Abstract
Premorbid risk for major depressive disorder (MDD) and predictors of an earlier onset and recurrent course were examined in two studies in a large, community-based sample of parents and offspring, prospectively assessed from late childhood into adulthood. In Study 1 (N = 2,764 offspring and their parents), parental psychiatric status, offspring personality at age 11, and age-11 offspring internalizing and externalizing symptoms predicted the subsequent development of MDD, as did poor quality parent-child relationships, poor academic functioning, early pubertal development, and childhood maltreatment by age 11. Parental MDD and adult antisocial behavior, offspring negative emotionality and disconstraint, externalizing symptoms, and childhood maltreatment predicted an earlier onset of MDD, after accounting for course; lower positive emotionality, trait anxiety, and childhood maltreatment predicted recurrent MDD, after accounting for age of onset. In Study 2 (N = 7,146), we examined molecular genetic risk for MDD by extending recent reports of associations with glutamatergic system genes. We failed to confirm associations with MDD using either individual SNP-based tests or gene-based analyses. Overall, results speak to the pervasiveness of risk for MDD, as well as specific risk for early-onset MDD; risk for recurrent MDD appears to be largely a function of its often earlier onset.
Keywords: Major depressive disorder, age of onset, recurrence, familial risk, personality, psychosocial functioning, glutamatergic system candidate genes
Major depressive disorder (MDD) is a major public health concern. It occurs frequently in the general population and is associated with serious, pervasive, and lasting impairment. The National Comorbidity Survey Replication (NCS-R), an epidemiological survey that assessed diagnoses of common psychiatric disorders using retrospective, cross-sectional assessment, found a lifetime prevalence estimate of 16.6% for MDD (Kessler, Berglund et al., 2005). Recent prospective, longitudinal studies suggest that MDD may be even more common, with lifetime prevalence estimates through early-to-middle adulthood ranging from 26.0% to 54.8% (Beesdo et al., 2009; Hamdi & Iacono, 2014; Moffitt et al., 2010; Olino et al., 2012). For many individuals, depressive disorders emerge well before adulthood—the 3- to 12-month prevalence estimate is relatively low at 2.8% for children under age 13, but the estimate for MDD of 5.7% for adolescents aged 13 to 18 (Costello, Erkanli, & Angold, 2006) is comparable to the 12-month estimate of 6.7% for adults (Kessler, Chiu, Demler, Merikangas, & Walters, 2005).
MDD has tremendous negative implications for society as a whole and for the individuals it affects. At a societal level, MDD is ranked by the World Health Organization as the fourth leading cause of disability worldwide, and overall lost work performance due to MDD is estimated at $30 to $50 billion dollars a year (Sartorius, 2001; Üstün, Ayuso-Mateos, Chatterji, Mathers, & Murray, 2004). At an individual level, MDD is associated with decreased quality of life, impaired academic and occupational functioning, problematic interpersonal relationships, psychiatric comorbidity, suicidality, negative health behaviors (e.g. smoking, drinking, obesity, poor medication compliance), and increased mortality (Kessler, 2012). There is considerable interest in better understanding the etiology and phenomenology of MDD with the aim of preventing its onset and recurrence and ameliorating its consequences. A large body of literature on risk for MDD has identified family (e.g., family environment, socioeconomic status) and individual (e.g., gender, psychiatric comorbidity, personality) risk factors (see Dobson & Dozois, 2008, for a review). There have also been attempts to differentiate factors that confer risk for the first onset of MDD from risk for recurrence after its onset (Burcusa & Iacono, 2007; Coryell, Endicott, & Keller, 1992; Lewinsohn, Allen, Seeley, & Gotlib, 1999). For example, being female, lower socioeconomic status, and experiencing divorce are associated with the onset of MDD but not its recurrence, while stressful life events and the personality trait negative emotionality/Neuroticism are associated with first onset, as well as subsequent recurrence, of MDD.
In the present paper, we examined premorbid risk for the development of MDD, as well as predictors of an earlier onset or recurrent course, in a large, community-based sample. We assessed a broad range of putative risk factors in late childhood, well before the onset of MDD, and assessed MDD prospectively at multiple points from late childhood through adolescence and into adulthood. Because a relatively large proportion of cases of MDD onsets by adolescence (Costello et al., 2006; Hasin, Goodwin, Stinson, & Grant, 2005), investigations of premorbid risk for MDD and particularly early-onset MDD require assessments prior to adolescence. Our longitudinal investigation spans 2 decades, from late childhood into adulthood, and offers a unique opportunity to identify premorbid risk factors for MDD.
Overview of the Present Paper
We considered several indicators of risk for MDD in two studies. The overarching aim of these studies was to identify risk factors for MDD and for its most severe forms, early-onset and recurrent MDD. We first provide operational definitions of the terms used in the present paper, including “MDD,” “early-onset” MDD, and “recurrent” MDD. We take the pragmatic approach of using age of onset and course categories that facilitate empirical investigation and clinical decision making, though we consider these features to be best conceptualized as dimensional constructs. We then consider the overlap in early-onset and recurrent MDD and highlight the difficulty inherent in disentangling these clinical features. Next, we review the existing literature on risk for MDD, focusing in particular on studies of early-onset and recurrent MDD. Finally, we present two empirical studies of risk for MDD. In Study 1, we examined a range of putative risk factors assessed in late childhood, well before the onset of MDD, with the aim of identifying risk factors that predict the subsequent development of MDD, as well as an early onset or recurrent course. We considered risk factors drawn from a number of important domains and assessed using multiple methods and reporters. In addition to indicators of risk at the family (parental psychiatric status, parent-child relationship quality) and individual (personality, internalizing and externalizing psychopathology, academic functioning, physical development, childhood maltreatment) levels, in Study 2, we extended our examination to the genetic level by examining associations between MDD and candidate genes involved in the glutamatergic system that were recently identified using a novel gene-set-based association analysis.
Definitions of Constructs Considered in the Present Paper
MDD
MDD is defined in the current psychiatric classification systems (DSM-5, American Psychiatric Association [APA], 2013; ICD-10, World Health Organization, 2010) and similarly in earlier editions of the DSM (DSM-III-R, APA, 1987; DSM-IV, APA, 2000) by the presence of a major depressive episode and absence of any manic or hypomanic episodes, with a major depressive episode defined as a period of 2 weeks or longer in which five or more symptoms (depressed mood, anhedonia, weight or appetite disturbance, sleep disturbance, psychomotor agitation or retardation, fatigue, feelings of worthlessness or guilt, difficulty concentrating or indecisiveness, suicidal ideation) are present most of the day nearly every day. Although some researchers have used symptom checklists or rating scales (e.g., Beck Depression Inventory; Beck, Steer, & Brown, 1996) to measure depressive symptomatology, we largely limit our review to studies that have used DSM or ICD criteria to make formal diagnoses.
“Early-onset” MDD
There is a considerable variability in how “early-onset” is defined across studies, with definitions ranging from onset in childhood or adolescence through age 60. Although an earlier onset generally indexes greater severity and impairment, there does not appear to be a clear demarcation between proximal developmental periods (e.g., childhood and adolescence, adolescence and early adulthood). For example, Zisook et al. (2007) examined a wide range of outcomes (quality of life, social and occupational functioning, physical and psychiatric comorbidity) among individuals with MDD who were classified into five discrete age-of-onset groups: childhood onset (by age 12), adolescent onset (between ages 12 and 17), early adult onset (between ages 18 and 44), middle adult onset (between ages 45 and 59), and late adult onset (after age 60)—earlier onset was associated with greater severity and impairment compared to later onset, but few outcomes differed markedly between age-of-onset groups. For ease of communication, in the present paper we use the term “early-onset” for MDD that onsets during childhood or adolescence.
“Recurrent” MDD
“Recurrent” MDD is defined as the presence of multiple MDD episodes. Standard definitions for MDD remission, recovery, relapse, and recurrence have been proposed (Frank et al., 1991; Kupfer & Frank, 2001), though few investigations formally assess or report this information. Many individuals with recurrent MDD fail to show complete interepisodic recovery, which takes on particular importance given evidence that the persistence of subthreshold interepisodic depressive symptoms is associated with shorter asymptomatic periods, more rapid and increased rates of relapse and recurrence, and a greater number of and more chronic MDD episodes (Judd et al., 2000), as well as significant social and occupational impairment (Romera et al., 2010). Much of the existing research on course has focused on factors that predict the recurrence of MDD among individuals who have already experienced an episode (see Burcusa & Iacono, 2007). In the present paper, we focus instead on identifying risk factors for MDD that follows a recurrent course relative to no MDD or single-episode MDD.
Age of Onset and Course of MDD
MDD often onsets early in life and frequently follows a recurrent course. Epidemiological studies indicate relatively low prevalence of MDD until early adolescence, at which point rates increase rapidly, then continue to increase at a more gradual rate into middle adulthood (Hasin et al., 2005; Kessler et al., 2003). Longitudinal studies with population-based and psychiatric samples indicate that about half of individuals with a first episode of MDD will experience a second or yet more episodes (estimates range from 40% to 85% depending on the sample and length of follow-up assessment; Eaton et al., 2008; M. B. Keller et al., 1992; Mattisson, Bogren, Horstmann, Munk-Jorgensen, & Nettelbladt, 2007; Rohde, Lewinsohn, Klein, Seeley, & Gau, 2013; Solomon et al., 2000). A recurrence of MDD typically occurs within five years of the first episode, whether assessed in samples of children (Kovacs et al., 1984), adolescents (Lewinsohn, Clarke, Seeley, & Rohde, 1994; Rao et al., 1995), or adults (Solomon et al., 2000), and individuals with MDD will experience an average of five separate episodes within their lifetimes (Hasin et al., 2005). Notably, individuals with early-onset MDD are more likely to experience recurrent episodes (Pettit, Lewinsohn, Roberts, Seeley, & Monteith, 2009; Rao, Hammen, & Daley, 1999; Rohde et al., 2013; Weissman et al., 1999; Wickramaratne, Warner, & Weissman, 2000; Zisook et al., 2007; Zisook et al., 2004). That these clinical features naturally co-occur means that investigations of early-onset MDD almost invariably include a large proportion of individuals with recurrent MDD, and investigations of recurrent MDD include many individuals with an early onset. This means that it can be difficult to discern from existing research whether risk or impairment is specific to one or both clinical features, or to individuals experiencing early-onset MDD that follows a recurrent course. In the present paper, we sought to differentiate these clinical features by identifying risk factors that predicted an early onset from a later onset of MDD and a recurrent from a single-episode course of MDD, while accounting for effects of age of onset on course and vice versa.
Risk Factors for MDD
In this section, we review the literatures on several putative risk factors for MDD, paying particular attention to indicators of risk that are associated with an earlier (versus later) age of onset or a recurrent (versus single-episode) course.
Genetic influences
Considerable research has sought to understand genetic influences on MDD. There is evidence from family and twin studies that depressive disorders aggregate in families (see Rice, Harold, & Thapar, 2002; Sullivan, Neale, & Kendler, 2000). The heritability of MDD is estimated at approximately 37% (Sullivan et al., 2000), and there is evidence that early-onset and recurrent MDD show even greater familial risk. For example, Weissman et al. (1984) found that early-onset (before age 20) MDD was associated with increased rates of MDD in family members, while later-onset MDD (after age 40) showed rates that were comparable to healthy controls. Klein, Lewinsohn, Seeley, and Rohde (2001) also found that early-onset MDD was associated with increased rates of MDD in family members, but a later report noted that rates were even higher for early-onset, recurrent MDD (Klein, Lewinsohn, Rohde, Seeley, & Durbin, 2002). Bland, Newman, and Orn (1986) and Pettit, Hartley, Lewinsohn, Seeley, and Klein (2013) found that, relative to single-episode MDD, recurrent MDD was associated with increased rates of MDD in family members, and McGuffin, Katz, Watkins, and Rutherford (1996) found that having 3 or more MDD episodes was associated with greater concordance among monozygotic than dizygotic twin pairs. In addition, there is evidence of psychopathology other than depressive disorders in the family members of probands with early-onset or recurrent MDD, including anxiety disorders, substance use disorders, and antisocial personality disorder (e.g., Klein et al., 2001; Klein et al., 2004; Kovacs, Devlin, Pollock, Richards, & Mukerji, 1997; Weller et al., 1994). However, few studies have attempted to differentiate the effects of MDD onset and course, meaning that it is difficult to determine their relative influences. For example, the increased rates of MDD in family members found by Klein et al. (2002) for probands with early-onset, recurrent MDD could be due to greater familial risk for early-onset MDD or for recurrent MDD, or may be specific to early-onset MDD that follows a recurrent course.
Evidence of the heritability of MDD from family and twin studies has prompted efforts to identify specific genetic variants associated with MDD. Earlier molecular genetics research included genome-wide linkage and candidate gene association studies of MDD, which showed little consistency in results across studies and multiple failures to replicate (see Bosker et al., 2011; Lopez-Leon et al., 2008). More recent genome-wide association studies (GWAS) of MDD, including early-onset and recurrent MDD, have likewise failed to identify genetic variants that achieve genome-wide significance (Kohli et al., 2011; Lewis et al., 2010; Muglia et al., 2010; Rietschel et al., 2010; Shi et al., 2011; Shyn et al., 2011; Sullivan et al., 2009; Wray et al., 2012), as did a recent mega-analysis of participants pooled across multiple GWAS studies (Sullivan et al., 2013). The etiology of MDD is likely heterogeneous and influenced by complex interactions of genetic and nongenetic factors, and these results suggest polygenic effects on MDD, with individually weak effects of specific genetic variants (see Lohoff, 2010, for a review). The failure of single nucleotide polymorphism (SNP)-based association tests typically used in current GWAS approaches to identify genetic variants for MDD has prompted the application of novel approaches, such as gene-set-based GWAS analysis, that hold considerable promise for identifying specific genetic risk factors and helping to elucidate the neurobiological basis of MDD and other psychiatric disorders (e.g., Glessner et al., 2009; Han et al., 2013; Holmans et al., 2009; Lee et al., 2012; Lips et al., 2012). However, the success of these alternative approaches hinges upon replication of the genetic variants identified, and attempts to confirm positive findings of associations between MDD and specific genetic variants identified in recent studies are needed.
Personality
A well-established body of cross-sectional literature demonstrates concurrent links between normal-range personality traits and psychiatric disorders (see Kotov, Gamez, Schmidt, & Watson, 2010, for a meta-analysis). Negative emotionality/Neuroticism (a tendency to experience negative mood states) appears to be a nonspecific risk factor for psychopathology in general, while (low) positive emotionality/Extraversion (a tendency to experience positive mood states) has been implicated as specific to depressive disorders (Clark & Watson, 1991; Mineka, Watson, & Clark, 1998; Watson & Tellegen, 1985). Higher negative emotionality and, less consistently, lower positive emotionality predict MDD and depressive symptoms in childhood and adolescence (Beevers, Rohde, Stice, & Nolen-Hoeksema, 2007; Lonigan, Phillips, & Hooe, 2003; Wetter & Hankin, 2009) and adulthood (de Graaf, Bijl, Ravelli, Smit, & Vollebergh, 2002; Fanous, Neale, Aggen, & Kendler, 2007; Kendler, Gatz, Gardner, & Pedersen, 2006; Kendler, Neale, Kessler, Heath, & Eaves, 1993; Ormel, Oldehinkel, & Vollebergh, 2004; Shea et al., 1996; Wilson, DiRago, & Iacono, 2014). Although less frequently considered, there is also evidence that (low) constraint/conscientiousness (a tendency to inhibit impulsive, risky behavior) is concurrently associated with MDD and depressive symptoms in childhood (John, Caspi, Robins, Moffitt, & Stouthamer-Loeber, 1994), adolescence (Wilson et al., 2014), and adulthood (Kotov et al., 2010). We are aware of only two studies, from our own group, that explicitly compared personality traits for early-onset and later-onset MDD. Although we found higher negative emotionality for both early- and later-onset MDD relative to no MDD (Wilson et al., 2014; Wilson et al., in press), and we also found that negative emotionality in adolescence prospectively predicted the onset of MDD in adulthood (Wilson et al., 2014), we found little evidence of differences in negative emotionality or positive emotionality in adulthood as a function of age of onset once course was accounted for (Wilson et al., in press). A handful of studies have considered effects of personality traits for the course of depressive disorders. Duggan, Lee, and Murray (1990) and Duggan, Sham, Lee, and Murray (1991) found that higher negative emotionality was prospectively associated with recurrent MDD. Wilson et al. (2014) found that negative emotionality was concurrently and prospectively associated with both recurrent/chronic MDD and nonrecurrent/nonchronic MDD, relative to no MDD, but positive emotionality was concurrently and prospectively associated only with recurrent/chronic MDD (regardless of whether it onset early or later). Wilson et al. (in press) explicitly compared personality traits for recurrent and single-episode MDD, and found that recurrent MDD predicted lower positive emotionality and higher negative emotionality. Notably, Wilson et al. (in press) found the lowest positive emotionality and highest negative emotionality for individuals with early-onset, recurrent MDD. Consistent with these results, Durbin, Klein, Hayden, Buckley, and Moerk (2005) found that positive emotionality was lower among young children of parents with early-onset or recurrent/chronic depressive disorders relative to parents with later-onset or nonrecurrent/nonchronic depressive disorders.
Psychopathology and psychosocial functioning
There is ample evidence that MDD is associated with comorbid internalizing and externalizing symptomatology, including anxiety, substance use, and antisocial behavior, as well as impaired functioning in important psychosocial domains, including conflictual interpersonal relationships, academic and work dysfunction, and poor physical health (see Chapman, Perry, & Strine, 2005; Hirschfeld et al., 2000; Kessler, 2012; Swendsen & Merikangas, 2000, for reviews). A number of studies suggest particularly severe dysfunction for early-onset and recurrent MDD, including concurrent and prospective associations with alcohol, nicotine, and illicit substance use and abuse, and antisocial behavior (Hammen, Brennan, Keenan-Miller, & Herr, 2008; Lewinsohn, Rohde, Seeley, Klein, & Gotlib, 2003; Sihvola et al., 2008; Wilson et al., in press); relationship impairment (Hammen, Brennan, & Keenan-Miller, 2008; Hammen, Brennan, Keenan-Miller et al., 2008; Jonsson et al., 2011; Lewinsohn et al., 2003; Puig-Antich et al., 1993; Weissman et al., 1999; Wilson et al., in press) and intimate partner violence (Jonsson et al., 2011); lower rates of graduation, less educational attainment, greater unemployment, and lower income (Breslau, Michael, Nancy, & Kessler, 2008; Fergusson, Boden, & Horwood, 2007; Fergusson & Woodward, 2002; Jonsson et al., 2011; Lewinsohn et al., 2003); and poorer mental health, more acute and chronic health conditions, and more visits to health professionals (Bardone, Moffitt, Caspi, Dickson, & Silva, 1996; Copeland, Shanahan, Worthman, Angold, & Costello, 2012; Keenan-Miller, Hammen, & Brennan, 2007; Lewinsohn, Seeley, Hibbard, Rohde, & Sack, 1996; Marmorstein, Iacono, & Legrand, in press; Wilson et al., in press).
Although these studies indicate that MDD is associated with comorbid psychopathology and impaired functioning in important domains, several methodologically rigorous studies carefully designed to examine whether MDD has lasting consequences suggest that dysfunction is limited to proximal (i.e., residual) depressive symptomatology and/or was apparent prior to the onset of MDD (see Burcusa & Iacono, 2007; Wichers, Geschwind, van Os, & Peeters, 2010, for reviews). For example, Beevers et al. (2007) found in a sample of adolescents that internalizing (depressive symptoms, lower self-esteem, rumination) and externalizing (substance use, antisocial behavior) problems, interpersonal dysfunction (less parent and peer support, greater parent control), and stressful life events were evident even before the onset of MDD. Ormel, Oldehinkel, Nolen, and Vollebergh (2004) similarly found in a sample of adults evidence of dysfunction in important social roles prior to the onset of MDD; notably, however, when participants with recurrent MDD were considered separately, there was greater dysfunction following MDD remission than had been evident prior to onset, suggesting that recurrent MDD may have a “scar” effect on functioning. This finding dovetails with evidence from our group and others that early-onset MDD is concurrently associated with psychosocial impairment in adolescence, but ongoing psychosocial impairment into adulthood is largely a function of a recurrent course (Hammen, Brennan, Keenan-Miller et al., 2008; Lewinsohn et al., 2003; Wilson et al., in press); moreover, we found that the negative psychosocial outcomes in adulthood found for recurrent MDD held over and above psychosocial impairment evident in adolescence, indicating that recurrent MDD predicts decrements in functioning over time (Wilson et al., in press). In one of the few studies to have examined whether premorbid dysfunction is evident prior to adolescence, or whether early-onset MDD shows greater premorbid dysfunction than later-onset MDD, Jaffee et al. (2002) found a number of premorbid risk factors assessed in childhood that predicted MDD onset by adolescence versus in adulthood, including internalizing and externalizing symptoms; however, there was no evidence of premorbid differences for early-onset MDD that recurred in adulthood versus nonrecurrent early-onset MDD.
Study 1
In Study 1, we examined a number of putative indicators of risk for MDD, including parental psychiatric disorders and premorbid functioning. Consistent with evidence of the familial aggregation of MDD (Rice et al., 2002; Sullivan et al., 2000), we expected that offspring with MDD would show increased rates of MDD and other psychiatric disorders in their parents. Previous research on premorbid risk for MDD has largely been conducted in adolescence and adulthood (Beevers et al., 2007; de Graaf et al., 2002; Fanous et al., 2007; Kendler et al., 2006; Kendler et al., 1993; Lonigan et al., 2003; Ormel, Oldehinkel, & Vollebergh, 2004; Shea et al., 1996; Wetter & Hankin, 2009). We extended this research to late childhood, and hypothesized that premorbid dysfunction would predict the subsequent development of MDD.
Our prospective assessment of MDD at multiple time points from childhood into adulthood also allowed us to examine indicators of risk that predict an earlier onset and recurrent course of MDD. We expected to find increased rates of parental MDD and other psychiatric disorders for offspring with early-onset and recurrent MDD, as suggested in earlier research (Bland et al., 1986; Klein et al., 2002; Klein et al., 2004; Pettit et al., 2013; Weissman et al., 1984). We further hypothesized that (a) premorbid negative emotionality would be highest for early-onset, recurrent MDD, as suggested by our findings in late adolescence and young adulthood (Wilson et al., 2014; Wilson et al., in press); (b) higher disconstraint would predict early-onset MDD, as suggested in our previous work (Wilson et al., 2014); and (c) lower premorbid positive emotionality would be specific to recurrent MDD. Our hypotheses for other domains of premorbid functioning on onset and course were more exploratory. Drawing on the results of the only similar study in childhood of which we are aware (Jaffee et al., 2002), we hypothesized that, in general, premorbid dysfunction would predict an earlier onset of MDD but not a recurrent course.
Methods
Participants and Procedures
Participants were 2,764 male and female twins from the Minnesota Twin Family Study (MTFS) (54% female) and their parents. The MTFS is an ongoing community-based, longitudinal study of reared-together twins and their parents, and is one of several ongoing studies that comprise the Minnesota Center for Twin and Family Research (MCTFR); the study design and sample have been described extensively elsewhere (see Iacono, Carlson, Taylor, Elkins, & McGue, 1999; Iacono & McGue, 2002; Iacono, McGue, & Krueger, 2006) and are only briefly reviewed here. The present study included two cohorts first recruited for participation at offspring age 11 (younger cohort, n = 1,512) or 17 (older cohort, n = 1,252). Consistent with the demographic makeup of Minnesota during the targeted birth years, families were predominately Caucasian (98%). The MTFS design includes assessments at offspring target ages of 11 (M = 11.72, SD = 0.43), 14 (M = 14.80, SD = 0.53), 17 (M = 17.83, SD = 0.69), 20 (M = 21.10, SD = 0.82), 24 (M = 25.01, SD = 0.90), and 29 years (M = 29.42, SD = 0.65). The present study includes data on offspring MDD diagnoses at each assessment. Parental psychiatric diagnoses were assessed for mothers and fathers of younger and older cohort offspring at study intake (the older cohort was used only in analyses of parental psychiatric disorders). Offspring functioning variables were assessed at age 11 for the younger cohort (analyses of premorbid functioning used only the younger cohort because only it was assessed at age 11, prior to the majority of first onset cases of MDD). Rates of retention across follow-up waves were universally high (ranging from 87% to 94% across assessments).
Offspring MDD Diagnoses
Offspring MDD diagnoses and information on the onset and course of depressive symptoms were assessed in semi-structured interviews using the Diagnostic Interview for Children and Adolescents—Revised (DICA-R; Reich & Welner, 1988) in offspring younger than age 17, and the Structured Clinical Interview for DSM-III-R (SCID; Spitzer, Williams, Gibbon, & First, 1987) in offspring aged 17 and older. Mothers also reported on offspring aged 17 and younger using the DICA-R, and a best-estimate procedure was used to assign MDD diagnoses if symptoms were endorsed by either offspring or their mothers. MDD diagnoses were based on DSM-III-R criteria to maintain continuity with the diagnostic system in use at the time of the MTFS intake. MDD diagnoses were assigned if criteria were met at a “definite” (i.e., full DSM-III-R criteria met) or “probable” (i.e., 1 criterion less than full DSM-III-R criteria met) level, following guidelines introduced in the Research Diagnostic Criteria (Spitzer, Endicott, & Robins, 1978) to account for the episodic nature of MDD and that participants were rarely acutely symptomatic at the time of assessment, meaning that they relied on memory when reporting on MDD symptoms. Diagnostic interviews were conducted by extensively trained interviewers with bachelor’s or master’s degrees in psychology or a related discipline. All diagnostic interviews were reviewed in case conferences with at least two advanced clinical psychology graduate students, and consensus was required prior to assigning each symptom. Computer algorithms were used to assign diagnoses at each time point. Interrater reliability for MDD diagnoses was assessed on a randomly selected subsample of 600 MTFS participants (kappa = .78 and above).
A total of 891 (40%) offspring reported a lifetime history of MDD1 through age 29 (i.e., criteria met for at least one MDD episode at any assessment). MDD age of onset was defined as early onset (n = 332) if onset was prior to the age-17 assessment and as later onset (n = 499) if onset was after the age-17 assessment. MDD course was defined as single episode (n = 403) if only one MDD episode was reported through age 29, and recurrent (n = 428) if multiple MDD episodes were reported through age 29. In addition, early-onset, recurrent depression (n = 193) was defined as MDD onset prior to the age-17 assessment with multiple MDD episodes reported through age 29; 584 participants were classified as having other forms of MDD (i.e., lifetime history of MDD but not early-onset, recurrent MDD). No MDD (n = 1,366) was defined as never meeting criteria for MDD (definite or probable) at any assessment point through age 292.
Parental Psychiatric Diagnoses
Parental psychiatric diagnoses were assessed in semi-structured interviews with mothers and fathers using the Structured Clinical Interview for DSM-III-R (SCID; Spitzer et al., 1987) at study intake. As for offspring, diagnoses were assigned if criteria were met at a “definite” or “probable” level, and diagnostic interviews with parents followed the same procedures to ensure consensus and reliability as followed for offspring. Parental psychiatric diagnoses included either mother or father meeting criteria for a lifetime history of MDD, anxiety disorder (panic disorder with or without agoraphobia, agoraphobia, social phobia, specific phobia, generalized anxiety disorder)3, substance use disorder (alcohol or drug abuse or dependence), conduct disorder, or adult antisocial behavior (the adult component of antisocial personality disorder).
Offspring Premorbid Functioning
Premorbid (age-11) functioning was assessed using multiple methods and informants (offspring, parents, teachers). The frequency distributions of all variables were examined and transformations were applied as appropriate. All continuous variables were transformed to z scores (mean = 0, standard deviation = 1); all binary variables were coded so that 0 = absence, 1 = presence.
Personality
Offspring positive emotionality, negative emotionality, and disconstraint were assessed at age 11 using mothers’ reports on the Multidimensional Personality Ratings (MPR; Cukrowicz, Taylor, Schatschneider, & Iacono, 2006; Oliva, Keyes, Iacono, & McGue, 2012; Tackett, Krueger, Iacono, & McGue, 2008) and teachers’ reports on the Teacher Rating Form (TRF; Hicks, Iacono, & McGue, 2014; Hicks et al., in press). The 34-item MPR was developed to approximate the adult Multidimensional Personality Questionnaire (Tellegen & Waller, 2008) and includes the three higher-order scales of positive emotionality (12 items; alpha = .82), negative emotionality (9 items; alpha = .74), and disconstraint (8 items; alpha = .73). The TRF includes 129 items that assess personality, behavior, and academic domains, and was completed by up to four teachers nominated by each participant. TRF positive emotionality (11 items; alpha = .88), negative emotionality (6 items; alpha = .88), and disconstraint (8 items; alpha = .93) scales were computed based on the results of a principal axis factor analysis with varimax rotation of the personality items. Composite positive emotionality, negative emotionality, and disconstraint variables were computed by transforming mother and teacher reports to z scores and averaging across raters (interrater r = .40 for positive emotionality, .25 for negative emotionality, and .37 for disconstraint).
Psychopathology
Offspring reported on their general level of anxiety at age 11 using a 20-item self-report anxiety scale (alpha = .83) (Spielberger, 1983). Offspring substance use and misuse at age 11 was assessed using offspring and their mothers’ reports of DSM-III-R symptoms of alcohol, nicotine, and drug abuse or dependence on the DICA-R (using a best-estimate procedure to assign symptoms if endorsed by either offspring or their mother), as well as offspring reports of the quantity and frequency of alcohol, nicotine, and drug use using a computer-administered substance-use assessment. All substance use/misuse variables were log (1 + x) transformed to reduce positive skew. Offspring alcohol use/misuse included the quantity of alcohol use in the past year, frequency of use in the past year, maximum number of drinks consumed in 24 hours, and alcohol abuse/dependence symptoms; the mean r between alcohol use/misuse variables was .71; variables were transformed to z scores and averaged to create an alcohol use/misuse composite variable. Offspring nicotine use/misuse included the quantity of nicotine use in the past year, frequency of use in the past year, and nicotine dependence symptoms; the mean r between nicotine use/misuse variables was .56; variables were transformed to z scores and averaged to create a nicotine use/misuse composite variable. Offspring drug use/misuse included ever using marijuana and abuse/dependence symptoms for the drug class with the highest number of reported symptoms; the r between drug use/misuse variables was .38; variables were transformed to z scores and averaged to create a drug use/misuse composite variable. The mean r between alcohol, nicotine, and drug use/misuse variables was .35; variables were transformed to z scores and averaged to create a substance use/misuse composite variable. Offspring externalizing symptoms at age 11 were assessed using offspring and their mothers’ reports of DSM-III-R oppositional defiant disorder, conduct disorder, and attention-deficit/hyperactivity disorder symptoms on the DICA-R (using a best-estimate procedure to assign symptoms). All externalizing symptom count variables were log (1 + x) transformed to reduce positive skew. The mean r between externalizing symptom variables was .35; variables were transformed to z scores and averaged to create an externalizing symptoms composite variable.
Psychosocial functioning
Offspring and their mothers reported on academic functioning (cumulative grade point average [GPA], academic engagement) at age 11 using the Academic History Questionnaire (AHQ; Johnson, McGue, & Iacono, 2006). The AHQ academic engagement scale includes 7 items (alpha = .83) that assess interest in and enjoyment of school, and study habits. The r between GPA and academic engagement was .53; variables were transformed to z scores and averaged to create an academic functioning composite variable, with higher values indicating poorer academic functioning. Offspring and their parents reported on the quality of the parent-child relationship (involvement, conflict, regard, structure) at age 11 using the 50-item Parental Environment Questionnaire (PEQ; Elkins, McGue, & Iacono, 1997). Cronbach’s alphas for the PEQ scales ranged from .69 to .82; the mean of offspring, mother, and father ratings on the first principal component among the PEQ scales (mean r across informants = .41) was used to create the parent-child relationship composite variable, with higher values indicating lower-quality parent-child relationship.
Physical development
Offspring reported on their pubertal development at age 11 using the Pubertal Development Scale (PDS; Petersen, Crockett, Richards, & Boxer, 1988). The PDS includes 5 (males) and 7 (females) items that assess Tanner stages, including (for males) pubic hair and voice changes, and (for females) breast development and menarche, with higher values indicating more advanced pubertal development. Offspring body mass index (BMI), a height-corrected weight-height index (weight in kilograms/height in meters2), was calculated by measuring height and weight in person in the laboratory.
Childhood maltreatment
Offspring reported on their history of physical abuse/assault or sexual abuse/assault using the Life Events Interview (LEI; Bemmels, Burt, Legrand, Iacono, & McGue, 2008; Billig, Hershberger, Iacono, & McGue, 1996) retrospectively at the age-29 assessment. Offspring reports of the age at which the abuse first occurred were used to create physical and sexual abuse/assault by age 11 variables.
Data Analyses
We conducted a series of two-level multilevel models that accounted for the interdependence of the family data (individual offspring at level 1, nested within families at level 2) to examine associations between indicators of risk and MDD. We calculated odds ratios (ORs) with 95% confidence intervals (CIs) to index the odds of offspring MDD when risk was present relative to when it was not (parental psychiatric disorders, childhood maltreatment), or the odds of offspring MDD for every one unit (i.e., one standard deviation) change in the risk variable (personality, psychopathology, psychosocial functioning, physical development). First, we considered any MDD diagnosis (i.e., regardless of age of onset or course). We fit a series of models predicting offspring MDD (0 = no MDD; 1 = MDD) as a function of a parental psychiatric disorder (0 = no disorder; 1 = disorder) and offspring functioning variables (in separate models). Second, we sought to differentiate risk for MDD onset and course by examining whether risk for MDD was specific to early-onset or recurrent MDD; to reduce the number of statistical tests conducted, only risk factors that showed significant associations with MDD were examined for early onset and course. We fit a series of models predicting MDD onset (0 = later onset; 1 = early onset) and course (0 = single episode; 1 = recurrent episodes) as a function of risk variables (in separate models); in order to account for the overlap between MDD onset and course, we included course in models predicting onset, and onset in models predicting course. We considered an effect specific to early-onset or recurrent MDD if two criteria were met: (a) the results were significant for either early-onset or recurrent MDD, but not the other; and (b) the 95% CI for the OR for the significant result did not include the OR for the nonsignificant result. Finally, we considered risk for the most severe form of MDD by examining whether risk was greatest for early-onset, recurrent MDD. We fit a series of models for the type of MDD (0 = other MDD; 1 = early onset, recurrent) as a function of risk variables (in separate models). All analyses that included premorbid offspring functioning variables were conducted excluding offspring with a definite or probable diagnosis of MDD at age 11 (n = 48). Offspring sex was included as a covariate in all models4. All analyses were conducted using Scientific Software International’s HLM 6.04 (Raudenbush, Bryk, & Congdon, 2004) with full maximum likelihood estimation. The significance level was set at p < .05 for all analyses.
Results
Risk Factors for MDD
The results of analyses examining risk factors for an MDD diagnosis are presented in the second column of Table 1. Offspring with psychiatric disorders in their parents, including MDD, anxiety disorders, substance use disorders, conduct disorder, and adult antisocial behavior, were at significantly greater risk of a diagnosis of MDD. Moreover, many indicators of premorbid dysfunction evident at age 11 predicted increased risk of a diagnosis of MDD, including lower positive emotionality, higher negative emotionality and disconstraint, higher trait anxiety, substance use/misuse, externalizing symptoms, poorer school functioning, poorer parent-child relationship quality, and more advanced pubertal development. Finally, childhood physical and sexual abuse/assault predicted increased risk of a diagnosis of MDD.
Table 1.
Effects of Parental Psychiatric Disorders and Premorbid (Age-11) Functioning on MDD
| Premorbid Risk | MDD | Early-Onset MDD | Recurrent MDD | Early-Onset, Recurrent MDD |
|---|---|---|---|---|
| Parental Psychiatric Disorder | ||||
| MDD | 1.73 (1.41, 2.11)*** | 1.65 (1.18, 2.29)** | 1.01 (0.73, 1.38) | 1.46 (1.03, 2.07)* |
| Anxiety disorders | 1.40 (1.02, 1.93)* | 0.87 (0.53, 1.42) | 0.81 (0.49, 1.34) | 0.82 (0.48, 1.40) |
| Substance use disorders | 1.56 (1.27, 1.93)*** | 1.16 (0.81, 1.67) | 1.00 (0.72, 1.40) | 1.17 (0.80, 1.73) |
| Conduct disorder | 1.42 (1.16, 1.74)*** | 0.97 (0.69, 1.36) | 1.03 (0.75, 1.42) | 1.06 (0.74, 1.52) |
| Adult antisocial behavior | 1.64 (1.30, 2.07)*** | 1.71 (1.19, 2.47)** | 0.98 (0.68, 1.40) | 1.69 (1.16, 2.47)** |
| Personality | ||||
| Positive emotionality | 0.84 (0.73, 0.95)** | 0.88 (0.70, 1.12) | 0.77 (0.62, 0.97)* | 0.85 (0.66, 1.08) |
| Negative emotionality | 1.27 (1.11, 1.46)*** | 1.43 (1.14, 1.79)** | 0.98 (0.79, 1.22) | 2.13 (1.23, 3.68)*** |
| Disconstraint | 1.36 (1.19, 1.55)*** | 1.34 (1.04, 1.72)* | 0.87 (0.69, 1.09) | 1.04 (0.81, 1.34) |
| Psychopathology | ||||
| Anxiety | 1.34 (1.14, 1.57)*** | 1.00 (0.78, 1.29) | 1.43 (1.10, 1.86)** | 1.26 (0.94, 1.68) |
| Substance use/misuse | 1.17 (1.03, 1.33)* | 1.08 (0.91, 1.28) | 0.97 (0.82, 1.15) | 1.08 (0.88, 1.33) |
| Externalizing symptoms | 1.38 (1.20, 1.58)*** | 1.44 (1.54, 1.80)** | 0.93 (0.74, 1.16) | 1.37 (1.10, 1.72)** |
| Psychosocial Functioning | ||||
| Poor academic functioning | 1.20 (1.05, 1.37)** | 1.04 (0.82, 1.32) | 0.96 (0.77, 1.19) | 1.05 (0.79, 1.40) |
| Poor parent-child relationship | 1.23 (1.07, 1.40)** | 1.19 (0.94, 1.51) | 0.83 (0.66, 1.04) | 1.03 (0.81, 1.31) |
| Physical Development | ||||
| Pubertal development | 1.31 (1.10, 1.55)** | 1.21 (0.88, 1.68) | 0.97 (0.72, 1.31) | 1.29 (0.89, 1.87) |
| BMI | 1.08 (0.95, 1.23) | - | - | - |
| Childhood Maltreatment | ||||
| Physical abuse/assault | 3.03 (1.22, 7.55)* | 10.34 (2.21, 48.38)** | 1.32 (0.31, 5.67) | 7.08 (1.88, 26.62)** |
| Sexual abuse/assault | 3.41 (1.55, 7.51)** | 1.93 (0.73, 5.09) | 5.92 (1.40, 24.95)* | 4.77 (1.69, 13.50)** |
Note. Results of multilevel models examining risk for MDD (0 = no MDD; 1 = MDD), early-onset MDD (0 = later onset; 1 = early onset), recurrent MDD (0 = single episode; 1 = recurrent episodes), and early-onset, recurrent MDD (0 = other MDD; 1 = early onset, recurrent) as a function of parental psychiatric disorder (0 = no disorder; 1 = disorder), premorbid (age-11) personality, psychopathology, psychosocial functioning, physical development, and childhood maltreatment (0 = no abuse/assault; 1 = abuse/assault) (in separate models); models for early-onset MDD include recurrence, and models for recurrent MDD include age of onset. Participant sex was entered as a covariate in all models. Statistics are odds ratios (ORs) with 95% confidence intervals (CIs); for binary variables (parental psychiatric disorders, childhood maltreatment), ORs index the odds of offspring MDD when the risk is present relative to when it is not; for continuous variables (personality, psychopathology, psychosocial functioning, physical development), ORs index the odds of offspring MDD for every one unit (i.e., one standard deviation) change in the risk variable. Only risk factors that were significantly associated with MDD were evaluated in models for early-onset, recurrent, and early-onset, recurrent MDD; risk factors that were not further evaluated due to nonsignificant associations with MDD are noted -. MDD = major depressive disorder. BMI = body mass index.
p < .05.
p < .01.
p < .001.
Risk Factors for Early-Onset and Recurrent MDD
The results of analyses examining risk factors for early-onset (versus later-onset) and recurrent (versus single-episode) MDD, accounting for the effects of onset on course, and course on onset, are presented in the third and fourth column of Table 1. Offspring with MDD or adult antisocial behavior in their parents were at significantly greater risk of an early onset of MDD (regardless of whether it ultimately followed a recurrent or single-episode course). In contrast, parental psychiatric disorders were not associated with risk for a recurrent course of MDD, after accounting for age of onset. Several indicators of premorbid dysfunction at age 11 predicted an earlier onset of MDD or a recurrent course. Higher negative emotionality, higher disconstraint, and externalizing symptoms, as well as childhood physical abuse/assault predicted an earlier onset of MDD, after accounting for course. Lower positive emotionality, higher trait anxiety, and childhood sexual abuse/assault predicted a recurrent course of MDD, after accounting for age of onset. Using the criteria for specificity given above, effects for parental MDD and adult antisocial behavior, negative emotionality and disconstraint, externalizing symptoms, and physical abuse/assault were specific to early-onset MDD, but only the effect for trait anxiety was specific to recurrent MDD.
Risk Factors for Early-Onset, Recurrent MDD
The results of analyses examining risk factors for early-onset, recurrent MDD (versus all other forms of MDD) are presented in the fifth column of Table 1. Offspring with MDD or adult antisocial behavior in their parents were at significantly greater risk for early-onset, recurrent MDD, and higher premorbid negative emotionality, externalizing symptoms, and childhood physical or sexual abuse/assault predicted increased risk for early-onset, recurrent MDD—notably, these risk factors were also found to be specific to early-onset MDD, after accounting for course.
Discussion
In Study 1, we considered premorbid risk factors for MDD, including parental psychiatric disorders, premorbid personality traits, psychopathology, psychosocial functioning, physical development, and childhood maltreatment. We identified a number of risk factors—in fact, almost all of the risk factors we considered predicted the subsequent development of MDD. This is a remarkable finding, considering the range of important domains considered and especially that premorbid risk was assessed in late childhood, well before the onset of MDD for most cases. The present results highlight the serious implications of familial risk, including parental MDD and other psychiatric disorders, and a poorer-quality parent-child relationship, and individual risk, including higher negative emotionality and disconstraint, lower positive emotionality, internalizing and externalizing psychopathology, poorer academic functioning, early pubertal development, and childhood maltreatment. The results also add to the existing literature by identifying several factors that predict an earlier onset or recurrent course of MDD. Given that early onset and recurrence frequently co-occur, much of the previous research on early-onset or recurrent MDD has actually included individuals with early-onset, recurrent MDD. We attempted to disentangle risk for these clinical features by statistically accounting for effects of onset on course and course on onset, and by explicitly considering risk for early-onset, recurrent MDD. We identified several risk factors that predicted an earlier onset of MDD, including parental MDD and adult antisocial behavior, higher premorbid negative emotionality and disconstraint, premorbid externalizing symptoms, and childhood physical abuse/assault, as well as several risk factors that predicted a recurrent course of MDD, including lower premorbid positive emotionality, premorbid trait anxiety, and childhood sexual abuse/assault. Notably, almost all of the risk factors for early-onset, recurrent MDD were specific risk factors for early-onset MDD, suggesting that risk for recurrent MDD is largely a function of the typically earlier onset of recurrent MDD. It is also notable that a number of factors that conferred risk for a diagnosis of MDD did not predict an earlier onset or recurrent course, including parental anxiety disorders, substance use disorders, or conduct disorder, and alcohol use, the parent-child relationship, academic functioning, and early pubertal development, suggesting that these are more general risk factors.
Our finding of increased rates of parental MDD for early-onset MDD is consistent with evidence from family and twin studies that early-onset depressive disorders are more heritable than later-onset depressive disorders (Bland et al., 1986; Klein et al., 2002; Klein et al., 2001; Klein et al., 2004; McGuffin et al., 1996; Pettit et al., 2013; Weissman et al., 1984). In contrast, our finding that MDD recurrence was not associated with increased rates of parental psychiatric disorders, after accounting for age of onset, suggests that previous evidence of greater heritability for recurrent MDD may be attributable to its typically earlier age of onset. Furthermore, our finding that parental adult antisocial behavior, in addition to parental MDD, was associated with increased risk for early-onset MDD, as well as early-onset, recurrent MDD, suggests possible genetic and environmental influences—increased liability for general psychopathology, as well as a dysfunctional rearing environment consequent to parental psychopathology, may lead to an earlier onset and more pernicious course of MDD.
It is notable that personality traits measured in late childhood, well before the onset of MDD, predicted its age of onset and course in adolescence and adulthood. The results of the present study add to the relatively large body of evidence that negative emotionality is a risk factor for the development of MDD (Beevers et al., 2007; de Graaf et al., 2002; Fanous et al., 2007; Kendler et al., 2006; Kendler et al., 1993; Lonigan et al., 2003; Ormel, Oldehinkel, & Vollebergh, 2004; Shea et al., 1996; Wetter & Hankin, 2009; Wilson et al., 2014), and extend prior evidence of higher premorbid negative emotionality to late childhood. Results also help clarify inconsistencies regarding positive emotionality (Fanous et al., 2007; Kendler et al., 2006; Kendler et al., 1993; Lonigan et al., 2003; Shea et al., 1996; Wetter & Hankin, 2009) in that effects appear to be specifically associated with recurrent MDD (see also Wilson et al., 2014; Wilson et al., in press), and add to a small body of research suggesting that disconstraint is associated with early-onset MDD (John et al., 1994; Wilson et al., 2014). Our finding that psychopathology and impaired functioning was apparent even prior to the onset of MDD extends previous research (Beevers et al., 2007; Ormel, Oldehinkel, Nolen et al., 2004) by showing that premorbid dysfunction is evident even in late childhood.
Although the present study identifies a number of factors that are associated with increased risk of MDD, and of early-onset or recurrent MDD, it is not necessarily the case that these are causal risk factors. That is, dysfunction may reflect an underlying liability to MDD and related psychopathology (see Burcusa & Iacono, 2007); increased liability marked by multiple indicators of risk may lead to an earlier onset or recurrent course of MDD. It is interesting that many of the factors considered in the present study that were associated with increased risk for an earlier onset of MDD reflected externalizing psychopathology—parental adult antisocial behavior, higher disconstraint, externalizing symptoms—whereas the factors associated with increased risk for a recurrent course of MDD reflected internalizing psychopathology, including lower positive emotionality and trait anxiety. This suggests potentially different mechanisms may play a role for an earlier onset and recurrent course.
The results of Study 1 identify several family- and individual-level risk factors for MDD. These results are bolstered by the inclusion of multiple indicators of risk from a range of important domains assessed using multiple methods and reporters. Importantly, parental MDD predicted the development of offspring MDD, as well as an earlier onset of MDD. This finding, coupled with the literature reviewed above indicating that the genetic influence on early-onset MDD may be particularly strong (Bland et al., 1986; Klein et al., 2002; Klein et al., 2001; Klein et al., 2004; McGuffin et al., 1996; Pettit et al., 2013; Weissman et al., 1984), prompted us to undertake a molecular genetic investigation of MDD to determine if we could identify specific genetic variants associated with MDD, and with early-onset MDD.
Study 2
Any study that endeavors to find genetic variants associated with MDD is confronted with the important challenge of identifying an analytic strategy likely to bear fruit given a literature that documents the great difficulty of the pursuit. As we noted above, candidate gene studies have largely proven unreplicable (see Bosker et al., 2011; Lohoff, 2010; Lopez-Leon et al., 2008). GWAS investigations have failed to turn up hits (Kohli et al., 2011; Lewis et al., 2010; Muglia et al., 2010; Rietschel et al., 2010; Shi et al., 2011; Shyn et al., 2011; Sullivan et al., 2013; Sullivan et al., 2009; Wray et al., 2012), and analyses based on pooled findings across studies have failed to detect genetic variants that show genome-wide significance (Hek et al., 2013). Nonetheless, it is clear that the SNPs covered by GWAS gene chips do collectively account for a major portion of the heritable variance in MDD (Lubke et al., 2012). Thus, the presence of the genetic signal is confirmed, but it is difficult to detect because it consists of many variants, each contributing small effects. Unfortunately, these true effects cannot be differentiated from the many false positive leads that arise when conducting the vast numbers of statistical tests required in GWAS. Against this backdrop, we sought to confirm recent findings of associations between MDD and several genes in the glutamatergic system, derived from a novel analytic technique, gene-set-based GWAS, hoping to circumvent the problems that thus far have rendered difficult identifying MDD-related genetic signals.
Gene-set-based GWAS analysis is an analytic approach that capitalizes upon increased power when pooling effects across SNPs. We attempted to confirm the positive findings of a recent application of gene-set-based association analysis of MDD. Lee et al. (2012) took a two-stage analytic approach to identify genes that were associated with MDD. They first applied statistical text-mining analysis (Raychaudhuri et al., 2009) to identify key biological mechanisms indexed by candidate genes selected from previous examinations of MDD, which were then used to compile a list of relevant gene sets (Ashburner et al., 2000). They then examined associations between these gene sets and MDD in a meta-analysis of three large study samples comprising a total of 8,776 participants (MDD: n = 4,346, 50%). Of the 178 target gene sets examined, only one, the glutamatergic synaptic transmission set, remained significant after correcting for multiple tests based on the number of gene sets examined. This glutamatergic synaptic transmission set consisted of 16 genes, of which 6 genes included significant linkage disequilibrium (LD)-independent associations with MDD.
Glutamate is the primary excitatory neurotransmitter in the mammalian nervous system, and it plays a major role in brain development and synaptic plasticity (see Niswender & Conn, 2010). Under pathological conditions, including brain injury or disease, excessive glutamate release or impaired glutamate uptake acts as a potent excitoxin, leading to neurotoxicity. Multiple lines of evidence speak to the pathophysiological role of the glutamatergic system for MDD (see Hashimoto, 2009; Paul & Skolnick, 2003, for reviews). Animal models of MDD indicate dysregulation in the metabolism of glutamate (Li et al., 2008); glutamate abnormalities in human brains have been found for MDD using post-mortem and in vivo measures (Capizzano, Jorge, Acion, & Robinson, 2007; Hashimoto, Sawa, & Iyo, 2007; Yildiz-Yesiloglu & Ankerst, 2006); and the administration of glutamatergic interventions, such as ketamine, show rapid antidepressant effects in animal (Autry et al., 2011) and human studies (Machado-Vieira, Salvadore, DiazGranados, & Zarate, 2009; Maeng & Zarate, 2007). Importantly, there is evidence of greater abnormalities in the glutamatergic system for early onset and recurrent MDD (Frodl et al., 2003; Mirza et al., 2004; Rosenberg et al., 2005; Sheline, Gado, & Price, 1998). Unexplored is the degree to which severe MDD, as indexed by early onset and recurrence, and which may have a stronger genetic loading than less severe MDD (Bland et al., 1986; Klein et al., 2002; Klein et al., 2001; Klein et al., 2004; McGuffin et al., 1996; Pettit et al., 2013; Weissman et al., 1984), shows association with genes in this system.
We attempted to confirm Lee et al.’s (2012) positive finding of associations of SNPs in 6 glutamatergic genes with MDD—SLC1A4, CACNA1A, GRM8, PARK2, UNC13A, and SHC3. Because molecular genetics studies benefit from using samples that are as large as possible, we included Study 1 participants in Study 2, and also expanded the sample to include other participants in the parent-offspring studies that comprise the MCTFR. Thus, we examined associations between SNPs in these gene regions in a large sample of 7,146 individuals clustered in 4-member families, almost 2,000 of whom had a lifetime diagnosis of MDD. Confirmation would provide further evidence of the etiological role of glutamate-mediated synaptic neurotransmission of MDD. Moreover, evidence of associations with early-onset and recurrent MDD would be consistent with greater heritability for these more severe forms of MDD.
Methods
Participants and Procedure
Study 2 included all Caucasian offspring and parents included in Study 1, as well as all other Caucasian participants (offspring and rearing parents) from the MCTFR with usable DNA data (see below) and the same MDD assessments, yielding a total sample of 7,146 individuals clustered in 2,295 families. Offspring included monozygotic twins, dizygotic twins, full biological siblings, adopted siblings, or mixed siblings with one biologically related to the parents and one adopted. The sample consisted of 3,331 offspring (52% female) and 3,815 parents (54% female). Only Caucasian participants were included in the present study because differences in allele frequencies among different ethnicities may present problems for genetic analyses. MCTFR offspring and their parents are predominately Caucasian (> 90%); ethnicity was determined using self-reports and information derived from birth records, and was confirmed by results from an Eigenstrat analyses (Price et al., 2006) (see below). (See M. B. Miller et al., 2012, for additional details regarding the MCTFR GWAS sample.) Offspring included the younger and older cohorts of twins from Study 1, a third cohort of twins enriched for childhood externalizing disorders (enrichment cohort), and a sample of biological and adoptive siblings (sibling sample) (see Iacono et al., 1999; Iacono & McGue, 2002; Iacono et al., 2006; McGue et al., 2007, for additional information on the MCTFR study design and samples). The present study includes data on offspring and parent MDD diagnoses assessed as in Study 1; younger and older cohort offspring were assessed through age 29, but enrichment cohort and sibling sample offspring were assessed through age 17 and a mean age of approximately 18, respectively, because these studies began later and longitudinal assessments are ongoing. Rates of retention across follow-up waves were universally high (ranging from 79% to 96% across assessments and cohorts).
Genotyping
The sample was genotyped using the Illumina Human660W-Quad array (Illumina, Inc., San Diego, CA), following standard protocol. Genotyping samples were primarily blood-based; a small number of participants refused a blood draw and provided saliva samples instead. Of 561,490 individual SNP markers on the array, a total of 527,829 (94%) markers were retained after eliminating 32,153 markers that were identified by Illumina as problematic; came from duplicate samples but failed to yield identical results; had call rates less than 99%, indicating that a large number of genotypes could not be determined for the marker; had minor allele frequency less than .01; showed significant deviation from Hardy-Weinberg equilibrium (p < 10−7); had more than two Mendelian inconsistencies across families, indicating that alleles for offspring were inconsistent with those for parents; were associated with the plate used for processing (p < 10−7), indicating systematic error in the processing procedure; or were associated with participant sex (p < 10−7), which would produce collinearity in regression models. In addition, 160 samples were excluded if more than 5,000 markers (~1%) could not be called, raising concerns about the quality of the DNA data; they had low Gen_Call scores, which provide an index of confidence in each call and, indirectly, in the quality of the DNA data; they showed extreme homozygosity or heterozygosity; or if we failed to confirm familial relationships or sex, suggesting that samples had been mixed. (See M. B. Miller et al., 2012, for additional details regarding genotyping and quality control procedures.)
MDD Diagnoses
Offspring and parent MDD diagnoses and information on the onset and course of depression symptoms were assessed as in Study 1, using the Diagnostic Interview for Children and Adolescents—Revised (DICA-R; Reich & Welner, 1988) and the Structured Clinical Interview for DSM-III-R (SCID; Spitzer et al., 1987). A total of 1,993 (28%) offspring and parents reported a lifetime history of MDD5, and 5,153 (72%) never met criteria for MDD (definite or probable) at any assessment point6.
Data Analysis
We attempted to confirm Lee et al.’s (2012) findings of associations between SNPs in SLC1A4, CACNA1A, GRM8, PARK2, UNC13A, and SHC3 and MDD using both individual SNP-based and gene-based analyses. First, we conducted individual SNP-based tests for SNPs in the MDD-associated genomic regions given for each of the 6 genes (Lee et al., 2012, Table 3). The genomic regions ranged in size from 6 Kb to 158 Kb. We had genotype data for 103 SNPs in those regions from an Illumina Human660W-Quad platform (see M. B. Miller et al., 2012), and we had dosages for an additional 464 SNPs imputed using a HapMap2 (Frazer et al., 2007) CEPH European (CEU) reference panel, with prephasing done using Beagle (Browning & Browning, 2009) and imputation completed using miniMac (Howie, Fuchsberger, Stephens, Marchini, & Abecasis, 2012). Eight imputed markers were dropped for low R2 (< .3), but most markers were imputed well (387 had R2 > .9). MDD diagnosis was regressed on each of these 567 usable markers. To account for the complex family structure of the MCTFR data, we used the rapid, feasible generalized least-squares (RFGLS) method (Li, Basu, Miller, Iacono, & McGue, 2011). The RFGLS algorithm models the covariance structure separately for the twin, sibling, and adoptive families in order to account for genetic and environmental contributions to phenotypic similarity among family members. The SNP effect was modeled as both an additive effect of the number of minor alleles and an interaction between the number of minor alleles and generation, in order to determine whether SNP effects differed for offspring and parents. Analyses included demographic covariates, including age, sex, birth year, generation (offspring versus parent), and the two-way interactions between age and generation, sex and generation, and birth year and generation, as well as the first 10 principal components from an Eigenstrat analysis (Price et al., 2006) of the SNP data in order to account for any residual population structure within the Caucasian sample. Second, we conducted gene-based analysis using the versatile gene-based association study (VEGAS; Liu et al., 2010) approach. The VEGAS approach assessed the degree to which SNPs in the 6 genes and their surrounding regions were associated with MDD. VEGAS uses the p values obtained in RFGLS, adjusts for the LD structure of the SNPs within each gene using simulations from the multivariate normal distribution, and yields a gene-based test statistic and its corresponding p value for each gene. To parallel the analytic approach we took in Study 1, we required the presence of a significant association between a genetic variant and MDD prior to extending the analyses to associations with early-onset and recurrent MDD.
Results
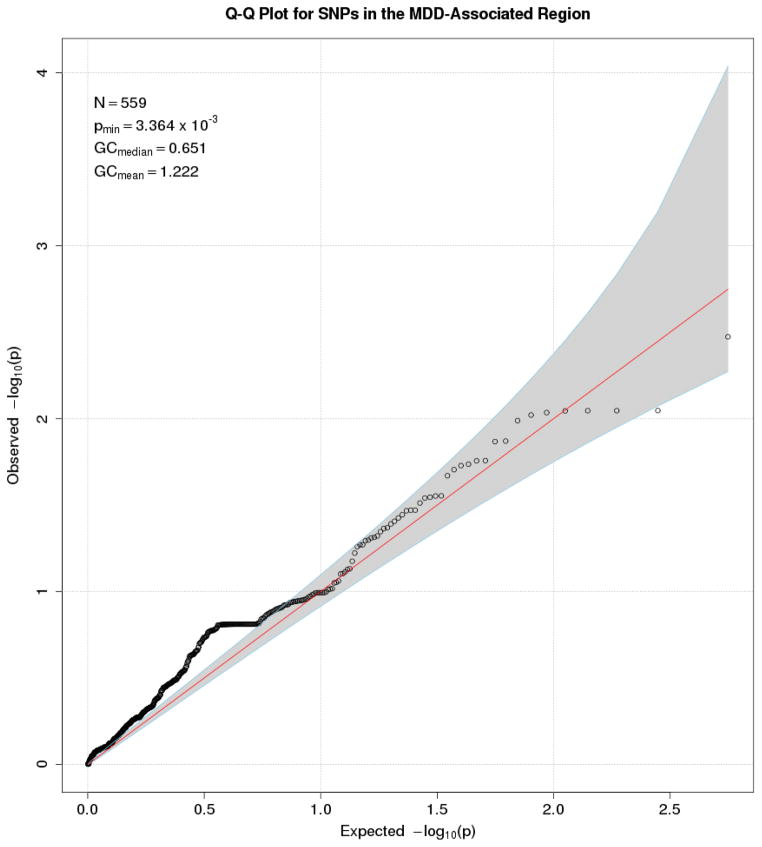
We first conducted individual SNP-based tests for SNPs in SLC1A4, CACNA1A, GRM8, PARK2, UNC13A, and SHC3 using RFGLS. A Q-Q plot of the expected distribution of p values for SNPs plotted against the observed p values (Figure 1) indicated that no p value for the association of any of the 567 SNPs with MDD approached significance after correction for multiple tests. We tested 567 SNPs, yielding a Bonferroni-corrected p value of 9 × 10−5. The smallest RFGLS p value for the association for the 567 SNPs with MDD was p = .00336. Thus, none of the SNPs we tested could be considered significant. We next conducted gene-based analysis of SLC1A4, CACNA1A, GRM8, PARK2, UNC13A, and SHC3 and their surrounding regions using VEGAS. The results of these analyses are presented in Table 2. We tested 6 genes, yielding a Bonferroni-corrected p value of 8 × 10−3. The smallest VEGAS p value for the association for the 6 genes with MDD was p = .09 for SLC1A4. Thus, none of the 6 genes we tested showed significant associations with MDD. Because neither the individual SNP-based or gene-based approach indicated any significant associations with a diagnosis MDD after correcting for multiple tests, we did not conduct further analysis of early-onset or recurrent MDD.
Figure 1.
Q-Q Plot for SNPs in the MDD-Associated Region Using Individual SNP-Based Analysis
Table 2.
Associations Between MDD and 6 Genes From the Glutamatergic System Using Gene-Based VEGAS Analysis
| Gene | p value | Best SNP | SNP p value |
|---|---|---|---|
| SLC1A4 | .087 | rs7601712 | .0034 |
| CACNA1A | .235 | rs4461194 | .0428 |
| GRM8 | .421 | rs17875031 | .0135 |
| PARK2 | .920 | rs9365313 | .3122 |
| UNC13A | .271 | rs2287851 | .1566 |
| SHC3 | .886 | rs9285046 | .3337 |
Note. The 6 genes considered in these analyses were identified by Lee et al. (2012) as showing significant associations with MDD. MDD = major depressive disorder. SNP = single nucleotide polymorphism.
Discussion
In Study 2, we attempted to confirm Lee et al.’s (2012) positive finding of associations between MDD and SNPs in 6 glutamatergic genes using individual SNP-based tests and gene-based analyses. Although we had a larger sample size than is typically seen in candidate gene analyses, and that included a large number of cases of MDD (n = 1,993), we found no evidence of significant associations with MDD for any of the genes after adopting conventionally accepted procedures applied in molecular genetics studies to correct for multiple tests.
It could be that had we used a different set of candidate genes, we would have found significant associations with MDD. However, the genes we examined emerged from a carefully reasoned analytic plan that included text-mining statistical analysis to identify 178 gene sets potentially implicated in the pathogenesis of MDD and multi-locus enrichment analysis of these gene sets using over 4,000 MDD cases and over 4,000 non-MDD controls (Lee et al., 2012). Using this approach, only one gene set produced significant effects, but missing from the literature was an attempted replication of these findings, thus leaving ambiguous how much confidence to place in them. Our interest was also stimulated by the possibility that especially severe MDD, such as that characterized by early onset or recurrent course, might be related to these genes. Unfortunately, our effort to confirm and extend the Lee et al. (2012) positive findings for a lifetime diagnosis of MDD in our sample failed; given the absence of a significant effect for MDD, we did not attempt to extend the findings by examining effects specific to an earlier onset or recurrent course. Gene × environment interaction (G × E) studies have shown some promise for identifying effects for MDD for certain genes (Dick et al., 2011; Rutter et al., 2006; Thapar et al., 2007), and it is possible that had we examined G × E effects for these glutamatergic genes, our results would have been different. However, in the absence of either a main effect for the genes of interest or a reasonable hypothesis supporting such a G × E analysis, we had no justification for undertaking such an analysis here.
General Discussion
In the present paper, we sought to identify risk factors for MDD and predictors of an earlier onset and recurrent course. We considered indicators of risk assessed at multiple levels of analysis, including family (parental psychiatric disorders, parent-child relationship), individual (personality, internalizing and externalizing psychopathology, academic functioning, physical development, childhood maltreatment), and genetics levels. We identified a number of risk factors for MDD, as well as several that were specific to early-onset and recurrent MDD.
Our examination of risk factors for MDD assessed at multiple levels of analysis reflects a growing appreciation for the importance of considering the interdependent biological, psychological, and social systems that underlie the development and manifestation of psychopathology. Study 1 showed that indicators of risk from a broad range of domains predicted the subsequent development of MDD. These results are notable in highlighting the severity and pervasiveness of dysfunction evident well before the onset of MDD, while suggesting important directions of future research. For example, does impairment in the parent-child relationship simply reflect parental psychopathology and offspring liability to MDD, or does it play a causal role in the development of MDD? Does successful intervention of premorbid risk, such as treatment of externalizing symptoms, protect against the subsequent development of MDD? What are the mechanisms by which early pubertal development confers risk for the development of MDD—does this finding reflect biological (e.g., hormonal) or social influences, or both? Answering these questions will further understanding of the etiology of MDD, while also informing the most targeted and effective prevention and intervention efforts.
Taking a multiple-levels approach allows us to go beyond descriptive study and has yielded tremendous leaps in our understanding of the etiology of psychopathology, as well as mechanisms of its development. This is an exciting time of discovery, but it is also clear that we have much to learn. The field of molecular genetics has made considerable advances in recent years, including novel analytic approaches such as that taken by Lee et al. (2012), but important work remains. Our failure to confirm the positive findings of Lee et al. (2012) joins many candidate gene replication failures since the recent explosion of candidate gene studies (see Sullivan, 2007). Indeed, the problem of finding common or rare variants that influence psychopathology is not just an issue when it comes to MDD, but a broader one in psychiatric genetics as a whole, where consistent replicable findings have proven hard to come by. Nonetheless, important advances in our understanding of the etiology and manifestation of MDD and other psychiatric disorders are being made, and continued interdisciplinary efforts that span fields of research, methodologies, and informants will lead to the most targeted and effective prevention and intervention efforts for these devastating diseases.
Strengths and Limitations
The present study has a number of strengths, including the examination of several putative risk factors for MDD assessed at multiple levels of analysis using multiple methods and informants in a large, community-based sample. Offspring were prospectively assessed from late childhood into early adulthood, with minimal attrition bias. However, there are also notable limitations that prompt caution in interpreting the results and suggest important directions for future research. Our assessment of offspring MDD at multiple time points through age 29 allowed us examine premorbid risk for offspring classified as having early-onset versus later-onset MDD, recurrent versus single-episode MDD, or no MDD. However, it is possible that some offspring classified as never depressed will eventually be diagnosed with MDD, and that some classified as having had only a single MDD episode of will ultimately experience a recurrence. Our assessment of MDD included information on age of onset and number of episodes, but the MTFS does not assess dysthymic disorder or the duration of MDD episodes; thus, we were able to examine MDD recurrence, but not duration or chronicity. The lack of racial or ethnic diversity in our sample limits the generalizability of the results of Study 1, though it allowed us to retain a large proportion of our sample for our candidate gene analysis in Study 2, which was limited to Caucasian participants.
Conclusions
Our longitudinal study of premorbid risk for MDD, spanning 2 decades and encompassing indicators of risk assessed at multiple levels of analysis, found evidence of both family- and individual-level risk for MDD that was evident in late childhood, well before the onset of MDD. Moreover, a number of risk factors, largely reflecting externalizing psychopathology, predicted an earlier onset of MDD, while risk for a recurrent course of MDD was largely specific to recurrent MDD that also showed an early onset. Continued examination of risk factors for an earlier age of onset and a recurrent course of MDD assessed at multiple levels of analysis will further our understanding of the etiology of MDD, its effects on functioning, and, ultimately, inform prevention and intervention efforts.
Acknowledgments
Research reported in this article was supported by the National Institute of Drug Abuse of the National Institutes of Health under award numbers R37DA005147, R01DA013240, and U01DA024417; the National Institute of Alcohol Abuse and Alcoholism of the National Institutes of Health under award numbers R01AA009367 and R01AA011886; and the National Institute of Mental Health of the National Institutes of Health under award number R01MH066140. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Bipolar I and II diagnoses (lifetime history through age 24) were also assessed. Given that the present study focused on risk for MDD, offspring who met lifetime criteria for bipolar I or II disorder at the definite or probable level (n = 17) were excluded from further analyses.
Due to missing diagnostic assessments, sample sizes for some MDD groups do not sum to the total sample size.
Parental anxiety disorders were assessed only for parents of female offspring.
With the exception of models that included parental anxiety disorders, which were assessed only for parents of female offspring.
The prevalence of MDD for Study 2 (younger and older cohort twins, enrichment cohort twins, sibling sample, parents of twins and siblings) is somewhat lower than that for Study 1 (younger and older cohort twins), reflecting different rates of MDD as a function of age and cohort. Analyses included age, birth year, and generation in order to account for age and cohort effects.
Offspring and parents who met lifetime criteria for bipolar I or II disorder at the definite or probable level (n = 32) were excluded from further analyses.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Arlington, VA: American Psychiatric Association; 1987. rev. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. text rev. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene Ontology: tool for the unification of biology. Nature Genetics. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardone AM, Moffitt TE, Caspi A, Dickson N, Silva PA. Adult mental health and social outcomes of adolescent girls with depression and conduct disorder. Development and Psychopathology. 1996;8:811–829. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Beesdo K, Hofler M, Leibenluft E, Lieb R, Bauer M, Pfennig A. Mood episodes and mood disorders: Patterns of incidence and conversion in the first three decades of life. Bipolar Disorders. 2009;11:637–649. doi: 10.1111/j.1399-5618.2009.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers CG, Rohde P, Stice E, Nolen-Hoeksema S. Recovery from major depressive disorder among female adolescents: A prospective test of the scar hypothesis. Journal of Consulting & Clinical Psychology. 2007;75:888–900. doi: 10.1037/0022-006X.75.6.888. [DOI] [PubMed] [Google Scholar]
- Bemmels HR, Burt SA, Legrand LN, Iacono WG, McGue M. The heritability of life events: An adolescent twin and adoption study. Twin Research and Human Genetics. 2008;11:257–265. doi: 10.1375/twin.11.3.257. [DOI] [PubMed] [Google Scholar]
- Billig JP, Hershberger SL, Iacono WG, McGue M. Life events and personality in late adolescence: Genetic and environmental relations. Behavior Genetics. 1996;26:543–554. doi: 10.1007/BF02361227. [DOI] [PubMed] [Google Scholar]
- Bland RC, Newman SC, Orn H. Recurrent and nonrecurrent depression. A family study. Archives of General Psychiatry. 1986;43:1085–1089. doi: 10.1001/archpsyc.1986.01800110071009. [DOI] [PubMed] [Google Scholar]
- Bosker FJ, Hartman CA, Nolte IM, Prins BP, Terpstra P, Posthuma D, et al. Poor replication of candidate genes for major depressive disorder using genome-wide association data. Molecular Psychiatry. 2011;16:516–532. doi: 10.1038/mp.2010.38. [DOI] [PubMed] [Google Scholar]
- Breslau J, Michael L, Nancy SB, Kessler RC. Mental disorders and subsequent educational attainment in a US national sample. Journal of Psychiatric Research. 2008;42:708–716. doi: 10.1016/j.jpsychires.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning BL, Browning SR. A unified approach to genotype imputation and haplotype-phase inference for large data sets of trios and unrelated individuals. American Journal of Human Genetics. 2009;84:210–223. doi: 10.1016/j.ajhg.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burcusa SL, Iacono WG. Risk for recurrence in depression. Clinical Psychology Review. 2007;27:959–985. doi: 10.1016/j.cpr.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capizzano AA, Jorge RE, Acion LC, Robinson RG. In vivo proton magnetic resonance spectroscopy in patients with mood disorders: A technically oriented review. Journal of Magnetic Resonance Imaging. 2007;26:1378–1389. doi: 10.1002/jmri.21144. [DOI] [PubMed] [Google Scholar]
- Chapman DP, Perry GS, Strine TW. The vital link between chronic disease and depressive disorders. Preventing Chronic Disease. 2005;2:1–10. [PMC free article] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Copeland WE, Shanahan L, Worthman C, Angold A, Costello EJ. Cumulative depression episodes predict later C-reactive protein levels: A prospective analysis. Biological Psychiatry. 2012;71:15–21. doi: 10.1016/j.biopsych.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coryell W, Endicott J, Keller MB. Major depression in a nonclinical sample: Demographic and clinical risk factors for first onset. Archives of General Psychiatry. 1992;49:117–125. doi: 10.1001/archpsyc.1992.01820020037005. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Erkanli A, Angold A. Is there an epidemic of child or adolescent depression? Journal of Child Psychology and Psychiatry. 2006;47:1263–1271. doi: 10.1111/j.1469-7610.2006.01682.x. [DOI] [PubMed] [Google Scholar]
- Cukrowicz KC, Taylor J, Schatschneider C, Iacono WG. Personality differences in children and adolescents with attention-deficit/hyperactivity disorder, conduct disorder, and controls. Journal of Child Psychology and Psychiatry. 2006;47:151–159. doi: 10.1111/j.1469-7610.2005.01461.x. [DOI] [PubMed] [Google Scholar]
- de Graaf R, Bijl RV, Ravelli A, Smit F, Vollebergh WA. Predictors of first incidence of DSM-III-R psychiatric disorders in the general population: Findings from the Netherlands Mental Health Survey and Incidence Study. Acta Psychiatrica Scandinavica. 2002;106:303–313. doi: 10.1034/j.1600-0447.2002.01397.x. [DOI] [PubMed] [Google Scholar]
- Dick DM. Gene-environment interaction in psychological traits and disorders. Annual Review of Clinical Psychology. 2011;7:383–409. doi: 10.1146/annurev-clinpsy-032210-104518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson KS, Dozois DJA. Risk factors in depression. San Diego, CA: Academic Press; 2008. [Google Scholar]
- Duggan CF, Lee AS, Murray RM. Does personality predict long-term outcome in depression? British Journal of Psychiatry. 1990;157:19–24. doi: 10.1192/bjp.157.1.19. [DOI] [PubMed] [Google Scholar]
- Duggan CF, Sham P, Lee AS, Murray RM. Does recurrent depression lead to a change in neuroticism? Psychological Medicine. 1991;21:985–990. doi: 10.1017/s0033291700029974. [DOI] [PubMed] [Google Scholar]
- Durbin CE, Klein DN, Hayden EP, Buckley ME, Moerk KC. Temperamental emotionality in preschoolers and parental mood disorders. Journal of Abnormal Psychology. 2005;114:28–37. doi: 10.1037/0021-843X.114.1.28. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Shao H, Nestadt G, Lee BH, Bienvenu OJ, Zandi P. Population-based study of first onset and chronicity in major depressive disorder. Archives of General Psychiatry. 2008;65:513–520. doi: 10.1001/archpsyc.65.5.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins IJ, McGue M, Iacono WG. Genetic and environmental influences on parent-son relationships: Evidence for increasing genetic influence during adolescence. Developmental Psychology. 1997;33:351–363. doi: 10.1037//0012-1649.33.2.351. [DOI] [PubMed] [Google Scholar]
- Fanous AH, Neale MC, Aggen SH, Kendler KS. A longitudinal study of personality and major depression in a population-based sample of male twins. Psychological Medicine. 2007;37:1163–1172. doi: 10.1017/S0033291707000244. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM, Horwood LJ. Recurrence of major depression in adolescence and early adulthood, and later mental health, educational and economic outcomes. British Journal of Psychiatry. 2007;191:335–342. doi: 10.1192/bjp.bp.107.036079. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Archives of General Psychiatry. 2002;59:225–231. doi: 10.1001/archpsyc.59.3.225. [DOI] [PubMed] [Google Scholar]
- Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder: Remission, recovery, relapse, and recurrence. Archives of General Psychiatry. 1991;48:851–855. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- Frazer KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, Gibbs RA, et al. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449:851–861. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Born C, Jager M, Groll C, et al. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biological Psychiatry. 2003;53:338–344. doi: 10.1016/s0006-3223(02)01474-9. [DOI] [PubMed] [Google Scholar]
- Glessner JT, Wang K, Cai GQ, Korvatska O, Kim CE, Wood S, et al. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature. 2009;459:569–573. doi: 10.1038/nature07953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdi NR, Iacono WG. Lifetime prevalence and co-morbidity of externalizing disorders and depression in prospective assessment. Psychological Medicine. 2014;44:315–324. doi: 10.1017/S0033291713000627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C, Brennan PA, Keenan-Miller D. Patterns of adolescent depression to age 20: The role of maternal depression and youth interpersonal dysfunction. Journal of Abnormal Child Psychology. 2008;36:1189–1198. doi: 10.1007/s10802-008-9241-9. [DOI] [PubMed] [Google Scholar]
- Hammen C, Brennan PA, Keenan-Miller D, Herr NR. Early onset recurrent subtype of adolescent depression: Clinical and psychosocial correlates. Journal of Child Psychology and Psychiatry. 2008;49:433–440. doi: 10.1111/j.1469-7610.2007.01850.x. [DOI] [PubMed] [Google Scholar]
- Han SZ, Yang BZ, Kranzler HR, Liu XM, Zhao HY, Farrer LA, et al. Integrating GWASs and human protein interaction networks identifies a gene subnetwork underlying alcohol dependence. American Journal of Human Genetics. 2013;93:1027–1034. doi: 10.1016/j.ajhg.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Research Reviews. 2009;61:105–123. doi: 10.1016/j.brainresrev.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biological Psychiatry. 2007;62:1310–1316. doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: Results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Archives of General Psychiatry. 2005;62:1097–1106. doi: 10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- Hek K, Demirkan A, Lahti J, Terracciano A, Teumer A, Cornelis MC, et al. A genome-wide association study of depressive symptoms. Biological Psychiatry. 2013;73:667–678. doi: 10.1016/j.biopsych.2012.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Iacono WG, McGue M. Identifying childhood characteristics that underlie premorbid risk for substance use disorders: Socialization and boldness. Development and Psychopathology. 2014;26:141–157. doi: 10.1017/S0954579413000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Johnson W, Durbin CE, Blonigen DM, Iacono WG, McGue M. Delineating selection and mediation effects among childhood personality and environmental risk factors in the development of adolescent substance abuse. Journal of Abnormal Child Psychology. doi: 10.1007/s10802-013-9831-z. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfeld RMA, Montgomery SA, Keller MB, Kasper S, Schatzberg AF, Moller HJ, et al. Social functioning in depression: A review. Journal of Clinical Psychiatry. 2000;61:268–275. doi: 10.4088/jcp.v61n0405. [DOI] [PubMed] [Google Scholar]
- Holmans P, Green EK, Pahwa JS, Ferreira MAR, Purcell SM, Sklar P, et al. Gene ontology analysis of GWA study data sets provides insights into the biology of bipolar disorder. American Journal of Human Genetics. 2009;85:13–24. doi: 10.1016/j.ajhg.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie B, Fuchsberger C, Stephens M, Marchini J, Abecasis GR. Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nature Genetics. 2012;44:955–959. doi: 10.1038/ng.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance-case disorders: Findings from the Minnesota Twin Family Study. Development and Psychopathology. 1999;11:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Iacono WG, McGue M. Minnesota Twin Family Study. Twin Research. 2002;5:482–487. doi: 10.1375/136905202320906327. [DOI] [PubMed] [Google Scholar]
- Iacono WG, McGue M, Krueger RF. Minnesota Center for Twin and Family Research. Twin Research and Human Genetics. 2006;9:978–984. doi: 10.1375/183242706779462642. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Moffitt TE, Caspi A, Fombonne E, Poulton R, Martin J. Differences in early childhood risk factors for juvenile-onset and adult-onset depression. Archives of General Psychiatry. 2002;59:215–222. doi: 10.1001/archpsyc.59.3.215. [DOI] [PubMed] [Google Scholar]
- John OP, Caspi A, Robins RW, Moffitt TE, Stouthamer-Loeber M. The “little five”: Exploring the nomological network of the five-factor model of personality in adolescent boys. Child Development. 1994;65:160–178. [PubMed] [Google Scholar]
- Johnson W, McGue M, Iacono WG. Genetic and environmental influences on academic achievement trajectories during adolescence. Developmental Psychology. 2006;42:514–532. doi: 10.1037/0012-1649.42.3.514. [DOI] [PubMed] [Google Scholar]
- Jonsson U, Bohman H, Hjern A, von Knorring L, Paaren A, Olsson G, et al. Intimate relationships and childbearing after adolescent depression: A population-based 15 year follow-up study. Social Psychiatry and Psychiatric Epidemiology. 2011;46:711–721. doi: 10.1007/s00127-010-0238-7. [DOI] [PubMed] [Google Scholar]
- Judd LL, Paulus MJ, Schettler PJ, Akiskal HS, Endicott J, Leon AC, et al. Does incomplete recovery from first lifetime major depressive episode herald a chronic course of illness? American Journal of Psychiatry. 2000;157:1501–1504. doi: 10.1176/appi.ajp.157.9.1501. [DOI] [PubMed] [Google Scholar]
- Keenan-Miller D, Hammen CL, Brennan PA. Health outcomes related to early adolescent depression. Journal of Adolescent Health. 2007;41:256–262. doi: 10.1016/j.jadohealth.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Mueller TI, Endicott J, Coryell W, Hirschfeld RMA, et al. Time to recovery, chronicity, and levels of psychopathology in major depression: A 5-year prospective follow-up of 431 subjects. Archives of General Psychiatry. 1992;49:809–816. doi: 10.1001/archpsyc.1992.01820100053010. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gatz M, Gardner CO, Pedersen NL. Personality and major depression: A Swedish longitudinal, population-based twin study. Archives of General Psychiatry. 2006;63:1113–1120. doi: 10.1001/archpsyc.63.10.1113. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A longitudinal twin study of personality and major depression in women. Archives of General Psychiatry. 1993;50:853–862. doi: 10.1001/archpsyc.1993.01820230023002. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The costs of depression. Psychiatric Clinics of North America. 2012;35:1–14. doi: 10.1016/j.psc.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DN, Lewinsohn PM, Rohde P, Seeley JR, Durbin CE. Clinical features of major depressive disorder in adolescents and their relatives: Impact on familial aggregation, implications for phenotype definition, and specificity of transmission. Journal of Abnormal Psychology. 2002;111:98–106. [PubMed] [Google Scholar]
- Klein DN, Lewinsohn PM, Seeley JR, Rohde P. A family study of major depressive disorder in a community sample of adolescents. Archives of General Psychiatry. 2001;58:13–20. doi: 10.1001/archpsyc.58.1.13. [DOI] [PubMed] [Google Scholar]
- Klein DN, Shankman SA, Lewinsohn PM, Rohde P, Seeley JR. Family study of chronic depression in a community sample of young adults. American Journal of Psychiatry. 2004;161:646–653. doi: 10.1176/appi.ajp.161.4.646. [DOI] [PubMed] [Google Scholar]
- Kohli MA, Lucae S, Saemann PG, Schmidt MV, Demirkan A, Hek K, et al. The neuronal transporter gene SLC6A15 confers risk to major depression. Neuron. 2011;70:252–265. doi: 10.1016/j.neuron.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Gamez W, Schmidt F, Watson D. Linking “big” personality traits to anxiety, depressive, and substance use disorders: A meta-analysis. Psychological Bulletin. 2010;136:768–821. doi: 10.1037/a0020327. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Devlin B, Pollock M, Richards C, Mukerji P. A controlled family history study of childhood-onset depressive disorder. Archives of General Psychiatry. 1997;54:613–623. doi: 10.1001/archpsyc.1997.01830190033004. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Feinberg TL, Crouse-Novak M, Paulauskas SL, Pollock M, Finkelstein R. Depressive disorders in childhood: II. A longitudinal study of the risk for a subsequent major depression. Archives of General Psychiatry. 1984;41:643–649. doi: 10.1001/archpsyc.1984.01790180013001. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Frank E. The interaction of drug- and psychotherapy in the long-term treatment of depression. Journal of Affective Disorders. 2001;62:131–137. doi: 10.1016/s0165-0327(00)00357-8. [DOI] [PubMed] [Google Scholar]
- Lee PH, Perlis RH, Jung JY, Byrne EM, Rueckert E, Siburian R, et al. Multi-locus genome-wide association analysis supports the role of glutamatergic synaptic transmission in the etiology of major depressive disorder. Translational Psychiatry. 2012:2. doi: 10.1038/tp.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, Allen NB, Seeley JR, Gotlib IH. First onset versus recurrence of depression: Differential processes of psychosocial risk. Journal of Abnormal Psychology. 1999;108:483–489. doi: 10.1037//0021-843x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Clarke GN, Seeley JR, Rohde P. Major depression in community adolescents: Age at onset, episode duration, and time to recurrence. Journal of the American Academy of Child & Adolescent Psychiatry. 1994;33:809–818. doi: 10.1097/00004583-199407000-00006. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Rohde P, Seeley JR, Klein DN, Gotlib IH. Psychosocial functioning of young adults who have experienced and recovered from major depressive disorder during adolescence. Journal of Abnormal Psychology. 2003;112:353–363. doi: 10.1037/0021-843x.112.3.353. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Seeley JR, Hibbard J, Rohde P, Sack WH. Cross-sectional and prospective relationships between physical morbidity and depression in older adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 1996;35:1120–1129. doi: 10.1097/00004583-199609000-00009. [DOI] [PubMed] [Google Scholar]
- Lewis CM, Ng MY, Butler AW, Cohen-Woods S, Uher R, Pirlo K, et al. Genome-wide association study of major recurrent depression in the UK population. American Journal of Psychiatry. 2010;167:949–957. doi: 10.1176/appi.ajp.2010.09091380. [DOI] [PubMed] [Google Scholar]
- Li X, Basu S, Miller MB, Iacono WG, McGue M. A rapid generalized least squares model for a genome-wide quantitative trait association analysis in families. Human Heredity. 2011;71:67–82. doi: 10.1159/000324839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips ES, Cornelisse LN, Toonen RF, Min JL, Hultman CM, Consortium TIS, et al. Functional gene group analysis identifies synaptic gene groups as risk factor for schizophrenia. Molecular Psychiatry. 2012;17:996–1006. doi: 10.1038/mp.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JZ, Mcrae AF, Nyholt DR, Medland SE, Wray NR, Brown KM, et al. A versatile gene-based test for genome-wide association studies. American Journal of Human Genetics. 2010;87:139–145. doi: 10.1016/j.ajhg.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohoff FW. Overview of the genetics of major depressive disorder. Current Psychiatry Reports. 2010;12:539–546. doi: 10.1007/s11920-010-0150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonigan CJ, Phillips BM, Hooe ES. Relations of positive and negative affectivity to anxiety and depression in children: Evidence from a latent variable longitudinal study. Journal of Consulting and Clinical Psychology. 2003;71:465–481. doi: 10.1037/0022-006x.71.3.465. [DOI] [PubMed] [Google Scholar]
- Lopez-Leon S, Janssens ACJW, Ladd AMGZ, Del-Favero J, Claes SJ, Oostra BA, et al. Meta-analyses of genetic studies on major depressive disorder. Molecular Psychiatry. 2008;13:772–785. doi: 10.1038/sj.mp.4002088. [DOI] [PubMed] [Google Scholar]
- Lubke GH, Hottenga JJ, Walters R, Laurin C, de Geus EJC, Willemsen G, et al. Estimating the genetic variance of major depressive disorder due to all single nucleotide polymorphisms. Biological Psychiatry. 2012;72:707–709. doi: 10.1016/j.biopsych.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Salvadore G, DiazGranados N, Zarate CA. Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacology & Therapeutics. 2009;123:143–150. doi: 10.1016/j.pharmthera.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA., Jr The role of glutamate in mood disorders: Results from the ketamine in major depression study and the presumed cellular mechanism underlying its antidepressant effects. Current Psychiatry Reports. 2007;9:467–474. doi: 10.1007/s11920-007-0063-1. [DOI] [PubMed] [Google Scholar]
- Marmorstein NR, Iacono WG, Legrand L. Obesity and depression in adolescence and beyond: reciprocal risks. International Journal of Obesity. doi: 10.1038/ijo.2014.19. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattisson C, Bogren M, Horstmann V, Munk-Jorgensen P, Nettelbladt P. The long-term course of depressive disorders in the Lundby Study. Psychological Medicine. 2007;37:883–891. doi: 10.1017/S0033291707000074. [DOI] [PubMed] [Google Scholar]
- McGue M, Keyes M, Sharma A, Elkins I, Legrand L, Johnson W, et al. The environments of adopted and non-adopted youth: Evidence on range restriction from the Sibling Interaction and Behavior Study (SIBS) Behavior Genetics. 2007;37:449–462. doi: 10.1007/s10519-007-9142-7. [DOI] [PubMed] [Google Scholar]
- McGuffin P, Katz R, Watkins S, Rutherford J. A hospital-based twin register of the heritability of DSM-IV unipolar depression. Archives of General Psychiatry. 1996;53:129–136. doi: 10.1001/archpsyc.1996.01830020047006. [DOI] [PubMed] [Google Scholar]
- Miller MB, Basu S, Cunningham J, Eskin E, Malone SM, Oetting WS, et al. The Minnesota Center for Twin and Family Research genome-wide association study. Twin Research and Human Genetics. 2012;15:767–774. doi: 10.1017/thg.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineka S, Watson D, Clark LA. Comorbidity of anxiety and unipolar mood disorders. Annual Review of Psychology. 1998;49:377–412. doi: 10.1146/annurev.psych.49.1.377. [DOI] [PubMed] [Google Scholar]
- Mirza Y, Tang J, Russell A, Banerjee SP, Bhandari R, Ivey J, et al. Reduced anterior cingulate cortex glutamatergic concentrations in childhood major depression. Journal of the American Academy of Child & Adolescent Psychiatry. 2004;43:341–348. doi: 10.1097/00004583-200403000-00017. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Taylor A, Kokaua J, Milne BJ, Polanczyk G, et al. How common are common mental disorders? Evidence that lifetime prevalence rates are doubled by prospective versus retrospective ascertainment. Psychological Medicine. 2010;40:899–909. doi: 10.1017/S0033291709991036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller TI, Leon AC, Keller MB, Solomon DA, Endicott J, Coryell W, et al. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. American Journal of Psychiatry. 1999;156:1000–1006. doi: 10.1176/ajp.156.7.1000. [DOI] [PubMed] [Google Scholar]
- Muglia P, Tozzi F, Galwey NW, Francks C, Upmanyu R, Kong XQ, et al. Genome-wide association study of recurrent major depressive disorder in two European case-control cohorts. Molecular Psychiatry. 2010;15:589–601. doi: 10.1038/mp.2008.131. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annual Review of Pharmacology and Toxicology. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino TM, Shankman SA, Klein DN, Seeley JR, Pettit JW, Farmer RF, et al. Lifetime rates of psychopathology in single versus multiple diagnostic assessments: Comparison in a community sample of probands and siblings. Journal of Psychiatric Research. 2012;46:1217–1222. doi: 10.1016/j.jpsychires.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva EM, Keyes M, Iacono WG, McGue M. Adolescent substance use groups: Antecedent and concurrent personality differences in a longitudinal study. Journal of Personality. 2012;80:769–793. doi: 10.1111/j.1467-6494.2011.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormel J, Oldehinkel AJ, Nolen WA, Vollebergh W. Psychosocial disability before, during, and after a major depressive episode: A 3-wave population-based study of state, scar, and trait effects. Archives of General Psychiatry. 2004;61:387–392. doi: 10.1001/archpsyc.61.4.387. [DOI] [PubMed] [Google Scholar]
- Ormel J, Oldehinkel AJ, Vollebergh W. Vulnerability before, during, and after a major depressive episode: A 3-wave population-based study. Archives of General Psychiatry. 2004;61:990–996. doi: 10.1001/archpsyc.61.10.990. [DOI] [PubMed] [Google Scholar]
- Paul IA, Skolnick P. Glutamate and depression: Clinical and preclinical studies. Annals of the New York Academy of Sciences. 2003;1003:250–272. doi: 10.1196/annals.1300.016. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pettit JW, Hartley C, Lewinsohn PM, Seeley JR, Klein DN. Is liability to recurrent major depressive disorder present before first episode onset in adolescence or acquired after the initial episode? Journal of Abnormal Psychology. 2013;122:353–358. doi: 10.1037/a0032655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit JW, Lewinsohn PM, Roberts RE, Seeley JR, Monteith L. The long-term course of depression: Development of an empirical index and identification of early adult outcomes. Psychological Medicine. 2009;39:403–412. doi: 10.1017/S0033291708003851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nature Genetics. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- Puig-Antich J, Kaufman J, Ryan ND, Williamson DE, Dahl RE, Lukens E, et al. The psychosocial functioning and family environment of depressed adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 1993;32:244–253. doi: 10.1097/00004583-199303000-00003. [DOI] [PubMed] [Google Scholar]
- Rao U, Hammen C, Daley SE. Continuity of depression during the transition to adulthood: A 5-year longitudinal study of young women. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38:908–915. doi: 10.1097/00004583-199907000-00022. [DOI] [PubMed] [Google Scholar]
- Rao U, Ryan ND, Birmaher B, Dahl RE, Williamson DE, Kaufman J, et al. Unipolar depression in adolescents: Clinical outcome in adulthood. Journal of the American Academy of Child & Adolescent Psychiatry. 1995;34:566–578. doi: 10.1097/00004583-199505000-00009. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS, Congdon RT. HLM 6 for Windows. Skokie, IL: Scientific Software International, Inc; 2004. [Google Scholar]
- Raychaudhuri S, Plenge RM, Rossin EJ, Ng ACY, Consortium IS, Purcell SM, et al. Identifying relationships among genomic disease regions: Predicting genes at pathogenic SNP associations and rare deletions. PLoS Genetics. 2009;5:1–15. doi: 10.1371/journal.pgen.1000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich W, Welner Z. Diagnostic Interview for Children and Adolescents--Revised: DSM-III-R version (DICA-R) St. Louis, OH: Washington University; 1988. [Google Scholar]
- Rice F, Harold G, Thapar A. The genetic aetiology of childhood depression: A review. Journal of Child Psychology and Psychiatry. 2002;43:65–79. doi: 10.1111/1469-7610.00004. [DOI] [PubMed] [Google Scholar]
- Rietschel M, Mattheisen M, Frank J, Treutlein J, Degenhardt F, Breuer R, et al. Genome-wide association-, replication-, and neuroimaging study implicates HOMER1 in the etiology of major depression. Biological Psychiatry. 2010;68:578–585. doi: 10.1016/j.biopsych.2010.05.038. [DOI] [PubMed] [Google Scholar]
- Rohde P, Lewinsohn PM, Klein DN, Seeley JR, Gau JM. Key characteristics of major depressive disorder occurring in childhood, adolescence, emerging adulthood, adulthood. Clinical Psychological Science. 2013;1:41–53. doi: 10.1177/2167702612457599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romera I, Perez V, Menchon JM, Delgado-Cohen H, Polavieja P, Gilaberte I. Social and occupational functioning impairment in patients in partial versus complete remission of a major depressive disorder episode. A six-month prospective epidemiological study. European Psychiatry. 2010;25:58–65. doi: 10.1016/j.eurpsy.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, MacMaster FP, Mirza Y, Smith JM, Easter PC, Banerjee SP, et al. Reduced anterior cingulate glutamate in pediatric major depression: A magnetic resonance spectroscopy study. Biological Psychiatry. 2005;58:700–704. doi: 10.1016/j.biopsych.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: Multiple varieties but real effects. Journal of Child Psychology and Psychiatry. 2006;47:226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Sartorius N. The economic and social burden of depression. Journal of Clinical Psychiatry. 2001;62:8–11. [PubMed] [Google Scholar]
- Shea MT, Leon AC, Mueller TI, Solomon DA, Warshaw MG, Keller MB. Does major depression result in lasting personality change? American Journal of Psychiatry. 1996;153:1404–1410. doi: 10.1176/ajp.153.11.1404. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9:2023–2028. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- Shi J, Potash JB, Knowles JA, Weissman MM, Coryell W, Scheftner WA, et al. Genome-wide association study of recurrent early-onset major depressive disorder. Molecular Psychiatry. 2011;16:193–201. doi: 10.1038/mp.2009.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyn SI, Shi J, Kraft JB, Potash JB, Knowles JA, Weissman MM, et al. Novel loci for major depression identified by genome-wide association study of Sequenced Treatment Alternatives to Relieve Depression and meta-analysis of three studies. Molecular Psychiatry. 2011;16:202–215. doi: 10.1038/mp.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihvola E, Rose RJ, Dick DM, Pulkkinen L, Marttunen M, Kaprio J. Early-onset depressive disorders predict the use of addictive substances in adolescence: A prospective study of adolescent Finnish twins. Addiction. 2008;103:2045–2053. doi: 10.1111/j.1360-0443.2008.02363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon DA, Keller MB, Leon AC, Mueller TI, Lavori PW, Shea MT, et al. Multiple recurrences of major depressive disorder. American Journal of Psychiatry. 2000;157:229–233. doi: 10.1176/appi.ajp.157.2.229. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. (rev. ed.) [Google Scholar]
- Spitzer RL, Endicott J, Robins E. Research diagnostic criteria: Rationale and reliability. Archives of General Psychiatry. 1978;35:773–782. doi: 10.1001/archpsyc.1978.01770300115013. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R (SCID) New York, NY: Biometrics Research Division, New York State Psychiatric Institute; 1987. [Google Scholar]
- Sullivan PF. Spurious genetic associations. Biological Psychiatry. 2007;61:1121–1126. doi: 10.1016/j.biopsych.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Daly MJ, Ripke S, Lewis CM, Lin DY, Wray NR, et al. A mega-analysis of genome-wide association studies for major depressive disorder. Molecular Psychiatry. 2013;18:497–511. doi: 10.1038/mp.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, de Geus EJC, Willemsen G, James MR, Smit JH, Zandbelt T, et al. Genome-wide association for major depressive disorder: A possible role for the presynaptic protein piccolo. Molecular Psychiatry. 2009;14:359–375. doi: 10.1038/mp.2008.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: Review and meta-analysis. American Journal of Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Swendsen JD, Merikangas KR. The comorbidity of depression and substance use disorders. Clinical Psychology Review. 2000;20:173–189. doi: 10.1016/s0272-7358(99)00026-4. [DOI] [PubMed] [Google Scholar]
- Tackett JL, Krueger RF, Iacono WG, McGue M. Personality in middle childhood: A hierarchical structure and longitudinal connections with personality in late adolescence. Journal of Research in Personality. 2008;42:1456–1462. doi: 10.1016/j.jrp.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellegen A, Waller NG. Exploring personality through test construction: Development of the Multidimensional Personality Questionnaire. In: Boyle GJ, Matthews G, Saklofske DH, editors. Handbook of personality theory and testing: Vol. II. Personality measurement and assessment. Thousand Oaks, CA: Sage; 2008. pp. 261–292. [Google Scholar]
- Thapar A, Harold G, Rice F, Langley K, O’Donovan M. The contribution of gene-environment interaction to psychopathology. Development and Psychopathology. 2007;19:989–1004. doi: 10.1017/S0954579407000491. [DOI] [PubMed] [Google Scholar]
- Üstün TB, Ayuso-Mateos JL, Chatterji S, Mathers C, Murray CJL. Global burden of depressive disorders in the year 2000. British Journal of Psychiatry. 2004;184:386–392. doi: 10.1192/bjp.184.5.386. [DOI] [PubMed] [Google Scholar]
- Watson D, Tellegen A. Toward a consensual structure of mood. Psychological Bulletin. 1985;98:219–215. doi: 10.1037//0033-2909.98.2.219. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Wickramaratne P, Merikangas KR, Leckman JF, Prusoff BA, Caruso KA, et al. Onset of major depression in early adulthood: Increased familial loading and specificity. Archives of General Psychiatry. 1984;41:1136–1143. doi: 10.1001/archpsyc.1984.01790230022003. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Wolk S, Goldstein RB, Moreau D, Adams P, Greenwald S, et al. Depressed adolescents grown up. JAMA. 1999;281:1707–1713. doi: 10.1001/jama.281.18.1707. [DOI] [PubMed] [Google Scholar]
- Weller RA, Kapadia P, Weller EB, Fristad M, Lazaroff LB, Preskorn SH. Psychopathology in families of children with major depressive disorders. Journal of Affective Disorders. 1994;31:247–252. doi: 10.1016/0165-0327(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Wetter EK, Hankin BL. Mediational pathways through which positive and negative emotionality contribute to anhedonic symptoms of depression: A prospective study of adolescents. Journal of Abnormal Child Psychology. 2009;37:507–520. doi: 10.1007/s10802-009-9299-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichers M, Geschwind N, van Os J, Peeters F. Scars in depression: Is a conceptual shift necessary to solve the puzzle? Psychological Medicine. 2010;40:359–365. doi: 10.1017/s0033291709990420. [DOI] [PubMed] [Google Scholar]
- Wickramaratne PJ, Warner V, Weissman MM. Selecting early onset MDD probands for genetic studies: Results from a longitudinal high-risk study. American Journal of Medical Genetics. 2000;96:93–101. [PubMed] [Google Scholar]
- Wilson S, DiRago AC, Iacono WG. Prospective inter-relationships between late adolescent personality and major depressive disorder in early adulthood. Psychological Medicine. 2014;44:567–577. doi: 10.1017/S0033291713001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S, Hicks BM, Foster KT, McGue M, Iacono WG. Age of onset and course of major depressive disorder: Associations with psychosocial funcitoning outcomes in adulthood. Psychological Medicine. doi: 10.1017/S0033291714001640. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. International statistical classification of diseases and related health problems. 10. Geneva: World Health Organization; 2010. [Google Scholar]
- Wray NR, Pergadia ML, Blackwood DHR, Penninx BWJH, Gordon SD, Nyholt DR, et al. Genome-wide association study of major depressive disorder: New results, meta-analysis, and lessons learned. Molecular Psychiatry. 2012;17:36–48. doi: 10.1038/mp.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz-Yesiloglu A, Ankerst DP. Review of 1-H magnetic resonance spectroscopy findings in major depressive disorder: A meta-analysis. Psychiatry Research: Neuroimaging. 2006;147:1–25. doi: 10.1016/j.pscychresns.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Zisook S, Lesser I, Stewart JW, Wisniewski SR, Balasubramani GK, Fava M, et al. Effect of age at onset on the course of major depressive disorder. American Journal of Psychiatry. 2007;164:1539–1546. doi: 10.1176/appi.ajp.2007.06101757. [DOI] [PubMed] [Google Scholar]
- Zisook S, Rush AJ, Albala A, Alpert J, Balasubramani GK, Fava M, et al. Factors that differentiate early vs. later onset of major depression disorder. Psychiatry Research. 2004;129:127–140. doi: 10.1016/j.psychres.2004.07.004. [DOI] [PubMed] [Google Scholar]