Abstract
Background
The current criteria for alcohol use disorders (AUD) do not include consumption (quantity/frequency) measures of alcohol intake, in part due to the difficulty of these measures in humans. Animal models of ethanol self-administration have been fundamental in advancing our understanding of the neurobiological basis of (AUD) and can address quantity/frequency measures with accurate measurements over prolonged periods of time. The non-human primate (NHP) model of voluntary oral alcohol self-administration has documented both binge drinking and drinking to dependence and can be used to test the stability of consumption measures over time.
Methods and Results
Here, an extensive set of alcohol intakes (g/kg/day) was analyzed from a large multi-cohort population of Rhesus (Macaca mulatta) monkeys (n=31). Daily ethanol intake was uniformly distributed over chronic (12 months) access for all animals. Underlying this distribution of intakes were subpopulations of monkeys that exhibited distinctive clustering of drinking patterns, allowing us to categorically define very heavy drinking (VHD), heavy drinking (HD), binge drinking (BD), and low drinking (LD). These categories were stable across the 12-month assessed by the protocol, but exhibited fluctuations when examined at shorter intervals.
Conclusions
The establishment of persistent drinking categories based on quantity/frequency suggests that consumption variables can be used to track long-term changes in behavioral, molecular or physiochemical mechanisms related to our understanding of diagnosis, prevention, intervention and treatment efficacies.
Keywords: alcohol, binge drinking, ethanol, self-administration, non-human primates
Introduction
Epidemiological studies have documented that about 9% of individuals that regularly drink alcohol will develop a diagnosis of alcohol use disorder (AUD) using the Diagnostic and Statistical manual of Mental Disorders (DSM-IV) criteria (Dawson et al., 2008a). This statistic reflects the phenomena that not all individuals who have regular access to alcohol are at equal risk for developing AUD. Epidemiological research also shows quantifiable levels of severity of AUDs suggesting a common underlying condition in the criterion for diagnosis (Hasin et al., 2013a). Of the eleven DSM-V criteria for Substance Use Disorders (SUD), using larger amounts over longer periods of time is the closest to a consumption measure, but may also relate to the development of tolerance. Although apparently considered, “quantity/frequency” (e.g., 5 or more drinks for men and four or more drinks for women at least weekly) worsened the model fitting the other criteria, did not generalize well to other substances and was difficult to measure reliably in humans (Hasin et al., 2013b). Nevertheless, alcohol consumption patterns are likely to predict the onset and offset of AUD and it is likely that these patterns also underlie the continuum of AUD severity, including the risk for alcohol-related harm (Saha et al. 2007; Dawson et al. 2005; Dawson, Stinson, et al. 2008). That is, there are clear associations between alcohol intake and the risk for developing an AUD as well as remission of an AUD even with continued drinking (Dawson et al., 2008b).
As noted above, it is not easy to reliably and accurately measure the quantity and frequency of alcohol intake in the human population. There are cultural differences in defining a “drink” and in assessing the accuracy of self-reporting in relation to drinking behavior or the associated timeframes related to drinking (Hasin et al. 2013). Nevertheless, there has been considerable effort in quantifying the amount of alcohol consumed as a fundamental measure in the development of AUDs. A threshold amount of quantity/frequency that defines “risk drinking”, “heavy episodic drinking” or “binge drinking” as synonymous terms is five or more alcoholic drinks/day for a male and 4 or more drinks/day for a female with an outcome of raising blood ethanol concentrations (BEC) above 80 mg/dl (Dawson 2011; Li et al. 2007). Several studies have shown the number of times an individual reports engaging in binge drinking per week is related to the severity of DSM criteria (Li et al. 2007; Dawson, Goldstein, et al. 2008; Saha et al. 2007). Further, Li et al (2007) argued that quantity consumed, patterns of drinking and drinking frequency are essential information needed to better understand the neurobiological processes at play in the etiology of AUD (Li et al., 2007).
The neurobiological basis of alcohol use disorders (AUD) has benefited greatly from interrogating animal models of ethanol exposure. As clearly articulated by Li and colleagues (Li et al., 2007), rodent and primate models have helped define molecular, physiological and behavioral correlates of AUDs and have modeled all seven of the original elements described by Edwards and Gross (Edwards and Gross, 1976) that served as a basis for defining an alcohol dependence syndrome in the DSM (Li et al., 2007). Although rodent models have been developed to study binge drinking, evident by attaining blood ethanol levels over 80 mg/dl, most models employ relatively short highly-prescriptive exposure to alcohol (a few hours/day for days or weeks) (Crabbe et al., 2012). Rodent models that rely on long-term free choice are uncommon, however, recent reports of mice selectively bred for alcohol preference have demonstrated high average BECs, metabolic tolerance and sustained intakes over a three week period (Matson and Grahame, 2013; Matson et al., 2013, 2014). In contrast, a non-human primate (NHP) model of voluntary oral alcohol consumption has documented daily ethanol consumption over the course of months to years (Cuzon-Carlson et al., 2011; Grant et al., 2014). In this monkey model reliable individual differences in drinking reflect a wide spectrum of intoxication (resultant BECs 30->300 mg/dl; Grant et al. 2008; Grant et al. 2014). The drinking patterns associated with this NHP model are accurate and reliable and offer an opportunity to quantify consumption patterns associated with developing an alcohol dependence syndrome (Cuzon Carlson et al. 2011; Welsh et al. 2011), and document harmful effects on the hepatic (Ivester et al., 2007), cardiovascular (Cheng et al., 2010), endocrine (Helms et al., 2013), immune (Messaoudi et al., 2013) and nervous systems (Kroenke et al., 2013). Further, organismal risk factors for heavy episodic drinking, such as the age of first intoxication, sex, in utero exposure, genetic polymorphisms, stressful environments, etc., can be compared directly to better understand unique opportunities for intervention or tailored treatments.
The present study aimed to define categories of alcohol consumption patterns related to a spectrum of outcome severity using a population of monkeys all subjected to the same self-administration protocol. Previously, we have categorically defined individual monkeys as chronic heavy drinkers provided that they attained an average ethanol intake equal to or greater than 3.0 g/kg/day (a 12-drink equivalent) over 12 months of open-access to ethanol (Grant et al., 2008). This classification was based on a small sample size of 10 animals, wherein 4 individuals commonly achieved BECs (100-300 mg/dl, 7 hours after the onset of drinking) associated with very heavy drinking in humans (Mello and Mendelson, 1972). Here, we report on an analysis of ethanol consumption patterns across 22hr of access a day using 31 male rhesus monkeys. We tested the robustness of our categorization, found evidence for a more granular classification of drinking than previously reported and determined the stability of this classification over time. Our analysis models addressed issues of describing the uniform distribution of daily ethanol intakes and found evidence that distinct categories of intake exist with increasing severity of outcomes related to these categories. Finally, we show that ethanol is consumed very differently from water and in a manner that either increases (in heavy drinking monkeys) or decreases (in light drinking monkeys) the probability of drinking to intoxication each day (i.e., attaining a BEC >80 mg/dl).
Methods and Materials
Animals
Animals (n=31) in this study were male Rhesus (Macaca mullata), derived from four cohorts designated as 4, 5, 7a, and 7b and studied chronologically in that numerical order. The breakdown of cohorts is as follows: Cohort 4 consisted of 10 males, with a mean age of 10.1 years; Cohort 5 was made up of 8 males, mean age of 7.2 years; Cohort 7a had 8 males with a mean age of 5.8 years; and, Cohort 7b had 5 males with a mean age of 7.2 years. The mean age of first intoxication for cohorts 4, 5, 7a and 7b were 8.5, 5.6, 4.4, and 5.9 years respectively, reflecting lifespan ages of late adolescence to full adulthood, with an aged rhesus monkey normally defined as >20 years. All animals were born into a pedigreed population at the Oregon National Primate Research Center (ONPRC) and remained with their mothers in a multigenerational troop until weaning at about 2 years or age. All monkeys were continually housed at the ONPRC and entered into an internal housing environment (i.e., laboratory building) within individual caging at least 3 months prior to the onset of ethanol self-administration. These subjects were not part of cohorts that initially defined chronic heavy drinking in our monkey model and reported in (Vivian et al., 2001) or (Grant et al., 2008).
Ethanol Self-Administration
The monkeys were induced to self-administer 4% ethanol (w/v in water) using a schedule-induced polydipsia (SIP) procedure previously described in detail (Grant et al. 2008; Vivian et al. 2001). A key aspect of the induction of ethanol self-administration was that the dose of ethanol the monkeys were required to consume each day increased every 30 days beginning from 0 g/kg/day (a volume of water equivalent to 1.5 g/kg ethanol), to 0.5 g/kg/day, to 1.0 g/kg/day, and finally 1.5 g/kg/day. In this manner all monkeys drank to levels that saturated metabolic capacity and increased BEC to >50 mg/dl. After the 30th session of 1.5 g/kg ethanol under schedule induced polydipsia the monkeys had concurrent access to ethanol (4% w/v) and water 22 hours/day (termed “open access”) and food was provided as at least three meals per day for over 12 months, as previously described (Grant et al., 2008).
Drinking Data Collection
Each drinking panel has two spouts for access to fluids, either 4% ethanol (w/v diluted in water) or water. Access to fluid through the spouts is controlled using miniature solenoid valves (Parker Fluidics, Cleveland, OH) activated by a computer interface (National Instruments Corporation, Austin, TX). Flexible tubing connects two fluid reservoirs through the valves to the spouts. The fluid reservoirs rest on balances with readability to 0.1 gram (O'Haus Corporation, Parsippany, NJ). The balances deliver data to the controlling computer through serial communication and the changing weight of the reservoirs reported by the balances is continuously monitored, with each gram of displacement equated to one ml of fluid (water or 4% w/v ethanol). Programming of panel control and data collection is done using data acquisition input/output hardware and custom programming within the Labview program development environment (National Instruments Corporation, Austin, TX). The computer systems are Dell computers (Dell Inc., Round Rock, TX) running Windows operating system (Microsoft Corporation, Redmond, WA). Collected data is processed further using SQL and Perl database interface scripts in custom Postgresql databases running on a MacPro Computer (Apple Corporation, Cupertino, CA). Technician ancillary notes and any related data are checked and cross-referenced to experimental data using Microsoft's Excel program.
BEC Data collection
The subjects were trained with fruit presentation to come to the front of their cage and present their leg for the 20 microliter blood sample for BEC measure. The blood was drawn into a capillary pipet after the saphenous vein was located, and a 22 gauge needle was used to puncture the vein. Thus, no anesthesia was used and all monkeys learned to comply with this procedure without showing signs of stress or discomfort. For example, prior to ethanol self-administration, ACTH and cortisol taken with this procedure has strong circadian rhythm and low evening nadirs that are not captured in stressed monkeys (Helms et al., 2013). During Induction blood samples (20 μl) were taken approximately every fifth day from every monkey at 30, 60, and 90 minutes after the start of the 0.5, 1.0, and 1.5 g/kg induction sessions, respectively. These time points were chosen because they are the time of peak BEC when 0.5, 1.0, and 1.5 g/kg is given in bolus by stomach gavage to cynomolgus macaques (Green et al., 1999). Blood samples were sealed in air-tight vials containing 0.5 ml of distilled water and 0.02 ml of isopropanol (10%; internal standard), and stored at −4°C un l assayed (Hewlett-Packard 5890 Series II, Avondale, PA, equipped with a headspace autosampler, flame ionization detector, and a Hewlett Packard 3392A integrator). During open-access self-administration blood samples (20 μl) were taken from the saphenous vein, approximately every 5-7 days throughout the 12 months, from every monkey 7 hours after the start of self-administration sessions, just before lights out for the night. Blood samples were sealed in air-tight vials containing 0.5 ml of distilled water and 0.02 ml of isopropanol (10%; internal standard), and stored at −4°C un l assayed as described above.
For both induction and self-administration periods the amount of ethanol solution consumed from session start to the time of each blood sample was recorded. Later, these volumes were compared with the volume of ethanol consumed over the entire 22 hr/day open session and recorded in that day's data file. Intake at the time of the blood sample was then calculated as a percentage of total daily intake to create a comprehensive view of drinking pattern related to BEC at 7 hrs into the 22 hr daily session.
MATRR
The Monkey Alcohol and Tissue Research Resource (MATRR) was developed to store tissues from ethanol and control subjects, empirical data relating to alcohol self-administration, data derived from tissue analysis, and data analytics (including data mining and data harmonization, and tissue dissemination) (Daunais et al. 2014). The MATRR serves to close the loop between tissue derived from this oral alcohol self-administration model and integrated analysis of behavioral, physiological, pharmacological and molecular data by tracking analyses conducted on distributed tissues, thus reinforcing tissue selection and experimental development by a network of laboratories. Metadata about all animals described in this study, as well as raw and semi-processed drinking and behavioral data, are imported in MATRR (http://www.MATRR.com) where analysis is performed using a suite of online and offline analytics.
Computing and Statistics
All computations were executed on MATRR's web and database servers. These are twin machines, each running 4 Intel Xeon E5620 (2.4 Ghz) with 4 cores each, 47 GB RAM and 1.7 TB disk space in a RAID configuration. Web services leverage the Django ORM for database access and several well-established python libraries for data processing, visualization, and statistics. In particular, statistical calculations were conducted using NumPy and SciPy python packages. NumPy arrays provide several basic statistical functions, such as Standard Deviation and Mean, while more complex statistical methods are provided in the SciPy library. Multivariate analysis of variance was performed using Wilks and Pillai tests in R (v.3.1.1).
Categorical Classification Strategy
Previously we defined “heavy”, “moderate” and “light” drinking in this monkey model based on definitions from the human literature including daily number of drinks, percent of daily calories in the form of ethanol, and average BECs measured (see (Vivian et al., 2001)). We further refined this categorization by showing how very heavy drinking days (days over 4.0 g/kg intake) helped demarcate the heavy from the non-heavy drinking monkeys (see Grant et al., 2008). Both of these initial attempts to provide a categorical level of quantity/frequency were based on single cohorts of monkeys. The data provided here are from a combined 4 cohorts of monkeys that were not previously characterized and help to replicate and extend these categorical levels.
Having defined these categories based on daily intakes, the current analysis was undertaken to test these classifications with observed demarcations (>2.0 g/kg/day, >3.0 g/kg/day, >4.0 g/kg/day) from >12,000 daily sessions in 31 monkeys. Specifically, based on a step-wise analysis, there is evidence for 4 distinct categories of drinkers: light, binge, heavy and very heavy. First, the aggregate daily intakes across all animals were investigated for qualitative measures of distribution. An affirmative qualitative test of bounded uniform distribution (mean=2.388, stdev=1.09) [Supplement, S1] enabled the assignment of testable classifications based on the mean and variance of the population intakes. Second, the classifications were then tested for stability over time. Third, the categorical specificity of ethanol was tested by comparing it to water intake within the same sessions. Finally, validation of these categories was attempted using supplemental BEC data because BEC is an objective measure used in human studies to define levels of intoxication.
Results
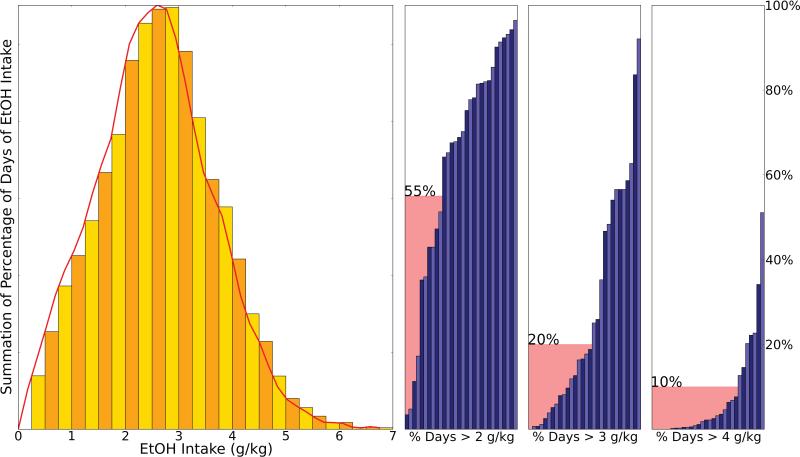
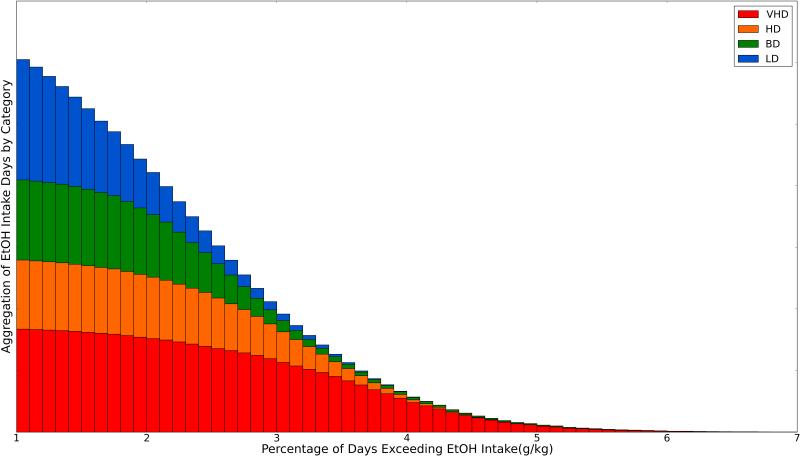
The aggregate summation of ethanol consumption for all animals over all sessions reflects a uniform distribution during open access (22 hr/day access, with a minimum of 341 days [cohort 7b] and a maximum of 407 days [cohort 4]) [Figure 1A]. For all monkeys, the percentage of days that exceed 2 g/kg (63.7%, 0.26 stdev), 3 g/kg (27.6%, 0.58 stdev), and 4 g/kg (7.29%, 1.48 stdev) are used as ethanol consumption thresholds for binge drinkers (BD), heavy drinkers (HD), and very heavy drinkers (VHD), respectively. Figure 1B illustrates the percentage of days in which each animal exceeded 2 g/kg, 3 g/kg, and 4 g/kg. Without an established percentile cutoff for category definition available in the literature, cutoff thresholds for each category were based on observed demarcations in the percentage of days any given animal might consume 2 g/kg, 3 g/kg and 4 g/kg. Cutoff thresholds for BD, HD, and VHD were set to > 55% at 2 g/kg, > 20% of days at 3 g/kg, and > 10% of days at 4 g/kg, respectively. Viewed differently, Figure 2 illustrates the aggregate summation for all animals, grouped by classification, and stacked. The classification schema demonstrates the consistency of drinking patterns of animals within groups, with VHD drinkers, for example, representing the largest share of animals consuming greater than 4 g/kg. Low drinking days (consumption < 0.5 g/kg) do not account for the discrimination between categories [Supplement, S2]. The data indicate that the overall number of low-drinking days is small and that only the lowest drinking animals have a prominent increase in the number of abstinent days.
Figure 1.
Distribution of daily ethanol intake during open access. Panel (A) represents the aggregate summation for all animals (31 drinking animals representing male Rhesus cohorts 4, 5, 7a, 7b) in the “open-access” (22 hr/day access) condition (min 341 – max 405 days). Ethanol intake (g/kg/day) is plotted along the x-axis, ranging from 0-7 g/kg/day. The distribution (mean=2.388, stdev=1.09) meets qualitative tests for bounded uniform distribution. For all monkeys, the number of days that exceed 2 g/kg is 63.7% (0. 26 stdev), the number of day that exceeded 3 g/kg is 27.6%, (0.58 stdev), and the number of days that exceeded 4 g/kg is 7.29% (1.48 stdev). Panel (B) illustrates the percentage of days where each animal exceeded 2 g/kg, 3 g/kg, and 4 g/kg (subpanels, left to right). Cutoff thresholds (2.0 g/kg, 3.0 g/kg and 4.0 g/kg) for BD, HD, and VHD were set to 55%, 20%, and 10%, respectively, based on previous publications for BD (Monti et al, 2004), HD and VHD (Grant et al., 2008, Grant et al., 2014).
Figure 2.
Distribution of EtOH intake over animals classified as LD, BD, HD, and VHD. This graph illustrates the aggregate summation for all animals, grouped by classification, and stacked ranging from 0-7 g/kg. The classification demonstrates group-dependent regularity within intake ranges as dose increase, with VHD drinkers, for example, representing the largest subset of animals consuming greater than 4 g/kg.
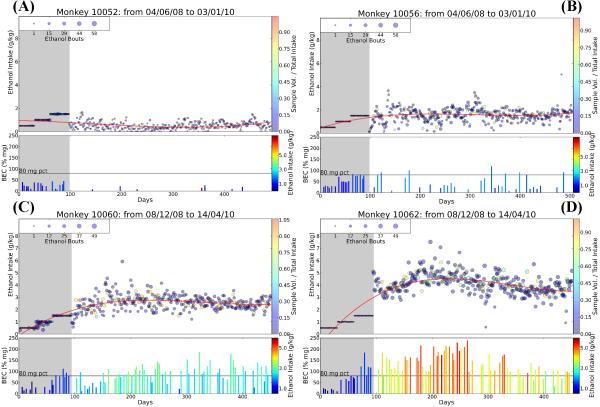
The choice of category names is intended to reflect observable daily intakes of consumption. For example, binge drinkers demonstrate an average ethanol intake 2.4 g/kg during 12 month open access with occasional episodes of intake > 3.0 g/kg (14% of days), and these intakes were associated with BECs>80 mg/dl (Figure 3, Panel B). In contrast, monkeys classified as heavy drinkers (HD) had an average ethanol intake of 2.8 g/kg over 12 month open access. While similar to binge drinkers on average, they demonstrate frequent episodes of intake > 3.0 g/kg (43% of days) resulting in an increased number of days with BECs >80 mg/dl and several documented days of greatly elevated BECs (Figure 3, panel C). In this regard, HD drinkers can be considered chronic binge drinkers. Given individual representative animals designated as LD, BD and HD, the LD animals have a visually more cohesive variance of daily ethanol intake and BEC than either BD or HD animals [S6]. While the day-to-day changes in ethanol intake amount between BD and HD are similar, their overall volume of intake differs markedly from the consistently high volume of intake observed in VHD monkeys [Figure 3, panel D]. For example, HD monkeys only had 3.8% of their days over 4.0 g/kg, whereas VHD animals averaged 26.3% of days (low=13.4%, high=52.5%) over 4gkg, and 67.7% of days (low=47.7%, high=94.6%) over 3gkg. VHD animals consistently reach elevated levels of BEC with only a fraction of their daily ethanol intake accounted for at time of BEC sample. HD animals, conversely, reach elevated BEC levels but do so only after consuming the majority of their daily ethanol intake (Figure 3, panel C, D). This reiterates an important point in our model: since animals are allowed open access to ethanol, the variability in the amount of ethanol consumed at the time of BEC monitoring complicates a correlative analysis of total daily (22 hr) ethanol consumption and daily BEC values (taken at 7 hrs).
Figure 3.
Representative categorical drinking patterns illustrate numerous differences between (A) LD, (B) BD, (C) HD, and (D) VHD individual animals. Each panel spans induction and open access timeframes. In the top graph of each panel is the daily ethanol intake over induction (shaded in gray) and during the open-access 22 hr session (no shading). The size of the circles in the upper graphs of each panel represents the number of bouts taken during the session and the color of the circles represents the sample volume as a percentage of the total intake. The lower graphs of each panel represents the BECs during induction (gray shading) and the 22 hr session (no shading). Ethanol intake at the time of the sample is depicted by color (left axis). A typical LD animal has small ethanol bout sizes, stable and low levels of alcohol consumption, and seldom reach 80 mg/% BEC levels indicative of intoxication. BD animals have relatively low alcohol consumption but, unlike LD animals, experience periodic bouts of ethanol consumption, elevated BEC levels, and days of abstinence. While VHD and HD animals exceed 80 mg/% BEC levels on numerous occasions, VHD animals consistently exceed this value with a lower percentage of daily ethanol intake at time of BEC measure.
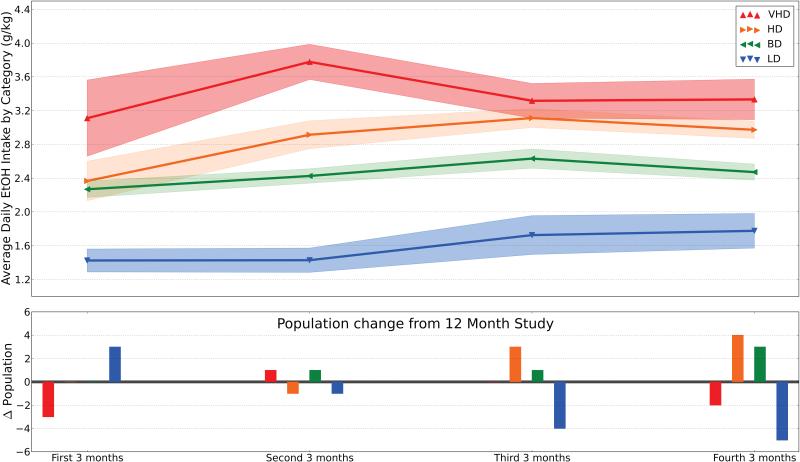
To demonstrate that our assigned categories reflect stability over time, we analyzed the ethanol consumption from each monkey in 3-month epochs [Figure 4]. As might be expected, there appears to be slight drifts in the means and standard deviations of each category during each sub-time frame. However, there is no change to the rank order of categories and only small changes to the delineations of the categories. Re-classifying all animals based on the original assignment criteria, but using the smaller 3-month time frame, was also used to examine categorical stability. Intervals less than 3 months did not appear informative. When the animals in these new categories were compared to their original placement in the 12-month categorization several interesting patterns appear. First, out of the 124 potential changes of categories [Supplement, S3], there was a tendency for monkeys to remain assigned to a particular drinking category throughout the experimental timeline. Monkeys changed classifications in about 32% of the opportunities presented at 3-month intervals across 12 months of consumption, while only 4.8% of changes crossed two or more drinking categories. The number of times a monkey changed categories remained consistent across categories: 11, 9, 10, and 10 changes for LD, BD, HD, and VHD animals, respectively. Secondly, there is no strong consensus in the temporal bias or direction of change. The number of changes to higher consumption categories (21 changes) balances with the total number of animals moving down consumption categories (19 changes).
Figure 4.
Stability of categorical drinking classifications over time. This figure illustrates the categorical classifications of animals examined in 3-month epochs within the 12-month open access protocol. (Top) Average ethanol consumption (lines) and standard error (shaded areas) for each category is plotted across smaller time frames. Categories defined by 12-month drinking history are very stable, with each category expressing the appropriate order and delineation during each time period. However, if categories were assigned using the original assignment criteria based on only 3 months of data, there is some localized variability when compared to the 12-month assignment (Bottom). For example, the first 3 months indicates an additional 3 low drinking animals and a loss of 3 VHD. Conversely, the final 3-month period indicates a loss of 5 LD and 2 VHD and the consequent gain of 4 HD and 3 BD.
Identifying characteristics of subjects that remain in a category of drinking (e.g., LD) or escalate to another category (e.g., VHD) could augment current approaches to using animal models to identify risk factors for AUD. It is also noteworthy that the movement away from LD and towards HD status increased in the second 6 months of the 12 months period of study. An increase in average daily intake occurred among all four groups during the first 3 months of access, except for LD monkeys. The increase among VHD monkeys was approximately 0.7 g/kg, of the equivalent of three drinks per day in three months, whereas HD monkeys increases their intake by 0.5 g/kg across subjects, a 2-drink equivalent. For comparison, the daily ethanol intake in the VHD monkeys for months 3-6 of the protocol represents an average of 15 human drink equivalents/day (using 17g/drink or 0.25g/kg as 1 drink, (Vivian et al., 2001)). This escalation has implications for the study of co-morbid disorders, and suggests that this monkey model can be used to assess both drinking severity (LD< BD< HD< VHD) and duration (6 vs. 12 months) to better predict the onset of AUD-related co-morbidities such as liver, heart, and immune system dysfunction (Messaoudi et al., 2013; Ivester et al. 2007, Chang et al. 2010).
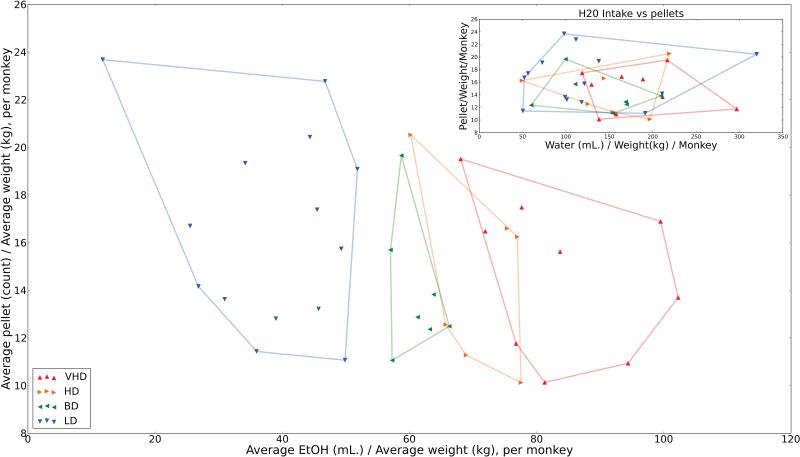
By analyzing these categories against a third parameter background, we can measure category stability in the presence of potentially confounding parameters. Figure 5 illustrates the correlation of ethanol consumption (g/kg) with the number of food pellets consumed for each animal normalized for weight. It is interesting to note that there is a significant partitioning of drinking categories based on ethanol consumption that is not reflected in the presence of water as determined by a multivariate analysis of variance (p<0.00186). Rather, in the case of normalized food intake versus water consumption, all four categories of ethanol drinkers clearly overlap and are indistinguishable.
Figure 5.
Categorical drinking assignment in ethanol and water consumption. This figure illustrates the correlation of ethanol consumption (g/kg) normalized for weight with the number of food pellets consumed for each animal normalized for weight. It is interesting to note an almost complete partitioning of drinking categories based on ethanol consumption. This same distribution is not viewed in the case of normalized food intake versus water consumption.
Discussion
The distribution of results suggest that under these controlled conditions, there is a normal level of ethanol consumption with a population mean equivalent to approximately 12 drinks per day in individually-housed rhesus macaques, having gone through the SIP induction and for whom there are little temporal or social constraints on drinking. The variation in intake from near abstinence to very heavy drinkers is similar to the human population, except this variation occurs in the absence of the key variables often considered to be related to ethanol intake. For example, these monkeys did not watch their parents drink, they were not exposed to alcohol in utero, and they do not experience loss of other resources due to their drinking. Thus, it is interesting that despite living in a uniform environment with identical treatment and diet, these monkeys also show a wide variation in inter-individual intake.
A majority of monkeys were consistent in their ethanol intake over time. Individuals were assigned to categories of alcohol consumption based on cut-offs identified in the frequency distributions of daily intake (Figures 1 and 2). These categories are important because they identify a pattern of intake amidst variation across days (Figure 3) such that even very heavy drinkers occasionally have low-drinking days. That is, the extended time frame studied here allowed identification of temporal changes in primate drinking. Specifically, whereas many subjects showed stable intakes over time, others showed an upward (escalation) or downward (deceleration) titration. Variation in the trajectory of intake despite identical procedures and environment suggest wide individual differences in homeostatic adaptation to chronic ethanol exposure that is likely to be the basis of the larger/longer dimension in the transition to or remission from dependence.
Studies on the development of heavy alcohol consumption in humans is limited and largely focused on “heavy drinking trajectories from adolescence to early adulthood” (Dawson et al., 2008b). Nevertheless, a few studies have examined the stability of an AUD diagnosis (Dawson, Goldstein, et al. 2008; Hasin et al. 1990). In general, studies of the US drinking population have documented similar trajectories as here, for example, transitions from a diagnosis of “alcohol dependence” to a diagnosis of “Alcohol Use Disorder” as well as between other categories of risky drinking over a 3-year time frame (Dawson et al., 2008b). Further, the monkey data suggest that categorical drinking levels have a strong biological basis. Overall, the monkey model, given the control over environmental variables, is an excellent model to help define genetic influences on the progression to (and stability of) categorical levels of alcohol drinking.
It is important to note that the conditions under which the monkeys have been first induced to drink alcohol and then have nearly continuous access to alcohol is very different from the experience of most humans. Although SIP is relatively easy to induce in human subjects under laboratory conditions (Doyle and Samson, 1985) and has been proposed to be operative in much of human society under various schedules of work, meals, social access and monetary reinforcement (Tang et al., 1984), the extreme control over alternative schedules and sources of reinforcement within our model require caution in extrapolating to the human condition. Future advances in the ability to precisely monitor human alcohol consumption and activity throughout the course of drinking will undoubtedly advance our knowledge of quantity/frequency as a diagnostic feature of AUD. Nevertheless, the lifetime history of alcohol quantity/frequency examined here suggests that this monkey model can help fill the gaps of underlying biological bases of drinking categories where human subject research is currently lacking.
Overall, a relatively low proportion of monkeys drink at the very highest categories of drinking. In this population of 31 monkeys, 13 were categorized as HD or VHD after 12 months of self-administration. Although this proportion of the heaviest drinkers is higher than the 8-9% proportion of US alcohol consumers categorized with AUD (Dawson et al., 2008a), as noted previously these monkeys have few limits on consumption compared to humans, which have legal, societal, religious and economic restrictions on drinking. An interpretation of the over estimate of HD and VHD drinking in this monkey sample compared to humans is that by minimizing environmental restrictions on access to alcohol, the biological risk for heavy alcohol consumption in old world macaques appears very high. By extension, the biological risk to human primates would also appear to be high, but curtailed by the norms imposed upon alcohol use. Social, economic and policy variables in the US appear to constrain alcohol drinking, rather than completely preventing very heavy or very low drinking. Thus, if similar constraints were applied to monkeys involved in these studies (e.g., an interval of work each day without access to ethanol, or punishment for intoxication during “working” hours) it is possible that most monkeys would decrease their intake similarly, perhaps to the level observed in US alcohol consumers.
In people, the failure of these constraints to control drinking are included in the diagnostic criteria, with the assumption that such constraints regulated drinking before problem drinking occurred. Some animal models are focused on ethanol self-administration despite punishment (e.g., (Vendruscolo et al., 2012)) to model the implied loss of control by social contingencies in humans. However, it is unclear how to operationally define loss of control in the transition to dependence in a laboratory setting. Specifically, ‘control’ in the context of an AUD could be defined never drinking, drinking below binge intoxication, or drinking less than a previous norm. A more useful concept in translational studies may be “risk” drinking that can be defined by harmful outcomes, particularly biomedical co-morbidities but also risky social choices if the experimental parameters allow for these variables (Eckhardt et al., 1998). But, ultimately, the data highlight that that amount and frequency that an individual drinks alcohol over a long term (3 months in this study) is a fundamental symptom that reflects the organism's propensity to become and remain intoxicated. It is from this basic symptom that all other diagnostic criteria of the DSM for AUD are developed over the course of addiction to alcohol.
In conclusion, our evidence indicates strong temporal stability of alcohol drinking categories, such that macaques that begin as, for example, light drinkers, largely continue to be light drinkers over 12 months. However, within these categories we also observed some variance with some individuals having temporary deviations from categorical drinking including low-drinking, no-drinking days (self-imposed abstinence), and binge days. Less common, we found some evidence for trends of a sufficient magnitude that resulted in a shift to a different drinking category. Further developing techniques to interrogate the covariates of these deviations are critical to understanding abstinence, binges, and increased drinking over time and should also have translational value to the human condition. Without the presence of many environmental restrictions on daily self-administration, biological factors are relatively more pronounced.
In the monkey model, identifiable and stable drinking categories can provide a backdrop against which we can examine potential indicators and causes of alcoholism. These include investigating potential such as sex, age at the onset of drinking, and genetic correlations. Robust categorization also organismal categorical determinants, enables exploration of correlations with relevant behavioral or social observations, such as the influences of social housing, social hierarchy, and maternal behavior.
Supplementary Material
Acknowledgments
This research was support by NIH grants AA013541, AA 0013510, AA 109431, OD 11092
Footnotes
Author Contribution:
EJB and KAG were responsible for the study concept and design. SG contributed to the curation of the data sets and JF and EJB performed analysis. JF created all figures. EJB, KAG and CH contributed to the drafting and critical review of the manuscript.
References
- Asquith M, Pasala S, Engelmann F, Haberthus K, Meyer C, Park B. Chronic ethanol consumption modulates growth factor release, mucosal cytokine production and microRNA expression in nonhuman primates. Alc. Clin. Exper. Res. doi: 10.1111/acer.12325. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang ET, Clarke CA, Canchola AJ, Lu Y, Wang SS, Ursin G, West DW, Bernstein L, Horn-Ross PL. Alcohol consumption over time and risk of lymphoid malignancies in the California Teachers Study cohort. Am. J. Epidemiol. 2010;172:1373–1383. doi: 10.1093/aje/kwq309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H-J, Grant KA, Han Q-H, Daunais JB, Friedman DP, Masutani S, Little WC, Cheng C-P. Up-regulation and functional effect of cardiac β 3-adrenoreceptors in alcoholic monkeys. Alcohol. Clin. Exp. Res. 2010;34:1171–1181. doi: 10.1111/j.1530-0277.2010.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Harkness JH, Spence SE, Huang LC, Metten P. Intermittent availability of ethanol does not always lead to elevated drinking in mice. Alcohol Alcohol. Oxf. Oxfs. 2012;47:509–517. doi: 10.1093/alcalc/ags067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzon Carlson VC, Seabold GK, Helms CM, Garg N, Odagiri M, Rau AR, Daunais J, Alvarez VA, Lovinger DM, Grant KA. Synaptic and morphological neuroadaptations in the putamen associated with long-term, relapsing alcohol drinking in primates. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2011;36:2513–2528. doi: 10.1038/npp.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daunais J, Davenport A, Helms C, Gonzales SW, Hemby S, Friedman D, Farro J, Baker EJ, Grant KA. Monkey Alcohol Tissue Research Resource: Banking Tissues for Alcohol Research. ACER. 2014 doi: 10.1111/acer.12467. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA. Defining risk drinking. Alcohol Res. Health J. Natl. Inst. Alcohol Abuse Alcohol. 2011;34:144–156. [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Li T-K. Quantifying the risks associated with exceeding recommended drinking limits. Alcohol. Clin. Exp. Res. 2005;29:902–908. doi: 10.1097/01.alc.0000164544.45746.a7. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcohol. Clin. Exp. Res. 2008a;32:2149–2160. doi: 10.1111/j.1530-0277.2008.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Stinson FS, Chou SP, Grant BF. Three-year changes in adult risk drinking behavior in relation to the course of alcohol-use disorders. J. Stud. Alcohol Drugs. 2008b;69:866–877. doi: 10.15288/jsad.2008.69.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle TF, Samson HH. Schedule-induced drinking in humans: a potential factor in excessive alcohol use. Drug Alcohol Depend. 1985;16:117–132. doi: 10.1016/0376-8716(85)90111-5. [DOI] [PubMed] [Google Scholar]
- Eckhardt CI, Barbour KA, Davison GC. Articulated thoughts of maritally violent and nonviolent men during anger arousal. J. Consult. Clin. Psychol. 1998;66:259–269. doi: 10.1037//0022-006x.66.2.259. [DOI] [PubMed] [Google Scholar]
- Edwards G, Gross MM. Alcohol dependence: provisional description of a clinical syndrome. Br. Med. J. 1976;1:1058–1061. doi: 10.1136/bmj.1.6017.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Leng X, Green HL, Szeliga KT, Rogers LSM, Gonzales SW. Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol. Clin. Exp. Res. 2008;32:1824–1838. doi: 10.1111/j.1530-0277.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Ferguson B, Helms C, McClintick M. Neurobiology of Alcohol Dependence. Elsevier; 2014. Risk factors in drinking to dependence in non-human primates. [Google Scholar]
- Green KL, Szeliga KT, Bowen CA, Kautz MA, Azarov AV, Grant KA. Comparison of Ethanol Metabolism in Male and Female Cynomolgus Macaques (Macaca fascicularis). Alcohol. Clin. Exp. Res. 1999;23:611–616. [PubMed] [Google Scholar]
- Hasin DS, Grant B, Endicott J. The natural history of alcohol abuse: implications for definitions of alcohol use disorders. Am. J. Psychiatry. 1990;147:1537–1541. doi: 10.1176/ajp.147.11.1537. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Auriacombe M, Borges G, Bucholz K, Budney A, Crowley T, Grant BF, O'Brien C, Petry NM, Schuckit M, et al. The DSM-5 field trials and reliability of alcohol use disorder. Am. J. Psychiatry. 2013a;170:442–443. doi: 10.1176/appi.ajp.2013.13010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, O'Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, Compton WM, Crowley T, Ling W, Petry NM, et al. DSM-5 criteria for substance use disorders: recommendations and rationale. Am. J. Psychiatry. 2013b;170:834–851. doi: 10.1176/appi.ajp.2013.12060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Gonzales SW, Green HL, Szeliga KT, Rogers LSM, Grant KA. Diurnal pituitary-adrenal activity during schedule-induced polydipsia of water and ethanol in cynomolgus monkeys (Macaca fascicularis). Psychopharmacology (Berl.) 2013;228:541–549. doi: 10.1007/s00213-013-3052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivester P, Roberts LJ, 2nd, Young T, Stafforini D, Vivian J, Lees C, Young J, Daunais J, Friedman D, Rippe RA, et al. Ethanol self-administration and alterations in the livers of the cynomolgus monkey, Macaca fascicularis. Alcohol. Clin. Exp. Res. 2007;31:144–155. doi: 10.1111/j.1530-0277.2006.00276.x. [DOI] [PubMed] [Google Scholar]
- Kroenke CD, Rohlfing T, Park B, Sullivan EV, Pfefferbaum A, Grant KA. Monkeys that Voluntarily and Chronically Drink Alcohol Damage their Brains: A Longitudinal MRI Study. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2013 doi: 10.1038/npp.2013.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T-K, Hewitt BG, Grant BF. The Alcohol Dependence Syndrome, 30 years later: a commentary. the 2006 H. David Archibald lecture. Addict. Abingdon Engl. 2007;102:1522–1530. doi: 10.1111/j.1360-0443.2007.01911.x. [DOI] [PubMed] [Google Scholar]
- Matson LM, Grahame NJ. Pharmacologically relevant intake during chronic, free-choice drinking rhythms in selectively bred high alcohol-preferring mice. Addict. Biol. 2013;18:921–929. doi: 10.1111/j.1369-1600.2011.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson L, Liangpunsakul S, Crabb D, Buckingham A, Ross RA, Halcomb M, Grahame N. Chronic free-choice drinking in crossed high alcohol preferring mice leads to sustained blood ethanol levels and metabolic tolerance without evidence of liver damage. Alcohol. Clin. Exp. Res. 2013;37:194–201. doi: 10.1111/j.1530-0277.2012.01873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson LM, Kasten CR, Boehm SL, Grahame NJ. Selectively bred crossed high-alcohol-preferring mice drink to intoxication and develop functional tolerance, but not locomotor sensitization during free-choice ethanol access. Alcohol. Clin. Exp. Res. 2014;38:267–274. doi: 10.1111/acer.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Drinking patterns during work-contingent and noncontingent alcohol acquisition. Psychosom. Med. 1972;34:139–164. doi: 10.1097/00006842-197203000-00007. [DOI] [PubMed] [Google Scholar]
- Messaoudi I, Asquith M, Engelmann F, Park B, Brown M, Rau A, Shaw J, Grant KA. Moderate alcohol consumption enhances vaccine-induced responses in rhesus macaques. Vaccine. 2013;32:54–61. doi: 10.1016/j.vaccine.2013.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha TD, Stinson FS, Grant BF. The role of alcohol consumption in future classifications of alcohol use disorders. Drug Alcohol Depend. 2007;89:82–92. doi: 10.1016/j.drugalcdep.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Kenny J, Falk JL. Schedule-induced ethanol dependence and phenobarbital preference. Alcohol Fayettev. N. 1984;1:55–58. doi: 10.1016/0741-8329(84)90037-5. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr, Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, et al. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J. Neurosci. Off. J. Soc. Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, Majerksy LS, Thomas BW, Shively CA, Tobin JR, Nader MA, Grant KA. Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): long-term characterization of sex and individual differences. Alcohol. Clin. Exp. Res. 2001;25:1087–1097. [PubMed] [Google Scholar]
- Welsh JP, Han VZ, Rossi DJ, Mohr C, Odagiri M, Daunais JB, Grant KA. Bidirectional plasticity in the primate inferior olive induced by chronic ethanol intoxication and sustained abstinence. Proc. Natl. Acad. Sci. U. S. A. 2011;108:10314–10319. doi: 10.1073/pnas.1017079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.