Abstract
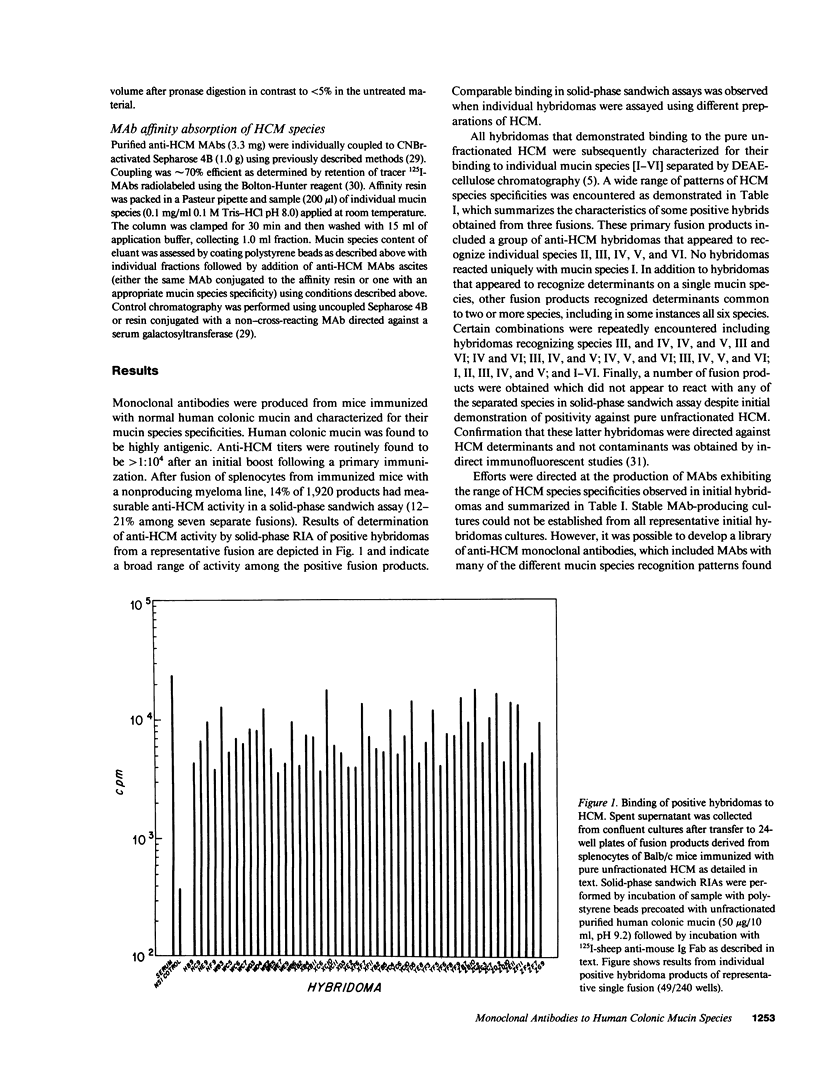
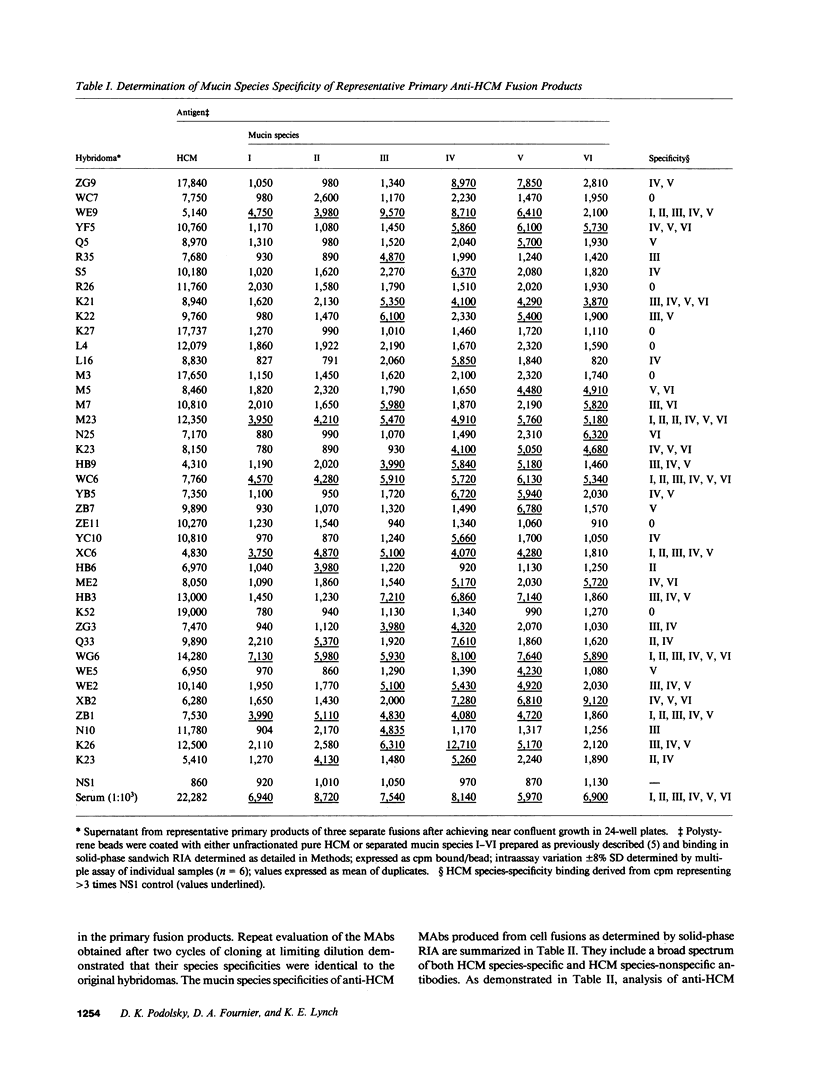
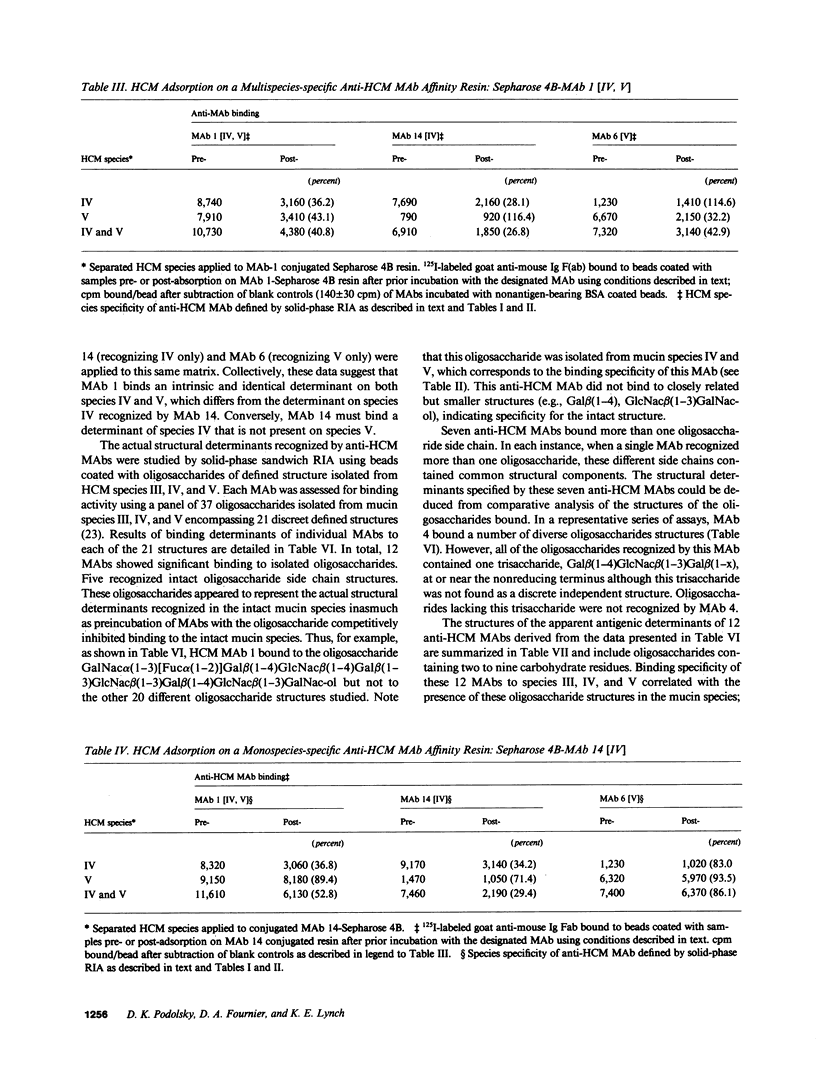
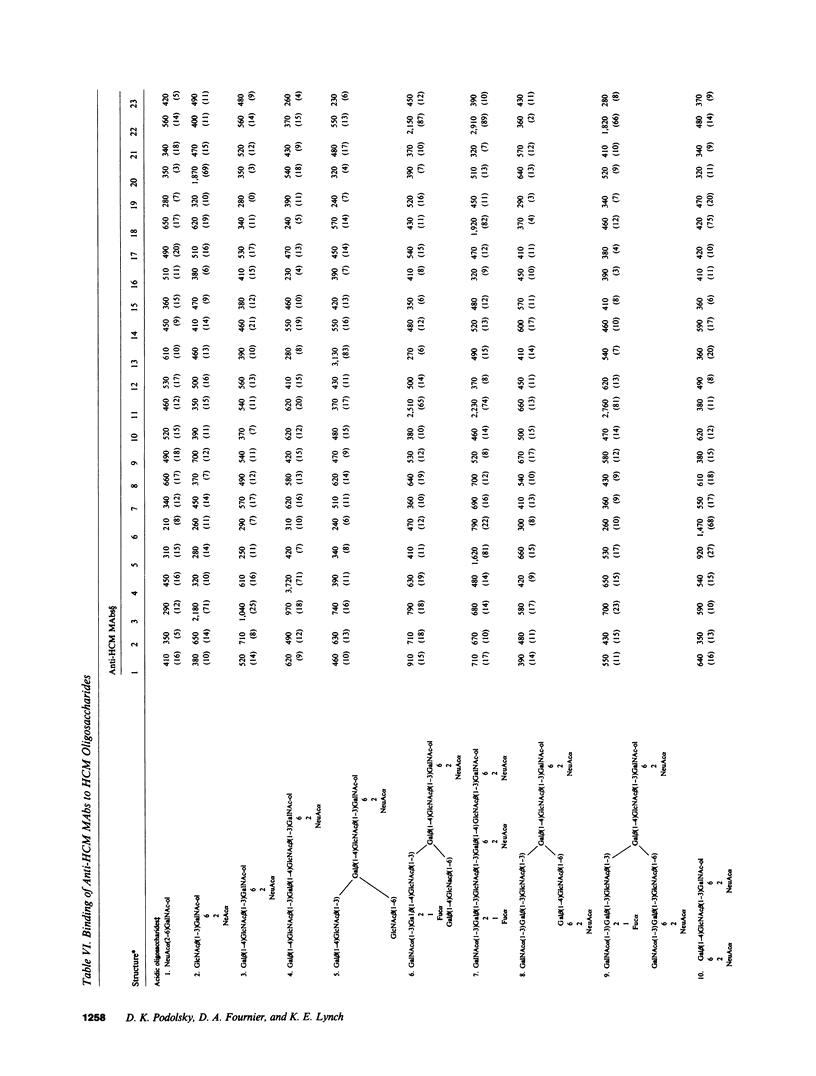
Structural relationships between colonic mucin species were assessed using a library of monoclonal antibodies (MAbs) directed against purified human colonic mucin (HCM). After immunization of mice with purified mucin from normal human colonic mucosa, 14% of 1,920 fusion products screened were positive for anti-HCM activity in a solid-phase assay. Patterns of selective binding by hybridomas to six discrete HCM species (I-VI) separated by DEAE-cellulose chromatography suggested the presence of both shared and species-specific antigenic determinants among HCM species I-VI. 23 anti-HCMs MAbs (7 IgM, 7 IgG1, and 9 IgG2) demonstrating a range of anti-HCM species specificities, were produced and used to study structural relationships between mucin species. Binding of various mucin species by individual anti-HCM MAbs was shown by competitive solid-phase radioimmunoassay to reflect the presence of identical epitopes on the different species. Adsorption of HCM species on a variety of affinity resins prepared with anti-HCM MAbs demonstrated that binding to multiple mucin species by a single MAb was related to intrinsic structural determinants. Four anti-HCM MAbs recognized protease-sensitive antigenic structures, which suggests that they may be directed to core HCM proteins. 12 of the anti-HCM MAbs were shown by solid-phase assay to recognize either complete (n = 5) or partial (n = 7) isolated colonic mucin oligosaccharide side chains of defined structure. Collectively, these data show the presence of both shared and unique antigenic structural determinants among colonic mucin species. Chromatographic heterogeneity of mucin glycoproteins seems to be related to the existence of biologically significant subclasses in the normal human colon.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A., Bell A., Mantle M., Pearson J. P. The structure and physiology of gastrointestinal mucus. Adv Exp Med Biol. 1982;144:115–133. doi: 10.1007/978-1-4615-9254-9_15. [DOI] [PubMed] [Google Scholar]
- Allen A. Structure of gastrointestinal mucus glycoproteins and the viscous and gel-forming properties of mucus. Br Med Bull. 1978 Jan;34(1):28–33. [PubMed] [Google Scholar]
- Bara J., Loisillier F., Burtin P. Antigens of gastric and intestinal mucous cells in human colonic tumours. Br J Cancer. 1980 Feb;41(2):209–221. doi: 10.1038/bjc.1980.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland C. R., Lance P., Levin B., Riddell R. H., Kim Y. S. Abnormal goblet cell glycoconjugates in rectal biopsies associated with an increased risk of neoplasia in patients with ulcerative colitis: early results of a prospective study. Gut. 1984 Dec;25(12):1364–1371. doi: 10.1136/gut.25.12.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Filipe M. I., Branfoot A. C. Abnormal patterns of mucus secretion in apparently normal mucosa of large intestine with carcinoma. Cancer. 1974 Aug;34(2):282–290. doi: 10.1002/1097-0142(197408)34:2<282::aid-cncr2820340211>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Filipe M. I., Dawson I. The diagnostic value of mucosubstances in rectal biopsies from patients with ulcerative colitis and Crohn's disease. Gut. 1970 Mar;11(3):229–234. doi: 10.1136/gut.11.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipe M. I. Value of histochemical reactions for mucosubstances in the diagnosis of certain pathological conditions of the colon and rectum. Gut. 1969 Jul;10(7):577–586. doi: 10.1136/gut.10.7.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner G., Wesley A., Forstner J. Clinical aspects of gastrointestinal mucus. Adv Exp Med Biol. 1982;144:199–224. doi: 10.1007/978-1-4615-9254-9_32. [DOI] [PubMed] [Google Scholar]
- Gad A. A histochemical study of human alimentary tract mucosubstances in health and disease. II. Inflammatory conditions. Br J Cancer. 1969 Mar;23(1):64–68. doi: 10.1038/bjc.1969.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold D. V., Shochat D., Miller F. Protease digestion of colonic mucin. Evidence for the existence of two immunochemically distinct mucins. J Biol Chem. 1981 Jun 25;256(12):6354–6358. [PubMed] [Google Scholar]
- Goldenberg D. M., Pant K. D., Dahlman H. L. Antigens associated with normal and malignant gastrointestinal tissues. Cancer Res. 1976 Sep;36(9 Pt 2):3455–3463. [PubMed] [Google Scholar]
- Inoue S., Yosizawa Z. Purification and properties of sulfated sialopolysaccharides isolated from pig colonic mucosa. Arch Biochem Biophys. 1966 Nov;117(2):257–265. doi: 10.1016/0003-9861(66)90410-3. [DOI] [PubMed] [Google Scholar]
- Jabbal I., Kells D. I., Forstner G., Forstner J. Human intestinal goblet cell mucin. Can J Biochem. 1976 Aug;54(8):707–716. doi: 10.1139/o76-102. [DOI] [PubMed] [Google Scholar]
- Jacobs L. R., Huber P. W. Regional distribution and alterations of lectin binding to colorectal mucin in mucosal biopsies from controls and subjects with inflammatory bowel diseases. J Clin Invest. 1985 Jan;75(1):112–118. doi: 10.1172/JCI111662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- LaMont J. T., Ventola A. S. Purification and composition of colonic epithelial mucin. Biochim Biophys Acta. 1980 Nov 20;626(1):234–243. doi: 10.1016/0005-2795(80)90214-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Listinsky C. M., Riddell R. H. Patterns of mucin secretion in neoplastic and non-neoplastic diseases of the colon. Hum Pathol. 1981 Oct;12(10):923–929. doi: 10.1016/s0046-8177(81)80198-0. [DOI] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Fink P., Gridley T., Raulet D. H., Bevan M. J., Gefter M. L. Properties and applications of monoclonal antibodies directed against determinants of the Thy-1 locus. J Immunol. 1979 Jun;122(6):2491–2497. [PubMed] [Google Scholar]
- Marshall T., Allen A. The isolation and characterization of the high-molecular-weight glycoprotein from pig colonic mucus. Biochem J. 1978 Aug 1;173(2):569–578. doi: 10.1042/bj1730569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty V. L., Downs F. J., Pigman W. Rat-colonic, mucus glycoprotein. Carbohydr Res. 1978 Mar;61:139–145. doi: 10.1016/s0008-6215(00)84474-2. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Fournier D. A., Lynch K. E. Human colonic goblet cells. Demonstration of distinct subpopulations defined by mucin-specific monoclonal antibodies. J Clin Invest. 1986 Apr;77(4):1263–1271. doi: 10.1172/JCI112429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky D. K., Isselbacher K. J. Characterization of monoclonal antibodies to serum galactosyltransferase. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2529–2533. doi: 10.1073/pnas.81.8.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky D. K., Isselbacher K. J. Composition of human colonic mucin. Selective alteration in inflammatory bowel disease. J Clin Invest. 1983 Jul;72(1):142–153. doi: 10.1172/JCI110952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky D. K., Isselbacher K. J. Glycoprotein composition of colonic mucosa. Specific alterations in ulcerative colitis. Gastroenterology. 1984 Nov;87(5):991–998. [PubMed] [Google Scholar]
- Podolsky D. K., Madara J. L., King N., Sehgal P., Moore R., Winter H. S. Colonic mucin composition in primates. Selective alterations associated with spontaneous colitis in the cotton-top tamarin. Gastroenterology. 1985 Jan;88(1 Pt 1):20–25. doi: 10.1016/s0016-5085(85)80127-x. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K. Oligosaccharide structures of human colonic mucin. J Biol Chem. 1985 Jul 15;260(14):8262–8271. [PubMed] [Google Scholar]
- Shamsuddin A. K., Trump B. F. Colon epithelium. I. Light microscopic, histochemical, and ultrastructural features of normal colon epithelium of male Fischer 344 rats. J Natl Cancer Inst. 1981 Feb;66(2):375–388. [PubMed] [Google Scholar]
- Slomiany B. L., Murty V. L., Slomiany A. Isolation and characterization of oligosaccharides from rat colonic mucus glycoprotein. J Biol Chem. 1980 Oct 25;255(20):9719–9723. [PubMed] [Google Scholar]
- Teague R. H., Fraser D., Clamp J. R. Changes in monosaccharide content of mucous glycoproteins in ulcerative colitis. Br Med J. 1973 Jun 16;2(5867):645–646. doi: 10.1136/bmj.2.5867.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Variyam E. P., Hoskins L. C. Mucin degradation in human colon ecosystems. Degradation of hog gastric mucin by fecal extracts and fecal cultures. Gastroenterology. 1981 Oct;81(4):751–758. [PubMed] [Google Scholar]
- Yonezawa S., Nakamura T., Tanaka S., Sato E. Glycoconjugate with Ulex europaeus agglutinin-I-binding sites in normal mucosa, adenoma, and carcinoma of the human large bowel. J Natl Cancer Inst. 1982 Oct;69(4):777–785. [PubMed] [Google Scholar]



