Abstract
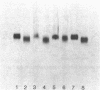
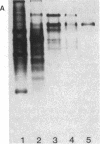
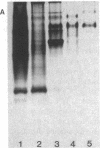
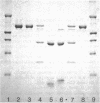
In response to global ischemia, tissue xanthine dehydrogenase was converted to xanthine oxidase in all tissues with half-times of conversion at 37 degrees C of approximately 3.6, 6, 7, and 14 h for the liver, kidney, heart, and lung, respectively. The time course of enzyme conversion at 4 degrees C was greatly extended with half-conversion times of 6, 5, 5, and 6 d for the respective tissues. Increases in xanthine oxidase activity were accompanied by the appearance of a distinct new protein species with greater electrophoretic mobility. The oxidase from ischemic rat liver was purified 781-fold and found to migrate with a higher mobility on native gels than the purified native dehydrogenase. Sodium dodecyl sulfate profiles revealed the presence of a single major band of 137 kD for the native dehydrogenase, whereas the oxidase had been partially cleaved generating polypeptides of 127, 91, and 57 kD. Polypeptide patterns for the oxidase resemble those seen following limited in vitro proteolysis of the native dehydrogenase supporting a proteolytic mechanism for the conversion of xanthine dehydrogenase to oxidase in ischemic rat liver.
Full text
PDF

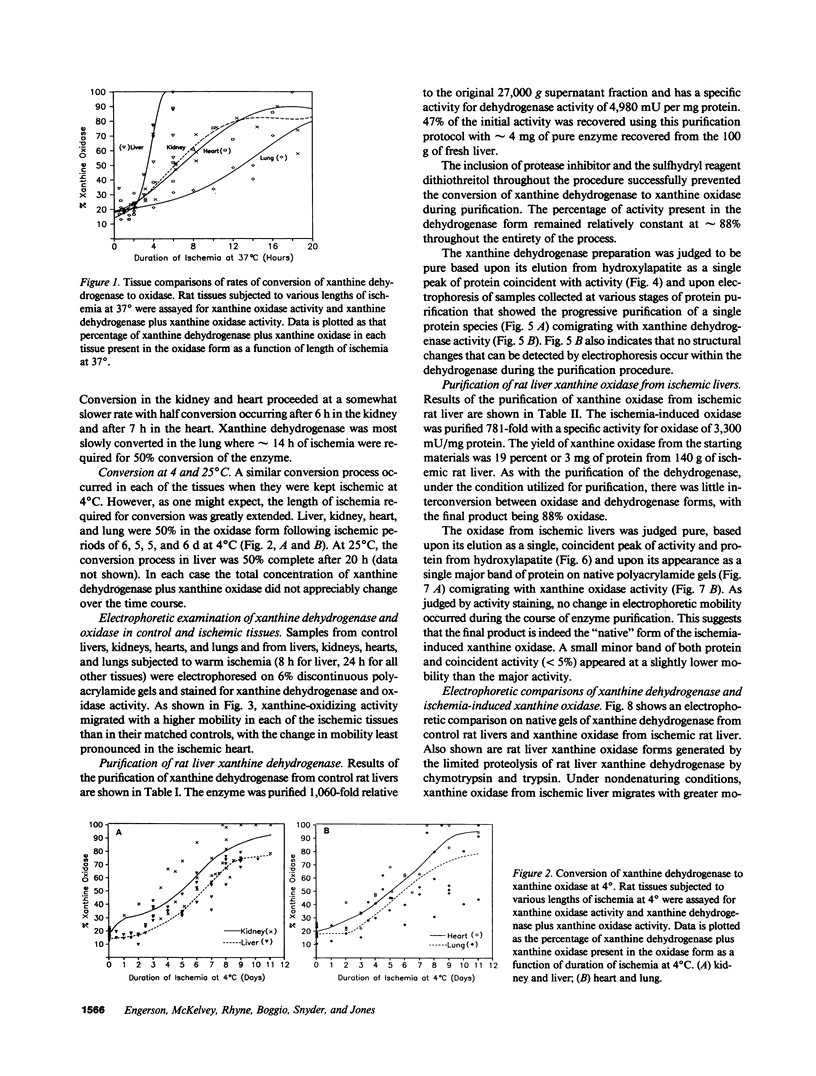



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkison D., Höllwarth M. E., Benoit J. N., Parks D. A., McCord J. M., Granger D. N. Role of free radicals in ischemia-reperfusion injury to the liver. Acta Physiol Scand Suppl. 1986;548:101–107. [PubMed] [Google Scholar]
- Battelli M. G., Lorenzoni E., Stripe F. Milk xanthine oxidase type D (dehydrogenase) and type O (oxidase). Purification, interconversion and some properties. Biochem J. 1973 Feb;131(2):191–198. doi: 10.1042/bj1310191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battelli M. G., Lorenzoni E., Stripe F. Milk xanthine oxidase type D (dehydrogenase) and type O (oxidase). Purification, interconversion and some properties. Biochem J. 1973 Feb;131(2):191–198. doi: 10.1042/bj1310191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Corte E. D., Stirpe F. The regulation of rat liver xanthine oxidase. Involvement of thiol groups in the conversion of the enzyme activity from dehydrogenase (type D) into oxidase (type O) and purification of the enzyme. Biochem J. 1972 Feb;126(3):739–745. doi: 10.1042/bj1260739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corte E. D., Stirpe F. The regulation of rat liver xanthine oxidase. Involvement of thiol groups in the conversion of the enzyme activity from dehydrogenase (type D) into oxidase (type O) and purification of the enzyme. Biochem J. 1972 Feb;126(3):739–745. doi: 10.1042/bj1260739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell J. W., Jones C. E., Smith E. E. Effect of allopurinol on hemorrhagic shock. Am J Physiol. 1969 Apr;216(4):744–748. doi: 10.1152/ajplegacy.1969.216.4.744. [DOI] [PubMed] [Google Scholar]
- De Martino G. N. Calcium-dependent proteolytic activity in rat liver: identification of two proteases with different calcium requirements. Arch Biochem Biophys. 1981 Oct 1;211(1):253–257. doi: 10.1016/0003-9861(81)90452-5. [DOI] [PubMed] [Google Scholar]
- Granger D. N., Höllwarth M. E., Parks D. A. Ischemia-reperfusion injury: role of oxygen-derived free radicals. Acta Physiol Scand Suppl. 1986;548:47–63. [PubMed] [Google Scholar]
- Granger D. N., Rutili G., McCord J. M. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981 Jul;81(1):22–29. [PubMed] [Google Scholar]
- Hansson R., Jonsson O., Lundstam S., Pettersson S., Scherstén T., Waldenström J. Effects of free radical scavengers on renal circulation after ischaemia in the rabbit. Clin Sci (Lond) 1983 Dec;65(6):605–610. doi: 10.1042/cs0650605. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Manning A. S., Downey J. M., Yellon D. M. Xanthine oxidase: a critical mediator of myocardial injury during ischemia and reperfusion? Acta Physiol Scand Suppl. 1986;548:65–78. [PubMed] [Google Scholar]
- Jolly S. R., Kane W. J., Bailie M. B., Abrams G. D., Lucchesi B. R. Canine myocardial reperfusion injury. Its reduction by the combined administration of superoxide dismutase and catalase. Circ Res. 1984 Mar;54(3):277–285. doi: 10.1161/01.res.54.3.277. [DOI] [PubMed] [Google Scholar]
- Koyama I., Bulkley G. B., Williams G. M., Im M. J. The role of oxygen free radicals in mediating the reperfusion injury of cold-preserved ischemic kidneys. Transplantation. 1985 Dec;40(6):590–595. doi: 10.1097/00007890-198512000-00003. [DOI] [PubMed] [Google Scholar]
- Krenitsky T. A., Tuttle J. V., Cattau E. L., Jr, Wang P. A comparison of the distribution and electron acceptor specificities of xanthine oxidase and aldehyde oxidase. Comp Biochem Physiol B. 1974 Dec 15;49(4):687–703. doi: 10.1016/0305-0491(74)90256-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Manning A. S., Coltart D. J., Hearse D. J. Ischemia and reperfusion-induced arrhythmias in the rat. Effects of xanthine oxidase inhibition with allopurinol. Circ Res. 1984 Oct;55(4):545–548. doi: 10.1161/01.res.55.4.545. [DOI] [PubMed] [Google Scholar]
- Owens M. L., Lazarus H. M., Wolcott M. W., Maxwell J. G., Taylor J. B. Allopurinol and hypoxanthine pretreatment of canine kidney donors. Transplantation. 1974 Apr;17(4):424–427. doi: 10.1097/00007890-197404000-00015. [DOI] [PubMed] [Google Scholar]
- Parks D. A., Bulkley G. B., Granger D. N., Hamilton S. R., McCord J. M. Ischemic injury in the cat small intestine: role of superoxide radicals. Gastroenterology. 1982 Jan;82(1):9–15. [PubMed] [Google Scholar]
- Parks D. A., Granger D. N. Ischemia-induced vascular changes: role of xanthine oxidase and hydroxyl radicals. Am J Physiol. 1983 Aug;245(2):G285–G289. doi: 10.1152/ajpgi.1983.245.2.G285. [DOI] [PubMed] [Google Scholar]
- Schaffer S. W., Roy R. S., McMcord J. M. Possible role for calmodulin in calcium paradox-induced heart failure. Eur Heart J. 1983 Dec;4 (Suppl H):81–87. doi: 10.1093/eurheartj/4.suppl_h.81. [DOI] [PubMed] [Google Scholar]
- Waud W. R., Rajagopalan K. V. Purification and properties of the NAD+-dependent (type D) and O2-dependent (type O) forms of rat liver xanthine dehydrogenase. Arch Biochem Biophys. 1976 Feb;172(2):354–364. doi: 10.1016/0003-9861(76)90087-4. [DOI] [PubMed] [Google Scholar]