., Guthrie L. A., Kopaniak M. M., Johnston R. B., Jr, Henson P. M. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985 Apr;119(1):101–110. [PMC free article] [PubMed] [Google Scholar]
- Heflin A. C., Jr, Brigham K. L. Prevention by granulocyte depletion of increased vascular permeability of sheep lung following endotoxemia. J Clin Invest. 1981 Nov;68(5):1253–1260. doi: 10.1172/JCI110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Larsen G. L., Webster R. O., Mitchell B. C., Goins A. J., Henson J. E. Pulmonary microvascular alterations and injury induced by complement fragments: synergistic effect of complement activation, neutrophil sequestration, and prostaglandins. Ann N Y Acad Sci. 1982;384:287–300. doi: 10.1111/j.1749-6632.1982.tb21379.x. [DOI] [PubMed] [Google Scholar]
- Issekutz A. C., Movat K. W., Movat H. Z. Enhanced vascular permeability and haemorrhage-inducing activity of rabbit C5ades arg: probable role of polymorphonuclear leucocyte lysosomes. Clin Exp Immunol. 1980 Sep;41(3):512–520. [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E. Elaboration of toxic oxygen by-products by neutrophils in a model of immune complex disease. J Clin Invest. 1976 Apr;57(4):836–841. doi: 10.1172/JCI108359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern P. A., Knedler A., Eckel R. H. Isolation and culture of microvascular endothelium from human adipose tissue. J Clin Invest. 1983 Jun;71(6):1822–1829. doi: 10.1172/JCI110937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRoy E. C., Ager A., Gordon J. L. Effects of neutrophil elastase and other proteases on porcine aortic endothelial prostaglandin I2 production, adenine nucleotide release, and responses to vasoactive agents. J Clin Invest. 1984 Sep;74(3):1003–1010. doi: 10.1172/JCI111467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J., Poore T. E., Zauber N. P., Oser R. S. Detection of endotoxin in the blood of patients with sepsis due to gran-negative bacteria. N Engl J Med. 1970 Dec 10;283(24):1313–1316. doi: 10.1056/NEJM197012102832404. [DOI] [PubMed] [Google Scholar]
- Martin W. J., 2nd Neutrophils kill pulmonary endothelial cells by a hydrogen-peroxide-dependent pathway. An in vitro model of neutrophil-mediated lung injury. Am Rev Respir Dis. 1984 Aug;130(2):209–213. doi: 10.1164/arrd.1984.130.2.209. [DOI] [PubMed] [Google Scholar]
- McIntire F. C., Barlow G. H., Sievert H. W., Finley R. A., Yoo A. L. Studies on a lipopolysaccharide from Escherichia coli. Heterogeneity and mechanism of reversible inactivation by sodium deoxycholate. Biochemistry. 1969 Oct;8(10):4063–4067. doi: 10.1021/bi00838a024. [DOI] [PubMed] [Google Scholar]
- Mecham R. P., Madaras J., McDonald J. A., Ryan U. Elastin production by cultured calf pulmonary artery endothelial cells. J Cell Physiol. 1983 Sep;116(3):282–288. doi: 10.1002/jcp.1041160304. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Kline L. F. Activation of the classical and properdin pathways of complement by bacterial lipopolysaccharides (LPS). J Immunol. 1977 Jan;118(1):362–368. [PubMed] [Google Scholar]
- Powers J. C., Gupton B. F., Harley A. D., Nishino N., Whitley R. J. Specificity of porcine pancreatic elastase, human leukocyte elastase and cathepsin G. Inhibition with peptide chloromethyl ketones. Biochim Biophys Acta. 1977 Nov 23;485(1):156–166. doi: 10.1016/0005-2744(77)90203-0. [DOI] [PubMed] [Google Scholar]
- Quie P. G., White J. G., Holmes B., Good R. A. In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J Clin Invest. 1967 Apr;46(4):668–679. doi: 10.1172/JCI105568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., Fox R. B., Berger E. M. Dimethyl sulfoxide inhibits killing of Staphylococcus aureus by polymorphonuclear leukocytes. Infect Immun. 1981 Jan;31(1):510–513. doi: 10.1128/iai.31.1.510-513.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STETSON C. A., GOOD R. A. Studies on the mechanism of the Shwartzman-phenomenon; evidence for the participation of polymorphonuclear leucocytes in the phenomenon. J Exp Med. 1951 Jan;93(1):49–63. doi: 10.1084/jem.93.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STETSON C. A., Jr Studies on the mechanism of the Shwartzman phenomenon; certain factors involved in the production of the local hemorrhagic necrosis. J Exp Med. 1951 May;93(5):489–504. doi: 10.1084/jem.93.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasada M., Johnston R. B., Jr Macrophage microbicidal activity. Correlation between phagocytosis-associated oxidative metabolism and the killing of Candida by macrophages. J Exp Med. 1980 Jul 1;152(1):85–98. doi: 10.1084/jem.152.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingu M., Yoshioka K., Nobunaga M., Yoshida K. Human vascular smooth muscle cells and endothelial cells lack catalase activity and are susceptible to hydrogen peroxide. Inflammation. 1985 Sep;9(3):309–320. doi: 10.1007/BF00916279. [DOI] [PubMed] [Google Scholar]
- Spitzer R. E., Vallota E. H., Forristal J., Sudora E., Stitzel A., Davis N. C., West C. D. Serum C'3 lytic system in patients with glomerulonephritis. Science. 1969 Apr 25;164(3878):436–437. doi: 10.1126/science.164.3878.436. [DOI] [PubMed] [Google Scholar]
- Tate R. M., Repine J. E. Hydrogen peroxide causes permeability edema and hypertension in isolated salt-perfused rabbit lungs. Chest. 1983 May;83(5 Suppl):48S–50S. doi: 10.1378/chest.83.5_supplement.48s. [DOI] [PubMed] [Google Scholar]
- Till G. O., Johnson K. J., Kunkel R., Ward P. A. Intravascular activation of complement and acute lung injury. Dependency on neutrophils and toxic oxygen metabolites. J Clin Invest. 1982 May;69(5):1126–1135. doi: 10.1172/JCI110548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnesen M. G., Smedly L. A., Henson P. M. Neutrophil-endothelial cell interactions. Modulation of neutrophil adhesiveness induced by complement fragments C5a and C5a des arg and formyl-methionyl-leucyl-phenylalanine in vitro. J Clin Invest. 1984 Nov;74(5):1581–1592. doi: 10.1172/JCI111574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J., Bowen J., Tewksbury D., Johnson D., Pannell R. Isolation of albumin from whole human plasma and fractionation of albumin-depleted plasma. Biochem J. 1976 Aug 1;157(2):301–306. doi: 10.1042/bj1570301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Webster R. O., Hong S. R., Johnston R. B., Jr, Henson P. M. Biologial effects of the human complement fragments C5a and C5ades Arg on neutrophil function. Immunopharmacology. 1980 Jun;2(3):201–219. doi: 10.1016/0162-3109(80)90050-8. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Regiani S. Neutrophils degrade subendothelial matrices in the presence of alpha-1-proteinase inhibitor. Cooperative use of lysosomal proteinases and oxygen metabolites. J Clin Invest. 1984 May;73(5):1297–1303. doi: 10.1172/JCI111332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Young J., LoBuglio A. F., Slivka A., Nimeh N. F. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981 Sep;68(3):714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada O., Moldow C. F., Sacks T., Craddock P. R., Boogaerts M. A., Jacob H. S. Deleterious effects of endotoxin on cultured endothelial cells: an in vitro model of vascular injury. Inflammation. 1981 Jun;5(2):115–126. doi: 10.1007/BF00914201. [DOI] [PubMed] [Google Scholar]







