Abstract
Introduction. Crotonis fructus (CF) is the mature fruit of Croton tiglium L. and has been used for the treatment of gastrointestinal disturbance in Asia. It is well known that the main component of CF is croton oil (CO). The present study is to investigate the effects of CF extracts (CFE) and CO on lipolysis in OP9 adipocytes. Methods. Glycerol release to the culture supernatants was used as a marker of adipocyte lipolysis. Results. Treatment with various concentrations of CFE and CO stimulates glycerol release in a dose-dependent manner. The increase in glycerol release by CFE is more potent than isoproterenol, which is a β-adrenergic agonist as a positive control in our system. The increased lipolysis by CFE and CO was accompanied by an increase of phosphorylated hormone sensitive lipase (pHSL) but not nonphosphorylated HSL protein and mRNA. Pretreatment with H89, which is a protein kinase A inhibitor, significantly abolished the CFE- and CO-induced glycerol release in OP9 adipocytes. These results suggest that CFE and CO may be a candidate for the development of a lipolysis-stimulating agent in adipocytes.
1. Introduction
Croton tiglium L. (family Euphorbiaceae) is a plant distributed in the tropical and subtropical zones of Asia. The fruit of Croton tiglium L. is Crotonis Fructus (CF), which is one of several medicines used for attenuating gastrointestinal diseases such as constipation, visceral pain, and intestinal inflammation [1, 2]. Additionally, the crude extracts of CF have been reported to activate M3 muscarinic receptor and Ca2+ influx through the L-type Ca2+ channel in isolated rabbit jejunum [3]. It is also well known that the main component of CF is the croton oil (CO). There is an abundance of linoleic acid, oleic acid, and eicosenoic acid in a methyl-esterified sample of CO [4]. It has been reported that CO may regulate gastrointestinal motility and induce intestinal inflammation related to immunological milieu and motor activity in mice [5].
Obesity is a medical condition in which excess body fat has accumulated to the extent that is associated with a variety of diseases, including metabolic syndrome and cardiovascular disease. White adipose tissue is the major fat reservoir in mammals storing the excess energy from the diet as triacylglycerol (TAG), a neural lipid composed of three fatty acids which is bound to the carbon backbone of a glycerol molecule [6]. One of the therapeutic methods for treating obesity is to reduce TAG in white adipocytes, which is termed lipolysis. Lipolysis is a complex process that is highly regulated and involves the coordinated participation of several lipases [7, 8]. Lipolysis occurs through the sequential hydrolysis of TAG by the action of three lipases: adipose triglyceride lipase (ATGL), hormone-sensitive lipase (HSL), and monoglyceride lipase (MGL) [9]. Among them, HSL is a key enzyme in the mobilization of fatty acids in adipocytes and its activity is regulated posttranscriptionally by reversible phosphorylation by protein kinase A (PKA) [10].
In this study, we used OP9 mouse stromal cells, first reported by Bickel and colleagues as a useful new model of adipocyte metabolism in 2006 [11]. In their study, OP9 cells initiated the same events, including lipid metabolism, insulin signaling, and glucose transport, much like 3T3-L1 cells [11]. We already reported about the inhibitory effects of Pericarpium Zanthoxyli extract on adipocyte differentiation by using OP9 cells [12].
In the present study, the effects of CF extract (CFE) and CO on lipolysis in OP9 cells were investigated by measuring glycerol release and evaluating HSL expression level.
2. Materials and Methods
2.1. Reagents
OP9 cells were purchased from the American Type Culture Collection (Manassas, VA, USA). Minimum essential medium alpha (MEMα) and fetal bovine serum (FBS) were purchased from Invitrogen (Carlsbad, CA, USA). Insulin, 3-isobutyl-1-methylxanthine (IBMX), dexamethasone (DEXA), isoguanosine, and croton oil (CO) were purchased from Sigma chemical (St. Louis, MO, USA). HA89, a protein kinase inhibitor, was purchased from Calbiochem (San Diego, CA). Antibodies against HSL and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibody against pHSL was obtained from Cell Signaling Technology (Beverly, MA, USA). All of the chemicals were of analytical grade.
2.2. Preparation of Crotonis Fructus Extracts (CFE)
Mature fruits of Croton tinglium L. were purchased from Kwangmyungdang Medicinal Herbs Co., Ltd. (Ulsan, Republic of Korea) and authenticated by Professor GS Lee, one of the authors. A voucher specimen (WKU030305-CT201305E) has been deposited at the Department of Herbology, College of Korean Medicine, Wonkwang University, Iksan, Korea.
The powdered Crotonis Fructus (100 g) was extracted using reflux method with 1000 mL of 70% aqueous ethanol for 2 hours. The extract was evaporated under 40 mmHg using a rotary evaporator and then freeze-dried. The yield of the final extract was 11.48% (w/w).
2.3. Cell Culture and Adipocyte Differentiation Induction
OP9 cells were cultured in MEMα containing 20% FBS, 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a 5% CO2 incubator. To induce differentiation, 1-day postconfluent preadipocytes were incubated in a differentiation medium containing 10% FBS, 0.5 mM IBMX, 0.25 μM DEXA, 175 nM insulin, 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin for 2 days. The medium was then changed to MEMα containing 10% FBS, 2 mM l-glutamine, and 175 nM insulin, and the cells were cultured for 3 days.
2.4. Determination of Cell Viability
The effect of CFE on OP9 cell viability was determined using an established MTT assay. The attached cells were kept untreated or treated with various concentrations of CFE (list the concentrations in parentheses) for 24 h at 37°C. The cells were washed with phosphate-buffered saline (PBS) prior to adding MTT (0.5 mg/mL PBS) and incubated at 37°C for 30 min. Formazan crystals were dissolved with dimethyl sulfoxide (100 μL/well) and detected at OD570 with a model Emax (Molecular Devices, Sunnyvale, CA, USA).
2.5. Glycerol Release
After treatment of differentiated OP9 cells with various concentrations of CFE and CO, free glycerol content in cell supernatants was quantified using a glycerol quantification kit according to the manufacturer's instructions (Sigma Chemicals). Glycerol was quantified at OD540 with a model Emax (Molecular Devices). Isoproterenol (Merck, Darmstadt, Germany), a known stimulator of lipolysis, was used as a positive control.
2.6. Western Blot Analysis
The differentiated OP9 cells were pretreated with 20 μM H89 for 2 h and then treated with 20 μg/mL CFE and 10 μg/mL CO for 12 h at 37°C. Cells were lysed with ice-cold M-PER mammalian protein extraction reagent (Pierce Biotechnology, Rockford, IL, USA), and the protein concentration in the lysate was determined using the Bradford method [13]. Samples (20 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis with 10% acrylamide and transferred to Hybond-P polyvinylidene fluoride (PVDF) membranes (GE Healthcare Life Sciences, Buckinghamshire, UK) using a western blot apparatus. Protein expression levels were determined by signal analysis using an image analyzer (Fuji-Film, Tokyo, Japan).
2.7. Quantitative Real-Time Polymerase Chain Reaction (PCR)
Total RNA was extracted from cells using a FastPure RNA kit (TaKaRa, Shiga, Japan). The RNA concentration and purity were determined by absorbance at 260/280 nm. cDNA was synthesized from 1 μg of total RNA using a PrimeScript RT reagent kit (TaKaRa). Adipocyte differentiation-related gene mRNA expressions were determined by real-time PCR using the ABI PRISM 7900 Sequence Detection System and SYBR Green I (Applied Biosystems, Foster City, CA, USA). The forward and the reverse primers for HSL were 5′-GGAGCACTACAAACGCAACGA-3′ and 5′-TCGGCCACCGGTAAAGAG-3′, respectively; the primers for GAPDH were 5′-CGTCCCGTAGACAAAATGGT-3′ and 5′-TTGATGGCAACAATCTCCAC-3′, respectively. All results were normalized to the GAPDH housekeeping gene to control for variation in mRNA concentrations. Relative quantification was performed using the comparative ΔΔC t method according to the manufacturer's instructions.
2.8. Statistical Analysis
Statistical analysis was performed using OriginPro 8.0 software. One way analysis of variance (ANOVA) followed by Duncan's test. Data were expressed in means ± SD. Differences with P values less than 0.05 were considered statistically significant.
3. Results and Discussion
Crotonis Fructus (CF) is the mature fruit of Croton tiglium L. which has been used in traditional medicine for the treatment of gastrointestinal (GI) diseases including constipation, abdominal pain, peptic ulcer, and intestinal inflammation in Asia [2, 3, 5]. The main components of CF comprise the great parts of the extracted essential oil from seed and bark (fruit) of Croton tiglium, which is named by Croton tiglium oil (CO), and modulate intestinal transit in mice [5]. There were many reports about the effects of CF extracts and CO on GI problems [2, 3]. Kim et al. [14] also reported that isoguanosine from Croton tiglium L. has antitumor activity against implanted S-180 ascitic tumor in mice. However, there are no studies on their lipolytic effects on adipocytes, particularly on the underlying mechanism.
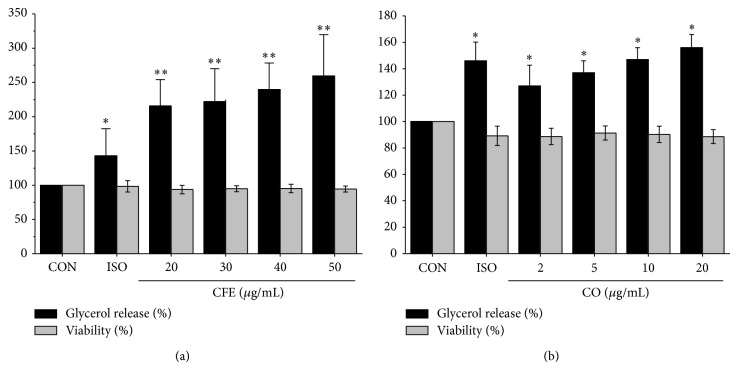
A significant increase in the amount of glycerol released into the media was observed in those OP9 adipocytes treated with CF extracts (CFE) (20–50 μg/mL; P < 0.01) for 12 h (Figure 1(a)) in a dose-dependent manner. The increase in glycerol release by 20 μg/mL CFE was observed after 6 h of treatment (1.6-fold, P < 0.05) and peaked after 12 h of treatment (2.2-fold, P < 0.01) (data not shown). Lipolysis of TAG into glycerol and fatty acids is activated by the β-adrenergic receptor agonist isoproterenol (ISO) and is used as a positive control in our system. ISO also increased glycerol release after 12 h of treatment (1.4-fold, P < 0.05). However, the effect of CFE on lipolysis is more potent than ISO, even at a concentration of 20 μg/mL. We also checked the effects of CFE on ISO-induced lipolysis and the data revealed that CFE did not have any additional effect on the lipolytic effect of ISO (data not shown). And then, we want to know what the main constituent of CFE increasing glycerol release is. As shown in Figure 1(b), CO significantly increased glycerol release into the media at all concentrations tested, but it was less effective than CFE. We also tested the effects of isoguanosine on lipolysis and there was none (data not shown). We could not exclude the lipolytic effects of other compounds in CFE. None of CFE, CO, and isoguanosine at the concentration ranges tested was cytotoxic according to the MTT (Figure 1).
Figure 1.
Effects of Crotonis Fructus extracts (CEF) and croton oil (CO) on glycerol release and viability. Differentiated OP9 adipocytes were treated with various concentrations of CFE (20–50 μg/mL), CO (2–20 μg/mL), and 200 nM isoproterenol (ISO) for 12 h. Glycerol release (black bar) was determined with a glycerol quantification kit and cell viability (gray bar) was quantified with MTT assay as described in Materials and Methods. Data are expressed as the mean ± SD of four independent experiments and a percentage of vehicle (DMSO-)treated control cells (CON). * P < 0.05, ** P < 0.01 versus CON.
In murine adipocytes, PKA phosphorylates HSL at several serine residues (563, 659, and 660), resulting in increased translocation of HSL to the lipid droplet surface and increased lipolytic activity [15]. To better elucidate the mechanisms underlying the lipolytic actions of CFE and CO, we investigated the effects of CFE and CO on HSL phosphorylation in Ser563. As shown in Figure 2, CFE (20 μg/mL) and CO (10 μg/mL) treatment for 12 h did not modify total protein and mRNA contents of HSL but significantly increased the phosphorylation of HSL at Ser563. However, the CFE- and CO-induced glycerol release and phosphorylation of HSL at Ser563 were significantly inhibited in the presence of the PKA inhibitor H89. These data suggest that CFE and CO stimulate lipolysis through HSL phosphorylation at Ser563 by PKA activation. In addition to the PKA-mediated phosphorylation, HSL may be phosphorylated by other kinases, such as extracellular signal-regulated kinase (ERK1/2), which activates HSL by phosphorylation [16]. Furthermore, AMP-activated protein kinase (AMPK) phosphorylates HSL at Ser565, which prevents phosphorylation induced PKA [17]. Other kinases such as ERK1/2 and AMPK may be involved in the underlying mechanism of CFE- and CO-induced glycerol release from lipid droplets.
Figure 2.
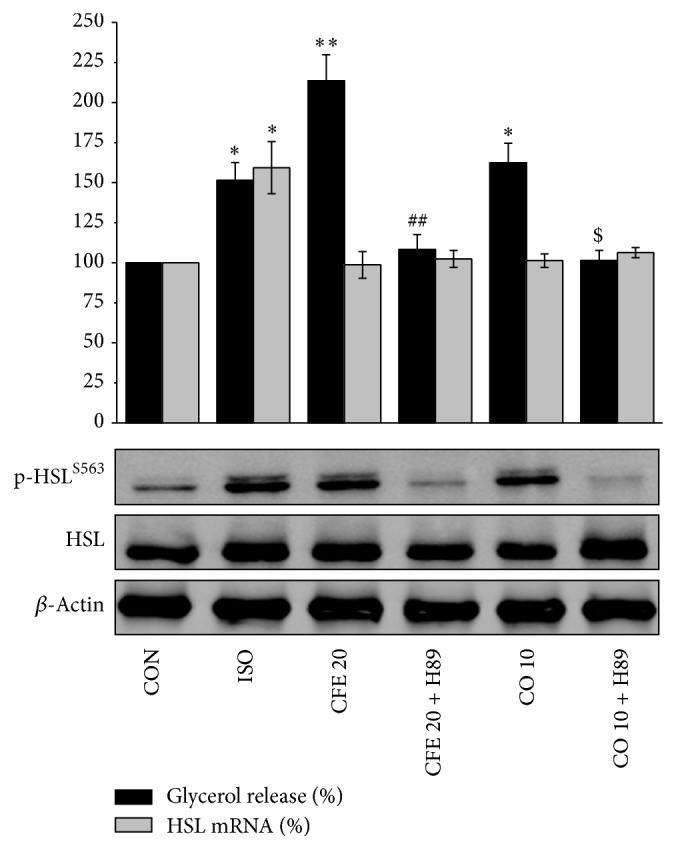
Signaling pathway involved in the lipolytic effects of CFE and CO. Differentiated OP9 adipocytes were pretreated with 20 μM H89 and then treated with 20 μg/mL of CFE, 10 μg/mL of CO, and 200 nM ISO for 12 h. Glycerol release (black bar) quantification is the same as Figure 1 legends and real-time PCR for hormone sensitive lipase (HSL) mRNA (gray bar) was carried out using a specific primer for HSL as described in Materials and Methods. Protein expression levels for p-HSLS563 and HSL (lower panel) were subjected to western blot analysis by using specific antibodies. Data are expressed as the mean ± SD of four independent experiments and a percentage of vehicle (DMSO-)treated control cells (CON). * P < 0.05, ** P < 0.01 versus CON; ## P < 0.01 versus CFE-treated group; and $ P < 0.05 versus CO-treated group.
Collectively, the present data demonstrate the ability of CFE to stimulate lipolysis in OP9 adipocytes, with CO as its major constituent. The lipolytic actions of CFE and CO are mainly mediated by the phosphorylation of HSL through PKA activation. These findings explain a mechanism by which CFE and CO induce lipolysis in adipocytes. This would further aid in the development of therapeutic strategy in preventing obesity.
Acknowledgment
This research was supported by a National Research Foundation of Korea (NRF) Grant, funded by the Korean Government (MEST) (no. 2011-0030130), Republic of Korea, and by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (no. 2011-0013528).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors’ Contribution
Mi-Seong Kim and Ha-Rim Kim contributed equally to this study.
References
- 1.Tsai J.-C., Tsai S., Chang W.-C. Effect of ethanol extracts of three Chinese medicinal plants with anti-diarrheal properties on ion transport of the rat intestinal epithelia. Journal of Pharmacological Sciences. 2004;94(1):60–66. doi: 10.1254/jphs.94.60. [DOI] [PubMed] [Google Scholar]
- 2.Wang X., Lan M., Wu H.-P., Shi Y.-Q., Lu J., Ding J., Wu K.-C., Jin J.-P., Fan D.-M. Direct effect of croton oil on intestinal epithelial cells and colonic smooth muscle cells. World Journal of Gastroenterology. 2002;8(1):103–107. doi: 10.3748/wjg.v8.i1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu J., Gao W.-Y., Ma L., Man S.-L., Huang L.-Q., Liu C.-X. Activation of M3 muscarinic receptor and Ca2+ influx by crude fraction from Crotonis Fructus in isolated rabbit jejunum. Journal of Ethnopharmacology. 2012;139(1):136–141. doi: 10.1016/j.jep.2011.10.041. [DOI] [PubMed] [Google Scholar]
- 4.Lan M., Wan P., Wang Z.-Y., Huang X.-L. GC-MS analysis of chemical components in seeds oil from Croton tiglium. Journal of Chinese Medicinal Materials. 2012;35(7):1105–1108. [PubMed] [Google Scholar]
- 5.Wang X., Zhang F., Liu Z., Feng H., Yu Z. B., Lu Y., Zhai H., Bai F., Shi Y., Lan M., Jin J., Fan D. Effects of essential oil from Croton tiglium L. on intestinal transit in mice. Journal of Ethnopharmacology. 2008;117(1):102–107. doi: 10.1016/j.jep.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 6.Jaworski K., Sarkadi-Nagy E., Duncan R. E., Ahmadian M., Sul H. S. Regulation of triglyceride metabolism. IV. Hormonal regulation of lipolysis in adipose tissue. American Journal of Physiology: Gastrointestinal and Liver Physiology. 2007;293(1):G1–G4. doi: 10.1152/ajpgi.00554.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duncan R. E., Ahmadian M., Jaworski K., Sarkadi-Nagy E., Sul H. S. Regulation of lipolysis in adipocytes. Annual Review of Nutrition. 2007;27:79–101. doi: 10.1146/annurev.nutr.27.061406.093734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watt M. J., Spriet L. L. Triacylglycerol lipases and metabolic control: implications for health and disease. American Journal of Physiology—Endocrinology and Metabolism. 2010;299(2):E162–E168. doi: 10.1152/ajpendo.00698.2009. [DOI] [PubMed] [Google Scholar]
- 9.Lass A., Zimmermann R., Oberer M., Zechner R. Lipolysis—a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Progress in Lipid Research. 2011;50(1):14–27. doi: 10.1016/j.plipres.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holm C. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochemical Society Transactions. 2003;31(6):1120–1124. doi: 10.1042/BST0311120. [DOI] [PubMed] [Google Scholar]
- 11.Wolins N. E., Quaynor B. K., Skinner J. R., Tzekov A., Park C., Choi K., Bickel P. E. OP9 mouse stromal cells rapidly differentiate into adipocytes: characterization of a useful new model of adipogenesis. Journal of Lipid Research. 2006;47(2):450–460. doi: 10.1194/jlr.D500037-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Kim H.-R., Kim J.-M., Kim M.-S., Hwang J.-K., Yang S.-H., Kim H.-J., Lee D.-S., Oh H., Kim Y.-C., Ryu D.-G., Lee Y.-R., Kwon K.-B. Inhibitory effects of Pericarpium zanthoxyli extract on adipocyte differentiation. International Journal of Molecular Medicine. 2014;33(5):1140–1146. doi: 10.3892/ijmm.2014.1667. [DOI] [PubMed] [Google Scholar]
- 13.Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry. 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 14.Kim J. H., Lee S. J., Han Y. B., Moon J. J., Kim J. B. Isolation of isoguanosine from Croton tiglium and its antitumor activity. Archives of Pharmacal Research. 1994;17(2):115–118. doi: 10.1007/BF02974234. [DOI] [PubMed] [Google Scholar]
- 15.Watt M. J., Holmes A. G., Pinnamaneni S. K., et al. Regulation of HSL serine phosphorylation in skeletal muscle and adipose tissue. American Journal of Physiology: Endocrinology and Metabolism. 2006;290(3):E500–E509. doi: 10.1152/ajpendo.00361. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg A. S., Shen W.-J., Muliro K., Patel S., Souza S. C., Roth R. A., Kraemer F. B. Stimulation of lipolysis and hormone-sensitive lipase via the extracellular signal-regulated kinase pathway. The Journal of Biological Chemistry. 2001;276(48):45456–45461. doi: 10.1074/jbc.M104436200. [DOI] [PubMed] [Google Scholar]
- 17.Anthony N. M., Gaidhu M. P., Ceddia R. B. Regulation of visceral and subcutaneous adipocyte lipolysis by acute AICAR-induced AMPK activation. Obesity. 2009;17(7):1312–1317. doi: 10.1038/oby.2008.645. [DOI] [PubMed] [Google Scholar]