Abstract
BACKGROUND
Prenatal alcohol exposure (PAE) has adverse effects on reproductive function and hypothalamic-pituitary-gonadal (HPG) activity. Kisspeptin neurons play a role in mediating feedback effects of estradiol (E2) and progesterone (P4) on the HPG axis. We hypothesized that PAE will have long-term effects on the response of kisspeptin neurons to E2 and P4.
METHODS
Adult female rats (53–58 days) from prenatal ad libitum-fed control (C), pair-fed (PF), and alcohol-exposed (PAE) groups were subjected to Sham ovariectomy (OVX) or OVX without or with replacement with low or high physiological levels of E2 and P4, and terminated under basal conditions. E2 and P4 levels, and the response of kisspeptin-ir neurons in the arcuate (ARC) and anteroventral periventricular (AVPV) nuclei to these hormones, were measured. As the E2 signal is conveyed to kisspeptin neurons via estrogen receptor-α (ERα), we investigated PAE effects on the number of kisspetin-ir/ERα-ir neurons. To determine if PAE alters interactions between kisspeptin and gonadotropin releasing hormone (GnRH) neurons, close contacts between kisspeptin-ir fibers and GnRH-ir cell bodies were examined.
RESULTS
Our data present the novel finding that kisspeptin-ir neurons in the ARC of PAE females show differential responses to E2 and to the combined treatment with E2 and P4 compared to controls: 1) OVX increased the number of kisspeptin-ir neurons in C and PF, but not PAE females compared to their Sham counterparts; 2) E2 replacement restored kisspeptin-ir cell numbers to Sham levels in C and PF females but caused a robust downregulation of kisspeptin-ir neurons below Sham levels in PAE females; 3) OVX and replacement with high physiological concentrations of E2 resulted in fewer kisspeptin-ir cells in PAE than C females; 4) OVX and replacement with high levels of both E2 and P4 markedly decreased the number of kisspeptin-ir neurons, below levels observed following E2 alone, in PF and C females, but had no significant effect in PAE females.
CONCLUSION
These data suggest that a possible mechanism underlying adverse effects of PAE on HPG function involves actions of alcohol on the kisspeptin system.
Keywords: kisspeptin, ethanol, prenatal alcohol exposure, estrogen, progesterone
1. Introduction
Alcohol use can have adverse effects on reproductive function in women, resulting in anovulatory cycles or luteal phase dysfunction, impaired fertility and increased risk of spontaneous abortion (Geisenburg et al., 2002). Likewise, alcohol treatment of adult female rats can disrupt the estrous cycle (Rettori et al., 1987), reduce hypothalamic-pituitary-gonadal (HPG) hormone levels (LaPaglia et al., 1997), inhibit the preovulatory surge of luteinizing hormone (LH) (Marco et al., 1984), and decrease neuronal activation of gonadotropin-releasing hormone (GnRH) neurons (Ogilvie and Rivier, 1997). Prenatal alcohol exposure (PAE) can also have long-term adverse effects on offspring HPG function, including reduced pulsatile secretion of LH, decreased and delayed E2-induced preovulatory-like surges of LH (Handa et al., 1985; Wilson et al., 1995), increased variation in LH levels across the estrous cycle (Lan et al., 2009b), delayed puberty onset (Creighton-Taylor and Rudeen, 1991; Lan et al., 2009b; McGivern et al., 1992; McGivern and Yellon, 1992; Wilson and Handa, 1997), and an earlier incidence of acyclicity (Wilson et al., 1995), all of which may shorten the window of fertility.
LH secretion occurs via both a tonic/pulsatile mode, which is responsible for follicular development and steroidogenesis, and a surge mode, which leads to ovulation. During tonic/pulsatile secretion, estrogen negative feedback inhibits GnRH/LH pulses to prevent excess gonadotropin secretion prior to follicular maturation. GnRH/LH surges, however, are induced by estrogen positive feedback, which stimulates ovulation. GnRH neurons express estrogen receptor-β (ER-β) but lack estrogen receptor-α (ER-α), the receptor subtype involved in normal reproductive function (Hrabovszky et al., 2001). Kisspeptin may be the missing link in this circuitry, mediating feedback effects of sex steroids on GnRH secretion (Tena-Sempere, 2005). Kisspeptin, derived from the Kiss1 gene, stimulates GnRH and LH secretion (Dhillo et al., 2005; Dhillo et al., 2007; Gottsch et al., 2004; Hoffman et al.; Irwig et al., 2004; Jayasena et al., 2009; Shahab et al., 2005), induces expression of the immediate early gene product Fos in GnRH neurons (Irwig et al., 2004), and evokes LH bursts at all phases of the estrous cycle (Roa et al., 2006). Kisspeptin antagonists suppress LH pulse frequency, and GnRH antagonists (Roseweir et al., 2009) block kisspeptin-mediate LH, testosterone and estradiol increases (Mikkelsen et al., 2009).
Kisspeptin-expressing neurons in the rodent hypothalamus are located in the arcuate (ARC) and the anteroventral periventricular (AVPV) nuclei (Smith et al., 2005a). Estradiol inhibits Kiss1 mRNA expression in the ARC through negative feedback and stimulates Kiss1 mRNA expression in the AVPV through positive feedback. The majority of Kiss1 mRNA-expressing cells in the ARC and AVPV express ER-α (Adachi et al., 2007), and neurons in the ARC also express progesterone receptors (PRs) (Auger and Blaustein, 1997; Lehman et al., 2010).
In view of the adverse effects of alcohol on HPG function and the role of kisspeptin in HPG circuitry, we hypothesized that PAE will alter the response of kisspeptin-immunoreactive (kisspeptin-ir) neurons to the sex steroids, estradiol (E2) and progesterone (P4), in adulthood. Adult females offspring from prenatal ad libitum-fed control (C), pair-fed (PF), and alcohol-exposed (PAE) groups were subjected to Sham ovariectomy (OVX), or OVX without or with replacement with low or high physiological levels of E2 and P4. Levels of E2 and the response of kisspeptin-ir neurons to E2 were measured. As progesterone plays a role in generation of the preovulatory surge, which is linked to kisspeptin neurons in the AVPV, P4 levels and the response to P4 were also measured. We also investigated PAE effects on the number of double-labelled kisspeptin-ir/ERα-ir neurons, and close contacts between kisspeptin-ir fibers and GnRH-ir cell bodies.
2. Materials and Methods
Experimental procedures followed the NIH Guide for the Care and Use of Laboratory Animals and were approved by the University of British Columbia Animal Care Committee.
2.1. Breeding and feeding
Detailed description of the breeding and feeding protocol is reported in (Lan et al., 2009a), as female offspring from that breeding were used in the present study. Female and male Sprague-Dawley rats (Charles River Laboratories, St Constant, PQ, Canada) were randomly paired and cages checked daily; presence of vaginal plugs, indicated gestation day 1 (GD1). On GD1, females were assigned to: (i) alcohol (ethanol - PAE), n=17: liquid ethanol diet (36% ethanol-derived calories); (ii) pair-fed (PF), n=14: liquid control diet (maltose-dextrin isocalorically substituted for ethanol), with amount matched to that consumed by a PAE partner (g/kg body weight/day of gestation); this controls for the reduced food intake that occurs with ethanol consumption; (iii) control (C), n=18: laboratory chow ad libitum. All groups had ad libitum access to water. Liquid diets were formulated to provide adequate nutrition to pregnant rats regardless of ethanol intake (Dyets Inc., Bethlehem, PA -Weinberg/Keiver High Protein Ethanol and Control Diets).
2.2. Experimental design
At 53–58 d, female offspring were weight-matched and assigned to adult treatment conditions (n=24 [8 from each of C, PF, PAE] per treatment) (Fig. 1): 1) Sham – bilateral incisions in dorsolateral flanks; 2) Ovariectomy (OVX); 3) OVX and replacement with a high concentration of E2 (OVX+E2H; 0.05 mg E2; 17-β estradiol pellet, Innovative Research of America, Sarasota, FL), implanted subcutaneously (SC); 4) OVX and replacement with high concentrations of E2 and P4 (OVX+E2H+P4H; 0.05 mg E2 pellet and 3 cm P4 capsule, SC; MP Biomedicals, LLC, Aurora, OH); 5) OVX and replacement with low concentrations of E2 and P4 (OVX+E2L+P4L; 0.01 mg E2 pellet and 1.5 cm P4 capsule, SC). Progesterone capsules utilized P4 packed into Silastic tubing (1.57mm ID, 3.18mm OD; Dow Corning, Midland, MI). Concentrations of E2 and P4 were within the physiological range, to study possible long-lasting effects of PAE-induced alterations in negative or positive feedback on the kisspeptin system.
Figure 1. Experimental design.
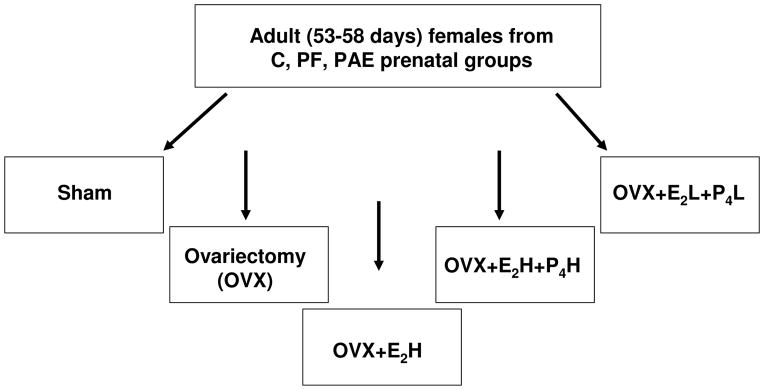
Prenatal groups: ad libitum-fed control (C), pair-fed (PF), prenatal alcohol-exposed (PAE).
Adult treatments: Sham (bilateral incisions in the dorsolateral flanks); Ovariectomy (OVX); OVX+E2H (OVX and replacement with a high concentration of estradiol [E2]); OVX+E2H+P4H (OVX and replacement with high concentrations of both E2 and progesterone [P4]); OVX+E2L+P4L - OVX and replacement with low concentrations of both E2 and P4.
2.3. Brain Perfusion and Blood Sampling
14 d post-surgery, animals were anesthetized and blood samples collected from the heart. Animals were perfused transcardially (Welch Pumps, VWR, Canada) with 0.9% saline, followed by 4% paraformaldehyde (PFA). Brains were removed and stored in PFA (4 h), then transferred into a 20% sucrose solution (4°C) until saturated. Coronal sections (30μm) were obtained throughout the entire hypothalamus in series (cryostat HM 505E; MICROM International GmbH, Germany), and stored in cryopreservative solution (−20°C). Blood samples were centrifuged (1880xg, 10 min, 0°C), and stored at −80°C.
2.4. Vaginal Smears
Vaginal smears were taken post-mortem to assess stages of the estrous cycle, diestrus (diestrus I and diestrus II), proestrus, and estrus, by two independent investigators blind to treatment conditions (Montes and Luque, 1988).
2.5. Estradiol and Progesterone Radioimmunoassays
Plasma E2 levels were measured using the Siemens Coat-A-Count Estradiol kit (TKE21, Siemens Healthcare Diagnostics Inc., Tarrytown, NY - calibration range 20–3,600 pg/mL, analytical sensitivity: 8 pg/mL). E2 levels were relatively high across treatment groups, including the OVX (mean ~20 pg/mL) condition. A subset of samples (n=64) was reanalyzed using the Siemens Double Antibody Estradiol kit (KE2D1 - calibration range of 5–500 pg/mL, analytical sensitivity 1.4 pg/mL), which yielded significantly lower E2 values, consistent with the comparative performance data provided by Siemens. We validated the Siemens linear regression formula that compares the two kits [(Double Antibody) = 1.1 × (Coat-A-Count) −15pg/mL, with r = 0.955], by running a new set of samples in a side-by-side comparison. Estradiol values were recalculated using the Siemens formula. Relative differences among groups remained exactly the same as with the Coat-A-Count assay.
Plasma P4 levels were measured using the ImmuChem Double Antibody Progesterone Kit (ICN Biomedicals Inc., Costa Mesa, CA); (minimum detectable P4 concentration of 0.2 ng/ml; intra- and inter-assay coefficients of variation of 3.6% and 6.7% respectively).
2.6. Immunohistochemistry
Brain sections in series were collected from C,PF and PAE females. One series of sections through the entire hypothalamus in Sham and OVX conditions was co-labelled for kisspeptin-ir/GnRH-ir to determine distribution of peptides. Sections containing the ARC, from rostral (rARC; Bregma −1.72 mm) to caudal (cARC; Bregma −4.08 mm) and AVPV (Bregma 0.12 to −0.12) were selected from a second series in all adult treatments, and total number of kisspeptin-ir cells was counted (Paxinos and Watson, 2008). A third series through the hypothalamus from the OVX and OVX+E2H conditions was processed for kisspeptin-ir/GnRH-ir. A fourth series through the ARC from the OVX and OVX+E2H conditions was processed for kisspeptin-ir/ER-α-ir.
Incubations were at room temperature, unless specified otherwise, with TBS + 4% normal donkey serum (Sigma-Aldrich) + 0.3% Triton X-100 (Fisher Biotech, Fairlawn, NJ). Sections were washed in TBS following each incubation step, treated with 1% hydrogen peroxide (Sigma-Aldrich Co., St. Louis) (10 min) and incubated (1 h) in TBS + 4% normal donkey serum and 0.3% Triton X-100.
Kisspeptin staining
Sections were incubated in a polyclonal rabbit anti-kisspeptin-10 antiserum, LOT # 566, 48 h; 1:5000 (provided by Dr. Alain Caraty, INRA, Nouzilly, France) and in donkey anti-rabbit Cy3 (1h; 1:100; Jackson ImmunoResearch Laboratories, Inc).
GnRH staining
Sections were incubated in anti-GnRH Sternburger mouse primary antibody (48 h; 1:1000; Invitrogen Canada Inc) and in donkey anti-mouse conjugated Alexa Fluor 488 (1:100; Invitrogen Canada, Inc).
ERα staining
Sections were incubated in monoclonal mouse anti-human ER-α clone 1D5 primary antibody (48 h; 1:10; DakoCytomation, Denmark) and in donkey anti-mouse conjugated Alexa Fluor 488.
Sections were mounted, and cover-slipped (ProLong Gold, Invitrogen Canada, Inc). Controls included omitting either the primary or secondary antibody, which resulted in the complete absence of staining for the corresponding antigen but not the other. Pre-absorption of kisspeptin antibody with the purified kisspeptin peptide (provided by Dr. Alain Caraty) for 24 h completely eliminated all kisspeptin-ir. All antibodies have been well characterized and utilized previously [e.g. (Avigdor et al., 2005; Franceschini et al., 2013; Nishihara et al., 2000; Rissman and Li, 2000)].
2.7. Quantification of data
Manual counting was performed by two independent and condition-blind experimenters (Zeiss Axioskop 2 Motorized Plus microscope (Jena, Germany)) equipped for epifluorescent microscopy. Photomicrographs were captured with a Retiga EXi Mono 12-bit digital CCD camera (Q Imaging, Burnaby, BC, Canada) using Northern Eclipse (EMPIX, Inc, Mississauga, Canada). For kisspeptin-ir: total number of kisspeptin-ir cells exhibiting cytoplasmic staining, with a round or oval cytoplasmic profile were counted. For kisspeptin-ir/ERα-ir: 2–3 sections from the mid-ARC (Bregma −2.52 mm to −2.64 mm) for animals in the OVX and OVX+E2H conditions (n=31, 5–6 from each of C, PF and PAE per adult treatment condition) were collected. Co-labeling was assessed using a 40x objective. For assessing close contacts between kisspeptin-ir fibers and GnRH-ir cell bodies: GnRH-ir cell bodies were identified under 40x in 2–3 sections per region of animals from the OVX and OVX+E2H conditions (n=33, 4–7 from each of C, PF and PAE adult treatment conditions); photomicrographs of GnRH-ir neurons and kisspeptin-ir fibers were superimposed (63x objective). GnRH cells identified as having close contacts with kisspeptin-ir fibers were examined using confocal microscopy (BioRad 200). Quantification was performed in an unbiased, blinded fashion.
Data are presented as mean (+SEM) of total cell counts and colocalization data are presented as percentage (+SEM) of cells that are double-labelled.
2.8. Statistical analysis
Analyses of variance (ANOVAs) for the factors of prenatal group, age (developmental data, age as a within subjects factor), estrous stage, and adult treatment condition (for hormone levels, kisspeptin-ir cell numbers, close contacts between GnRH-ir cell bodies and kisspeptin-ir fibers and kisspeptin-ir/ERα-ir cell numbers) were utilized. Significant main or interaction effects were followed by targeted Fisher’s LSD a priori comparisons based on hypotheses. Statistical significance was set at p<0.05.
3. Results
3.1. Developmental data
Female offspring from this study were part of larger breeding, and the developmental data have been presented in detail (Lan et al., 2009a). Birth weights (PND1) of female offspring from PAE (5.8±0.1 g) and PF (5.4±0.2 g) dams were lower than those of their C (6.3±0.1 g) counterparts (p<0.01). By weaning, there was catch up growth in PF (48.5±1.6) but not in PAE (46.5±0.9) compared to C (51.0±1.2 g) females (PAE<C, p<0.05; PF not different from C).
3.2. Effects of adult treatment but not prenatal group on plasma estradiol and progesterone levels
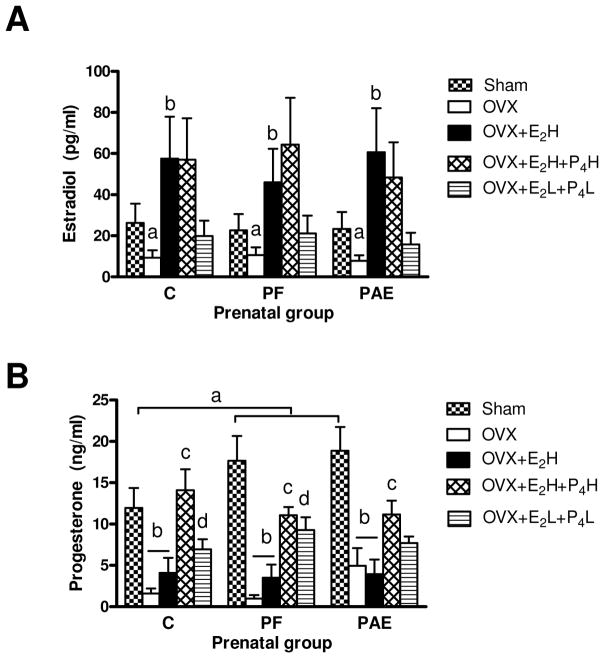
Animals in the Sham condition were in different stages of the estrous cycle, with approximately half the animals (5 C, 4 PF, 3 PAE) in diestrus and the remaining in early proestrus or estrus. ANOVAs (3 prenatal group × 3 estrous stage) revealed that neither E2 nor P4 levels differed as a function of either prenatal group (p=0.722 and p=0.415, respectively) or stage of the estrous cycle (p=0.506 and p=0.198, respectively) (Figs. 2A–B). This is not surprising given the low numbers of animals (n=3–5) in any one stage of the cycle and the timing of sample collection (09:00–12:00 h), which was not optimal for obtaining the high E2 levels typical of proestrus.
Figure 2. Plasma levels (mean+SEM) of estradiol [E2] (A) and progesterone [P4] (B) in ad libitum-fed control (C), pair-fed (PF), and prenatal alcohol-exposed (PAE) adult females.
(A) As expected, E2 levels were lowest in OVX compared to all other conditions, regardless of prenatal group (ap’s<0.05). E2 levels were higher in OVX+E2H compared to Sham, OVX and OVX+E2L+P4L conditions (bp’s<0.0001), regardless of prenatal group; n=8 rats per prenatal group per adult treatment.
(B) Under Sham conditions, P4 levels in PAE and PF females were higher than C (PAE=PF>C and ap<0.05). As expected, P4 levels were lower in the OVX and OVX+E2H conditions compared to all other adult treatment conditions, regardless of prenatal group (bp’s<0.05). In the OVX+E2H+P4H condition, P4, levels were higher than those in OVX and OVX+E2H females across prenatal group (cp’s<0.01), and were restored to Sham levels in C but not PAE and PF females. Finally, in the OVX+E2L+P4L condition, PF females had higher (dp<0.01) and C females had marginally higher (dp=0.06) P4 levels than their C counterparts in the OVX condition, whereas P4 levels in PAE females were not different from those in their OVX counterparts (p=0.34); n=8 rats per prenatal group per adult treatment.
Two-way ANOVA (3 prenatal group × 5 adult treatment) for E2 levels indicated a main effect of adult treatment (F(4, 105)=41.103, p<0.0001; Fig. 2A). OVX animals had the lowest E2 levels, regardless of prenatal group (p<0.05), and OVX females replaced with high levels of E2 had higher plasma E2 levels than both OVX and Sham (p’s<0.0001), as well as females in the low level E2 replacement condition (p’s<0.0001).
Consistent with the E2 data, a two-way ANOVA revealed a main effect of adult treatment on P4 levels (F(4, 80)=24.358, p<0.000001; Fig. 2B). Interestingly, P4 levels were higher in Sham PAE and PF compared to Sham C females (p’s<0.05; Fig. 2B). OVX and OVX+E2H animals were not different from each other and had the lowest P4 levels compared to all other adult treatment conditions (p’s<0.05, Fig. 2B). In the OVX+E2H+P4H condition, P4, levels were higher than those in OVX and OVX+E2H females across prenatal groups (p’s<0.01), and were restored to Sham levels in C but not PAE and PF females. In addition, in the OVX+E2L+P4L condition, P4 levels were higher in PF (p<0.01) and marginally higher in C (p=0.06) compared to their OVX counterparts, whereas P4 levels did not differ between the OVX and OVX+E2L+P4L conditions in PAE females (p=0.34).
Neither E2 nor P4 levels were at 0 in OVX females, likely due to the calibration range (5–500 pg/mL) and analytical sensitivity (1.4 pg/mL) of the assay. Analysis of post-mortem vaginal smears in OVX animals confirmed that OVX animals were in diestrus. Thus, measurable E2 levels (mean ~ 10 pg/mL) in OVX animals were likely due to the parameters of the assay.
3.3. Distribution of kisspeptin-ir neurons
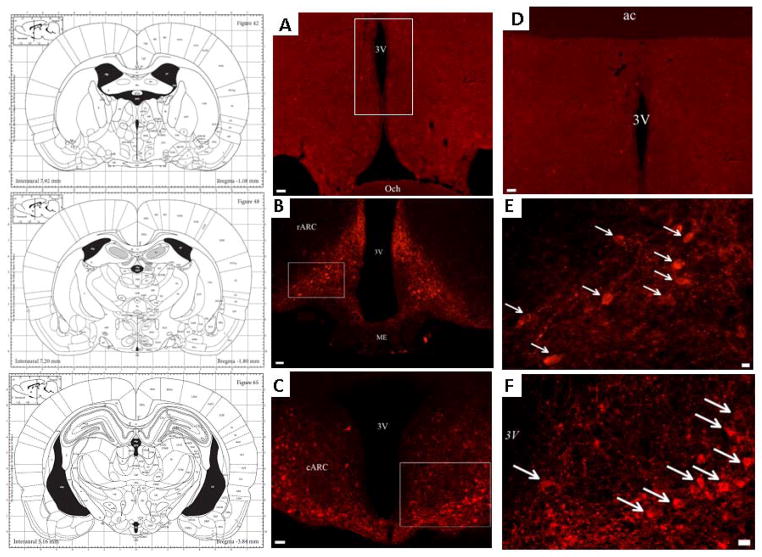
Kisspeptin-ir cells were identified throughout the ARC from rostral (Figs. 3B and E) to caudal levels (Figs. 3C and F), and were surrounded by a dense network of fibers. We also found kisspeptin-ir fiber staining in the AVPV (Figs. 3A and D). In contrast to the ARC, but in agreement with previous findings (Overgaard et al., 2013), we did not detect kisspeptin-ir cell bodies within the AVPV.
Figure 3. Distribution of kisspeptin-ir cell bodies and fibers in the anteroventral periventricular nucleus (AVPV) (A, D) and the arcuate nucleus (ARC) (B, C, E, F) of the hypothalamus in adult OVX female control rats.
Low power images (A–C) and high power images (D–F) of corresponding boxed areas. In E and F, arrows indicate kisspeptin-ir cell bodies; 3v –third ventricle; Och – optic chiasm; ac – anterior commissure; rARC - rostral arcuate nucleus; cARC - caudal arcuate nucleus; ME - median eminence. Scale bars: A, D, 100 μm; B–C, 50 μm; E–F, 10 μm. Panels at left show sections from (Paxinos and Watson, 2008) to illustrate the levels from which these sections were taken.
3.4. Ovariectomy and hormone replacement differentially altered numbers of kisspeptin-ir neurons in PAE compared to PF and C females
A two-way ANOVA to investigate the effects of E2 and P4 on the number of kisspeptin-ir neurons indicated a main effect of adult treatment [F(4,65)=36.10, p<0.0001] (Fig. 4). Consistent with our hypothesis that PAE would alter the response of kisspeptin-ir neurons to the sex hormones, targeted Fisher’s LSD a priori comparisons indicated that OVX increased kisspeptin-ir cell numbers compared to Sham for C (p=0.05) and PF (p=0.02), but not PAE (p=0.66) females. Replacement with high concentrations of E2 restored kisspeptin-ir cell numbers to Sham levels in C (p=0.86) and PF (p=0.34) females, but downregulated the number of kisspeptin-ir neurons below Sham (p<0.05) and OVX (p<0.01) levels in PAE females (Fig. 4). Comparisons within the OVX+E2H treatment revealed that under constant high E2 levels, there were fewer (by 29%, p<0.02) kisspeptin-ir cells in PAE compared to C females (Fig. 4). PF females had an intermediate number of kisspeptin-ir cells and were not different from either C or PAE.
Figure 4. Total number of kisspeptin-ir cells in the arcuate nucleus (ARC) in adult female rats from ad libitum-fed control (C), pair-fed (PF), and prenatal alcohol-exposed (PAE) groups following OVX without or with replacement with estradiol [E2] and progesterone [P4].
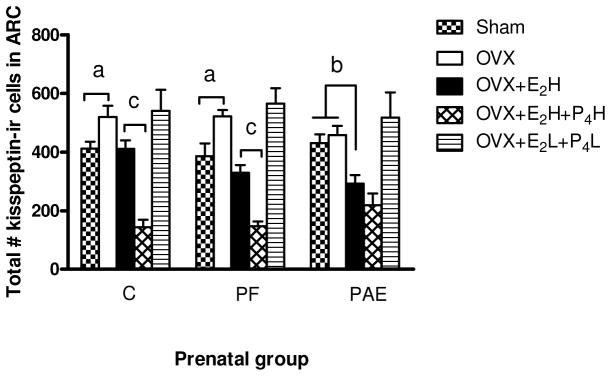
a OVX females in C (p=0.05) and PF (p=0.02) groups had higher total numbers of kisspeptin-ir cells than their Sham counterparts, whereas kisspeptin-ir cell numbers for PAE females (p=0.66) did not differ in OVX and Sham conditions;
b Ovariectomy and replacement with high E2 concentrations restored kisspeptin-ir cell numbers to Sham levels in C (p=0.86) and PF (p=0.34) groups, but significantly reduced kisspeptin-ir cell numbers in PAE compared to Sham (p<0.05) and OVX (p<0.01) females.
c Ovariectomy and replacement with high levels of E2 and P4 significantly reduced numbers of kisspeptin-ir cells in females from C (p<0.0001) and PF (p<0.003) groups below those in their OVX+E2H counterparts, whereas kisspeptin-ir cell numbers showed no significant change in PAE females (OVX+E2H vs OVX+E2H+P4H, p=0.21).
OVX (ovariectomized); OVX+ E2H (OVX + E2 replacement at high physiological levels); OVX+ E2 H+P4H (OVX + replacement of both E2 and P4 at high physiological levels); OVX+E2 L+P2L (OVX + replacement of both E2 and P4 at low physiological levels).
Replacement with high concentrations of P4 and E2 resulted in robust decrease in kisspeptin-ir neurons, beyond that observed with estradiol alone, for C (p<0.0001) and PF (p<0.003) females, but not for PAE (p=0.21) females (Fig. 4). By contrast, replacement with low concentrations of P4 and of E2 had no effect on the number of kisspeptin-ir neurons.
3.5. No effect of PAE or adult treatment on number of close contacts of kisspeptin-ir fibers to GnRH-ir neurons in the preoptic area (POA) of the hypothalamus
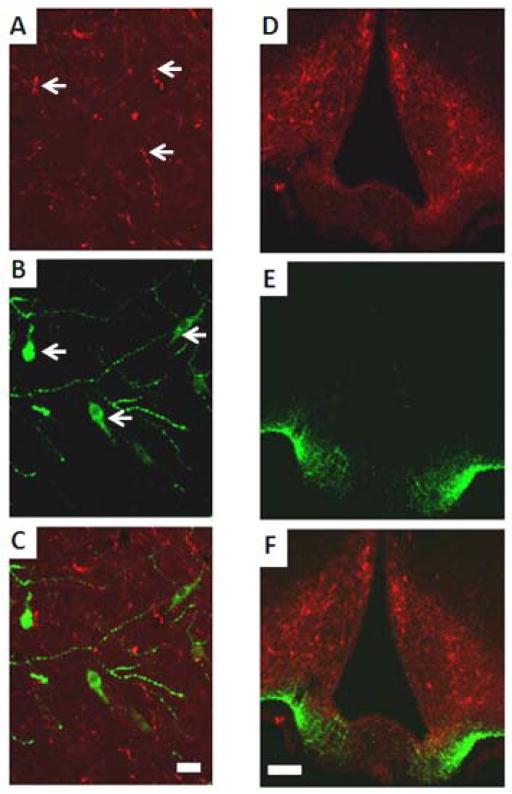
Strong immunoreactivity for GnRH neurons and kisspeptin fibers was detected at the median eminence (ME). Kisspeptin-ir fiber varicosities were observed in both the internal and external layers of the ME, including around the deeper part of the infundibular recess. Kisspeptin-ir fibers were most abundant in the internal layer of the ME (Figs. 5D and F), whereas GnRH-ir fibers were located mainly in the external zone (Figs. 5E–F).
Figure 5. Localization of kisspeptin- and GnRH-immunoractivity in the preoptic area (POA) (A–C), and the median eminence (ME) (D–F), of the hypothalamus of ad libitum-fed OVX control females.
A–C: Confocal images of: kisspeptin-ir fibers in the POA (in red; A); GnRH-ir cells and fibers in the POA (in green; B) overlay of A and B (C). Arrows indicate kisspeptin-ir fibers (A) and GnRH-ir neurons (B). GnRH-ir neurons were surrounded by kisspeptin-ir fibers (C). Confocal analysis revealed that a subpopulation of GnRH-ir cells were in close contact with kisspeptin-ir fibers. No effect of prenatal group or adult treatment on number of close contacts between kisspeptin-ir fibers and GnRH-ir cells.
D–F: Confocal images of: kisspeptin-ir fibers located mainly in internal layer of ME (in red, D); GnRH-ir fibers located in the external zone of the ME (in green, E); overlay of D and E (F); scale bars: A–C, 20 μm; D–F, 100 μm.
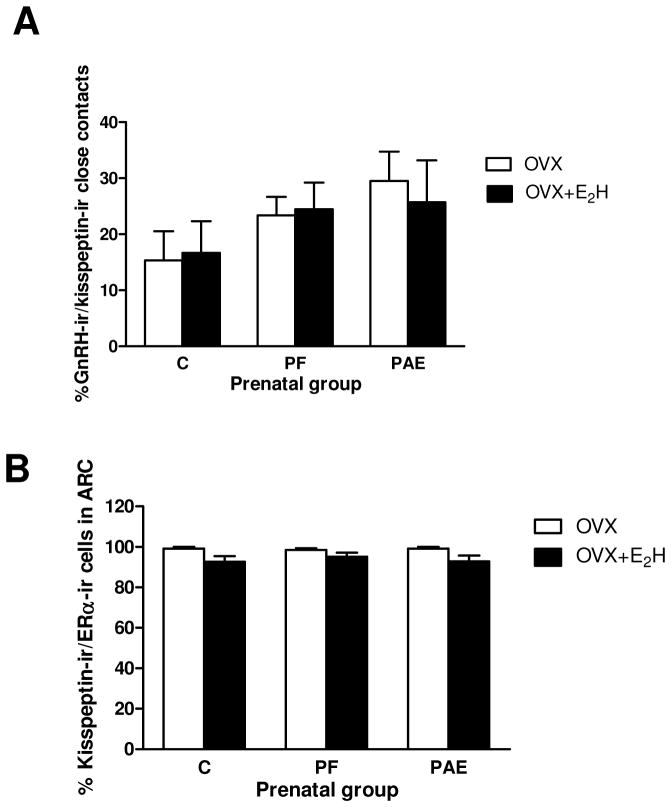
To test the hypothesis that PAE would differentially alter the number of close contacts between kisspeptin-ir fibers (Fig. 5A) and GnRH-ir cells (Fig. 5B), a total of 482 GnRH-ir cells were analyzed in the hypothalamus, the vast majority of which had their neuronal soma located in the POA (Figs. 5B–C). GnRH cells and kisspeptin-ir fibers were counted, and an averge across sections for each animal was calculated to yield a single unit of determination for statistical analysis. For all prenatal groups, a subset of GnRH cells was in close contact with kisspeptin-ir fibers (15%±5.2 and 16%±5.6 for C OVX and C OVX+E2H; 23%±3.3 and 24%±4.8 for PF OVX and PF OVX+E2H; 29%±5.2 and 25%±7.4, for PAE OVX and PAE OVX+E2H) (Fig. 6A). There were no effects of either prenatal group [(F(2,27)=1.51, p=0.240)] or adult treatment [F (1,27)=0.33, p=0.857] on the number of close contacts.
Figure 6. Quantification of: (A) close contacts between GnRH-immunoreacitve (−ir) cell bodies and kissppetin-ir fibers; (B) kisspeptin-ir/ERα-ir co-labeled cells in the arcuate nucleus (ARC), in OVX and OVX+E2H females across prenatal groups.
(A) There was no effect of prenatal group or adult treatment on number of close contacts between kisspeptin-ir fibers and GnRH-ir neurons in the preoptic area (POA) of the hypothalamus.
(B) Replacement with high concentrations of E2 reduced the percent of kisspeptin-ir/ERα-ir cells compared to those in the OVX condition (p=0.003). OVX (ovariectomized); OVX+ E2H (OVX + E2 replacement at high physiological levels).
3.6. Ovariectomy and replacement with high E2 concentrations reduced the percent of kisspeptin-ir/ERα-ir co-labeled cells in the ARC
As the E2 signal is conveyed to kisspeptin neurons via ER-α receptors, we investigated whether the number of double-labelled kisspetin-ir/ERα-ir neurons in the ARC was altered by PAE. A total of 775 kisspeptin-ir cells was identified (Fig. 7A), the majority of which (745) showed ERα-immunoreactivity (Figs. 7B–C). Replacement with high concentrations of E2 caused a small but significant reduction in the percent of kisspeptin-ir/ERα-ir cells compared to the OVX condition across prenatal groups [F(1,25)=10.489, p=0.003], (Fig. 6B). There were no differences in the intensity of ERα-ir staining among groups.
Figure 7. Confocal images of: kisspeptin-ir cells (A); ERα-ir cells (B); overlay of A and B (C), in the arcuate nucleus (ARC) of OVX ad libitum-fed control females.
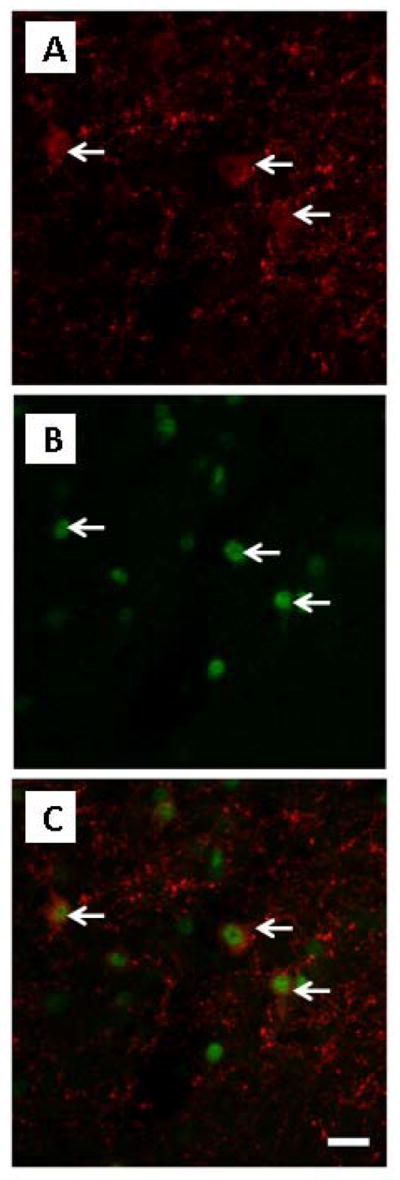
Arrows indicate: kisspeptin-ir cells (in red, A), ERα-ir cells (in green, B), cells co-expressing kisspeptin-ir/ERα-ir (red/green, C); scale bar: A–C, 20 μm. Here we can visualize only kisspeptin-ir neurons in a single focal plane: OVX (ovariectomized).
4. Discussion
Our data present the novel finding that a possible mechanism underlying the adverse effects of PAE on the adult HPG axis involves actions of alcohol at the level of the kisspeptin system. Kisspeptin-ir neurons in PAE animals showed an altered response to estradiol and to progesterone in the presence of estradiol compared to controls: 1) OVX increased the number of kisspeptin-ir neurons in C and PF, but not PAE females compared to their Sham counterparts; 2) E2 replacement restored kisspeptin-ir cell numbers to Sham levels in C and PF females but caused a robust downregulation of kisspeptin-ir neurons below Sham levels in PAE females; 3) OVX and replacement with high physiological concentrations of E2 resulted in fewer kisspeptin-ir cells in PAE than C females; 4) OVX and replacement with high concentrations of both E2 and P4 significantly decreased the number of kisspeptin-ir neurons in C and PF females but had no significant effect in PAE females, whereas replacement with low concentrations of both sex steroids restored the number of kisspeptin-ir cell numbers to approximately Sham levels.
The differential effects of the gonadal hormones on kisspeptin-ir cell numbers in PAE females were concentration-dependent, and revealed only following replacement with high physiological concentrations of hormones. These findings extend our previous showing differential effects of high concentrations of E2 on PAE compared to control females. Under most conditions, basal corticosterone levels do not differ among prenatal groups, but during proestrus, when E2 levels are high, PAE females have increased basal corticosterone levels compared to controls (Lan et al., 2009b; Sliwowska et al., 2008).
We found that kisspeptin-ir neurons were distributed throughout the ARC, which parallels previous work (Desroziers et al., 2010), using the same well characterized KP-10 antibody, and data from both male (Bentsen et al., 2010; Overgaard et al., 2013) and female (Kinoshita et al., 2005) rats using other antibodies. We confirm observations on the pattern of kisspeptin-ir fibers in the ME and the AVPV, and lack of kisspeptin-ir cell bodies in the AVPV (Desroziers et al., 2010; Overgaard et al., 2013). In contrast, in mice dense staining for kisspeptin-ir cell bodies in the AVPV was found (Clarkson et al., 2009; Overgaard et al., 2013). Kiss1 mRNA has been detected in the AVPV of both female and male rats (Kauffman et al., 2007; Overgaard et al., 2013), a finding that we have recently corroborated using in situ hybridization in females (Sliwowska et al, in preparation). Kisspeptin-ir cell bodies have been detected in the AVPV in colchicine-treated animals. As colchicine interrupts transport from cell bodies to nerve terminals (Adachi et al., 2007; Iijima et al., 2010; Kinoshita et al., 2005; Takase et al., 2009), it is possible that, in the rat, kisspeptin is quickly transported from cell bodies in the AVPV to the nerve terminals. However, Kiss 1 mRNA has also been detected in the AVPV in rats (Kauffman et al., 2007; Overgaard et al., 2013). Thus it is also possible that limited translation of mRNA to mature peptide occurred.
Kisspeptin- ir neurons in the ARC are targets of estrogen negative feedback, are involved in GnRH/LH pulse generation, and provide tonic and direct stimulatory drive to GnRH neurons (Adachi et al., 2007; Dungan et al., 2006; Kinoshita et al., 2005; Smith, 2008). In agreement, our data show that for C and PF females, when circulating levels of sex steroids declined (OVX), the number of kisspeptin neurons in the ARC increased over Sham levels, and conversely, when circulating levels of estradiol increased (OVX+E2H), numbers kisspeptin-ir neurons in the ARC decreased back to Sham levels, reflecting the role of E2 in negative feedback in the ARC. By contrast, in PAE females, replacement with high physiological concentrations of E2 resulted in downregulation in the number of kisspeptin-ir neurons below Sham levels, and fewer kisspeptin-ir cells in PAE than C females. This suggests that PAE adversely affects sex steroid feedback regulation in females, possibly via the kisspeptin system. As noted, high levels of alcohol use in adult women can have adverse effects on reproductive function, resulting in anovulatory cycles or luteal phase dysfunction, impaired fertility and increased risk of spontaneous abortion (Geisenburg et al., 2002). We speculate that disruption of HPG axis activity may occur in women with FASD, potentially mediated by alterations in the kisspeptin system.
As the levels of estradiol replacement were similar in all prenatal groups, the robust decrease in kisspeptin-ir neurons in PAE females in the OVX+E2H condition suggests an increased response of kisspeptin neurons to estradiol. PF females showed an intermediate number of kisspeptin-ir neurons and were not different from PAE or C females following E2 replacement, suggesting that pair-feeding caused alteration in the response to E2. Although pair-feeding is a control for the reduced food intake that occurs with ethanol consumption, it is a treatment itself (Weinberg, 1984). Because PF dams are matched in intake to PAE partners, they receive a reduced ration, below what they would consume if given ad libitum access to food. They are hungry, and consume their food within a few hours of receiving it, remaining deprived until the next feeding. This may introduce of mild prenatal stress for PF fetuses, which could play a role in the altered responsiveness observed, as stress may decrease Kiss1 mRNA expression (Kinsey-Jones et al., 2009).
We postulate that PAE may alter HPG feedback regulation at the level of the hypothalamus through an E2-related decrease in the number of kisspeptin-ir neurons in the ARC. This could contribute to alterations in LH release in PAE animals (Handa et al., 1985; Lan et al., 2009b; Wilson et al., 1995). Srivstava et al. (Srivastava et al., 2009) showed that in prepubertal females, short-term alcohol exposure results in decreased Kiss1 mRNA expression levels in both the ARC and the AVPV, with an associated decrease in levels of LH and E2. Because estradiol downregulates Kiss1 expression in the ARC and upregulates Kiss1 in the AVPV (Smith et al., 2005b) one would have predicted that decreased levels of E2 would differentially alter Kiss1 neurons in these two nuclei. It is possible that stress played a role in the Srivastiva et al. (2009) study, as alcohol was administered via intragastric injections. Stress was shown to decrease Kiss1 mRNA expression within the POA and the ARC, which was associated with decreased LH levels (Kinsey-Jones et al., 2009). Hinley et al. (2010) showed that in immature females, short-term alcohol exposure blocks IGF-1-induced kisspeptin expression. Whether stress or altered IGF-1 signalling underlies the effects of kisspeptin in our study on PAE females remains to be determined.
Although PRs are expressed by ARC neurons (Auger and Blaustein, 1997; Chappell and Levine, 2000), the role of P4 in the regulation of kisspeptin neurons in the ARC is not known. Studies using PR blockade demonstrate a role for PR signalling in the control of LH responses to kisspeptin and the endogenous preovulatory LH surge (Plagemann et al., 2008), which is linked to kisspeptin neurons in the AVPV. PR activation in the AVPV appears to be an obligatory event in the stimulation of the GnRH surge by E2 (Auger and Blaustein, 1997; Chappell and Levine, 2000). The fact that PRs have been found in the ARC suggests that the action of P4 could occur at this level (Chappell and Levine, 2000).
P4 levels were higher in Sham PAE and PF compared to Sham C females. This finding of alterations under basal conditions extends our previous data showing that after 10 days of chronic mild stress, P4 levels were higher in PAE compared to C females (Hellemans et al., 2008), suggesting differential effects of stress on P4 in PAE compared to C. Recent data (Hueston and Deak, 2014) indicate that CORT and P4 were highly correlated following stress in male rats. It would be of interest to study the response to different stress paradigms to investigate the relationship between CORT and P4 in PAE females.
We also found that progesterone in the presence of estradiol markedly decreased (beyond that seen in females replaced with E2 alone [OVX+E2H]) the number of kisspeptin-ir neurons in the ARC in PF and C but not PAE females. This suggest that alcohol may decrease responsiveness of kisspeptin-ir neurons to progesterone in PAE females. However, this suggestion is made with caution. A limitation of our study is that progesterone was given only in concert with estradiol. Because estradiol alone produced reductions in kisspeptin immunoreactivity in PAE animals, it is possible that there is a “floor effect” and further reductions in kisspeptin-ir cells could not be observed in PAE animals following replacement with both E2 and P4. Further studies are needed to understand fully the role of progesterone in regulation of the kisspeptin system in females.
Another limitation in our study is that for females in the Sham condition, there were few (n=3–5) animals in any one stage of the estrous cycle in any one prenatal group, such that we could not directly assess effects of estrous cycle stage per se on kisspeptin neuron numbers. Examination of how natural variations in E2 and P4 levels across the estrous cycle might differentially affect kisspeptin neurons in PAE could provide further insight into PAE effects on the kisspeptin system. That we did not assess possible changes in total numbers of arcuate neurons also limits our conclusions. We are not aware of any study assessing effects of PAE on neuron numbers in the hypothalamus. However, using Neu N, we have shown that PAE decreased hippocampal neurogenesis in males but not females (Sliwowska et al., 2010; Uban et al., 2010). Thus, we can speculate that there were likely no changes in total numbers of hypothalamic neurons other than kisspeptin neurons. Further studies are needed to resolve this issue.
A goal of the present study was to investigate possible site(s) of action of E2 on GnRH neurons. Consistent with (Kinoshita et al., 2005) we reported that the majority of kisspeptin-ir cells in the ARC express ER-α, and E2 replacement decreased the number of kisspeptin-ir/ER-α-ir cells in the ARC compared to OVX. However, there were no effects of prenatal treatment on the number of kisspeptin-ir/ER-α-ir cells. Thus, the altered response to estradiol in PAE females is likely not related to changes in the number of co-localized kisspeptin-ir/ER-α-ir neurons. We examined two possible sites where kisspeptin and GnRH neurons make contact. Although we identified close contacts between kisspeptin-ir terminals and GnRH-ir cell bodies in the POA, prenatal treatment had no effect on the number of contacts. We also detected kisspeptin-ir fibers in the ME. However, it seems unlikely that direct contacts between kisspeptin and GnRH neurons would occur at this level, as kisspeptin-ir fibers were found to be located mainly in the internal zone, whereas GnRH-ir fibers were located mainly in the external zone of the ME, in parallel with previous work (Desroziers et al., 2010; Iijima et al., 2010). While communication between kisspeptin fibers and GnRH neurons appears to occur at the level of the POA, our data suggest that prenatal treatment has no effect on the number of kisspeptin-GnRH contacts and that the ME is not a key region mediating kisspeptin-GnRH communication.
In summary, our data suggest that a possible mechanism underlying adverse effects of PAE on the adult HPG axis involves actions of alcohol at the level of the kisspeptin system. We propose that in utero exposure to alcohol, acting as an early homeostatic challenge, may have dysregulatory effects on HPG activity that extend beyond embryonic and fetal development, and give rise to altered neuroendocrine function into adulthood.
Acknowledgments
Role of funding
Grants NIH/NIAAA R37 AA007789 to JW; a Fellowship from the National Science and Engineering Research Council (NSERC CGS-D) and a University Graduate Fellowship to TSB.
References
- Auger AP, Blaustein JD. Progesterone treatment increases Fos-immunoreactivity within some progestin receptor-containing neurons in localized regions of female rat forebrain. Brain Res. 1997;746(1–2):164–70. doi: 10.1016/s0006-8993(96)01190-0. [DOI] [PubMed] [Google Scholar]
- Avigdor M, Sullivan SD, Heideman PD. Response to selection for photoperiod responsiveness on the density and location of mature GnRH-releasing neurons. Am J Physiol Regul Integr Comp Physiol. 2005;288(5):R1226–36. doi: 10.1152/ajpregu.00562.2004. [DOI] [PubMed] [Google Scholar]
- Bentsen AH, Ansel L, Simonneaux V, Tena-Sempere M, Juul A, Mikkelsen JD. Maturation of kisspeptinergic neurons coincides with puberty onset in male rats. Peptides. 2010;31(2):275–83. doi: 10.1016/j.peptides.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Chappell PE, Levine JE. Stimulation of gonadotropin-releasing hormone surges by estrogen. I. Role of hypothalamic progesterone receptors. Endocrinology. 2000;141(4):1477–85. doi: 10.1210/endo.141.4.7428. [DOI] [PubMed] [Google Scholar]
- Clarkson J, d’Anglemont de Tassigny X, Colledge WH, Caraty A, Herbison AE. Distribution of kisspeptin neurones in the adult female mouse brain. J Neuroendocrinol. 2009;21(8):673–82. doi: 10.1111/j.1365-2826.2009.01892.x. [DOI] [PubMed] [Google Scholar]
- Creighton-Taylor JA, Rudeen PK. Prenatal ethanol exposure and opiatergic influence on puberty in the female rat. Alcohol. 1991;8(3):187–91. doi: 10.1016/0741-8329(91)90790-4. [DOI] [PubMed] [Google Scholar]
- Desroziers E, Mikkelsen J, Simonneaux V, Keller M, Tillet Y, Caraty A, Franceschini I. Mapping of kisspeptin fibres in the brain of the pro-oestrous rat. J Neuroendocrinol. 2010;22(10):1101–12. doi: 10.1111/j.1365-2826.2010.02053.x. [DOI] [PubMed] [Google Scholar]
- Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, McGowan BM, Amber V, Patel S, Ghatei MA, Bloom SR. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90(12):6609–15. doi: 10.1210/jc.2005-1468. [DOI] [PubMed] [Google Scholar]
- Dhillo WS, Chaudhri OB, Thompson EL, Murphy KG, Patterson M, Ramachandran R, Nijher GK, Amber V, Kokkinos A, Donaldson M, Ghatei MA, Bloom SR. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrinol Metab. 2007;92(10):3958–66. doi: 10.1210/jc.2007-1116. [DOI] [PubMed] [Google Scholar]
- Dungan HM, Clifton DK, Steiner RA. Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology. 2006;147(3):1154–8. doi: 10.1210/en.2005-1282. [DOI] [PubMed] [Google Scholar]
- Franceschini I, Yeo SH, Beltramo M, Desroziers E, Okamura H, Herbison AE, Caraty A. Immunohistochemical evidence for the presence of various kisspeptin isoforms in the mammalian brain. J Neuroendocrinol. 2013;25(9):839–51. doi: 10.1111/jne.12069. [DOI] [PubMed] [Google Scholar]
- Geisenburg E, Mello N, Mendelson J. Alcohol Abuse: Endocrine Concomitants. In: Pe, et al., editors. Hormones, Brain & Behaviour. Vol. 5. Elsevier; Science: 2002. pp. 747–780. [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145(9):4073–7. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- Handa RJ, McGivern RF, Noble ES, Gorski RA. Exposure to alcohol in utero alters the adult patterns of luteinizing hormone secretion in male and female rats. Life Sci. 1985;37(18):1683–90. doi: 10.1016/0024-3205(85)90295-4. [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Verma P, Yoon E, Yu W, Weinberg J. Prenatal alcohol exposure increases vulnerability to stress and anxiety-like disorders in adulthood. Ann N Y Acad Sci. 2008;1144:154–75. doi: 10.1196/annals.1418.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman GE, Le WW, Franceschini I, Caraty A, Advis JP. Expression of fos and in vivo median eminence release of LHRH identifies an active role for preoptic area kisspeptin neurons in synchronized surges of LH and LHRH in the ewe. Endocrinology. 152(1):214–22. doi: 10.1210/en.2010-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabovszky E, Steinhauser A, Barabas K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z. Estrogen receptor-beta immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2001;142(7):3261–4. doi: 10.1210/endo.142.7.8176. [DOI] [PubMed] [Google Scholar]
- Hueston CM, Deak T. On the time course, generality and regulation of plasm progesterone release in male rats by stress exposure. Endocrinology:en. 2014:20141060. doi: 10.1210/en.2014-1060. [DOI] [PubMed] [Google Scholar]
- Iijima N, Takumi K, Sawai N, Ozawa H. An Immunohistochemical Study on the Expressional Dynamics of Kisspeptin Neurons Relevant to GnRH Neurons Using a Newly Developed Anti-kisspeptin Antibody. J Mol Neurosci. 2010 doi: 10.1007/s12031-010-9433-y. [DOI] [PubMed] [Google Scholar]
- Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80(4):264–72. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- Jayasena CN, Nijher GM, Chaudhri OB, Murphy KG, Ranger A, Lim A, Patel D, Mehta A, Todd C, Ramachandran R, Salem V, Stamp GW, Donaldson M, Ghatei MA, Bloom SR, Dhillo WS. Subcutaneous injection of kisspeptin-54 acutely stimulates gonadotropin secretion in women with hypothalamic amenorrhea, but chronic administration causes tachyphylaxis. J Clin Endocrinol Metab. 2009;94(11):4315–23. doi: 10.1210/jc.2009-0406. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148(4):1774–83. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146(10):4431–6. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- Kinsey-Jones JS, Li XF, Knox AM, Wilkinson ES, Zhu XL, Chaudhary AA, Milligan SR, Lightman SL, O’Byrne KT. Down-regulation of hypothalamic kisspeptin and its receptor, Kiss1r, mRNA expression is associated with stress-induced suppression of luteinising hormone secretion in the female rat. J Neuroendocrinol. 2009;21(1):20–9. doi: 10.1111/j.1365-2826.2008.01807.x. [DOI] [PubMed] [Google Scholar]
- Lan N, Hellemans KG, Ellis L, Viau V, Weinberg J. Role of testosterone in mediating prenatal ethanol effects on hypothalamic-pituitary-adrenal activity in male rats. Psychoneuroendocrinology. 2009a;34(9):1314–28. doi: 10.1016/j.psyneuen.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan N, Yamashita F, Halpert AG, Sliwowska JH, Viau V, Weinberg J. Effects of prenatal ethanol exposure on hypothalamic-pituitary-adrenal function across the estrous cycle. Alcohol Clin Exp Res. 2009b;33(6):1075–88. doi: 10.1111/j.1530-0277.2009.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPaglia N, Steiner J, Kirsteins L, Emanuele MA, Emanuele N. The impact of acute ethanol on reproductive hormone synthesis, processing, and secretion in female rats at proestrous. Alcohol Clin Exp Res. 1997;21(9):1567–72. [PubMed] [Google Scholar]
- Lehman MN, Merkley CM, Coolen LM, Goodman RL. Anatomy of the kisspeptin neural network in mammals. Brain Res. 2010;1364:90–102. doi: 10.1016/j.brainres.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco J, Parafita MA, Alfonso M, Espinosa J. Changes in serum LH and FSH following preovulatory administration of ethanol in rats. Drug Alcohol Depend. 1984;14(2):215–8. doi: 10.1016/0376-8716(84)90047-4. [DOI] [PubMed] [Google Scholar]
- McGivern RF, Raum WJ, Handa RJ, Sokol RZ. Comparison of two weeks versus one week of prenatal ethanol exposure in the rat on gonadal organ weights, sperm count, and onset of puberty. Neurotoxicol Teratol. 1992;14(5):351–8. doi: 10.1016/0892-0362(92)90042-9. [DOI] [PubMed] [Google Scholar]
- McGivern RF, Yellon SM. Delayed onset of puberty and subtle alterations in GnRH neuronal morphology in female rats exposed prenatally to ethanol. Alcohol. 1992;9(4):335–40. doi: 10.1016/0741-8329(92)90077-n. [DOI] [PubMed] [Google Scholar]
- Mikkelsen JD, Bentsen AH, Ansel L, Simonneaux V, Juul A. Comparison of the effects of peripherally administered kisspeptins. Regul Pept. 2009;152(1–3):95–100. doi: 10.1016/j.regpep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Montes GS, Luque EH. Effects of ovarian steroids on vaginal smears in the rat. Acta Anat (Basel) 1988;133(3):192–9. doi: 10.1159/000146639. [DOI] [PubMed] [Google Scholar]
- Nishihara E, Nagayama Y, Inoue S, Hiroi H, Muramatsu M, Yamashita S, Koji T. Ontogenetic changes in the expression of estrogen receptor alpha and beta in rat pituitary gland detected by immunohistochemistry. Endocrinology. 2000;141(2):615–20. doi: 10.1210/endo.141.2.7330. [DOI] [PubMed] [Google Scholar]
- Ogilvie KM, Rivier C. Effect of alcohol on the proestrous surge of luteinizing hormone (LH) and the activation of LH-releasing hormone (LHRH) neurons in the female rat. J Neurosci. 1997;17(7):2595–604. doi: 10.1523/JNEUROSCI.17-07-02595.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard A, Tena-Sempere M, Franceschini I, Desroziers E, Simonneaux V, Mikkelsen JD. Comparative analysis of kisspeptin-immunoreactivity reveals genuine differences in the hypothalamic Kiss1 systems between rats and mice. Peptides. 2013;45:85–90. doi: 10.1016/j.peptides.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6. Elsevier; 2008. [DOI] [PubMed] [Google Scholar]
- Plagemann A, Harder T, Dudenhausen JW. The diabetic pregnancy, macrosomia, and perinatal nutritional programming. Nestle Nutr Workshop Ser Pediatr Program. 2008;61:91–102. doi: 10.1159/000113179. [DOI] [PubMed] [Google Scholar]
- Rettori V, Skelley CW, McCann SM, Dees WL. Detrimental effects of short-term ethanol exposure on reproductive function in the female rat. Biol Reprod. 1987;37(5):1089–96. doi: 10.1095/biolreprod37.5.1089. [DOI] [PubMed] [Google Scholar]
- Rissman EF, Li X. Olfactory bulbectomy blocks mating-induced ovulation in musk shrews (Suncus murinus) Biol Reprod. 2000;62(4):1052–8. doi: 10.1095/biolreprod62.4.1052. [DOI] [PubMed] [Google Scholar]
- Roa J, Vigo E, Castellano JM, Navarro VM, Fernandez-Fernandez R, Casanueva FF, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Hypothalamic expression of KiSS-1 system and gonadotropin-releasing effects of kisspeptin in different reproductive states of the female Rat. Endocrinology. 2006;147(6):2864–78. doi: 10.1210/en.2005-1463. [DOI] [PubMed] [Google Scholar]
- Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29(12):3920–9. doi: 10.1523/JNEUROSCI.5740-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci U S A. 2005;102(6):2129–34. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwowska JH, Barker JM, Barha CK, Lan N, Weinberg J, Galea LA. Stress-induced suppression of hippocampal neurogenesis in adult male rats is altered by prenatal ethanol exposure. Stress. 2010;13(4):301–13. doi: 10.3109/10253890903531582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwowska JH, Lan N, Yamashita F, Halpert AG, Viau V, Weinberg J. Effects of prenatal ethanol exposure on regulation of basal hypothalamic-pituitary-adrenal activity and hippocampal 5-HT1A receptor mRNA levels in female rats across the estrous cycle. Psychoneuroendocrinology. 2008;33(8):1111–23. doi: 10.1016/j.psyneuen.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT. Kisspeptin signalling in the brain: steroid regulation in the rodent and ewe. Brain Res Rev. 2008;57(2):288–98. doi: 10.1016/j.brainresrev.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005a;146(9):3686–92. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005b;146(7):2976–84. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- Srivastava VK, Hiney JK, Dees WL. Short-term alcohol administration alters KiSS-1 gene expression in the reproductive hypothalamus of prepubertal female rats. Alcohol Clin Exp Res. 2009;33(9):1605–14. doi: 10.1111/j.1530-0277.2009.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takase K, Uenoyama Y, Inoue N, Matsui H, Yamada S, Shimizu M, Homma T, Tomikawa J, Kanda S, Matsumoto H, Oka Y, Tsukamura H, Maeda KI. Possible role of oestrogen in pubertal increase of Kiss1/kisspeptin expression in discrete hypothalamic areas of female rats. J Neuroendocrinol. 2009;21(6):527–37. doi: 10.1111/j.1365-2826.2009.01868.x. [DOI] [PubMed] [Google Scholar]
- Tena-Sempere M. Hypothalamic KiSS-1: the missing link in gonadotropin feedback control? Endocrinology. 2005;146(9):3683–5. doi: 10.1210/en.2005-0652. [DOI] [PubMed] [Google Scholar]
- Uban KA, Sliwowska JH, Lieblich S, Ellis LA, Yu WK, Weinberg J, Galea LA. Prenatal alcohol exposure reduces the proportion of newly produced neurons and glia in the dentate gyrus of the hippocampus in female rats. Horm Behav. 2010;58(5):835–43. doi: 10.1016/j.yhbeh.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J. Nutritional issues in perinatal alcohol exposure. Neurobehav Toxicol Teratol. 1984;6(4):261–9. [PubMed] [Google Scholar]
- Wilson ME, Handa RJ. Gonadotropin secretion in infantile rats exposed to ethanol in utero. Alcohol. 1997;14(5):497–501. doi: 10.1016/s0741-8329(97)00037-2. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Marshall MT, Bollnow MR, McGivern RF, Handa RJ. Gonadotropin-releasing hormone mRNA and gonadotropin beta-subunit mRNA expression in the adult female rat exposed to ethanol in utero. Alcohol Clin Exp Res. 1995;19(5):1211–8. doi: 10.1111/j.1530-0277.1995.tb01603.x. [DOI] [PubMed] [Google Scholar]