Abstract
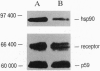
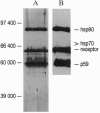
The nonactivated estrogen receptor of human MCF-7 mammary carcinoma cells was investigated with respect to stoichiometry of protein subunits. The native receptor complex stabilized by molybdate had a molecular mass of approximately 300 kDa. Chemical cross-linking with several bifunctional reagents resulted in complete stabilization of the same receptor form of approximately 300 kDa and was achieved both in cell extracts and in intact cells. Incubation of the cross-linked receptor with a receptor-specific monoclonal IgG1 antibody increased the molecular mass by approximately 135 kDa--i.e., no more than one immunoglobulin molecule bound to the complex. Partial and progressive cross-linking of affinity-labeled receptors revealed patterns of labeled bands upon denaturing gel electrophoresis indicative of a heteromeric structure. The completely cross-linked receptor was purified to homogeneity and analyzed for protein components. In addition to the receptor polypeptide of approximately 65 kDa, we detected the heat shock proteins hsp90 and p59; the hsp90 band was roughly twice as intense as the p59 band. The heat shock protein hsp70 and the 40-kDa cyclophilin were not detected as components of the highly purified cross-linked receptor of approximately 300 kDa. We suggest a heterotetrameric structure consisting of one receptor polypeptide, two hsp90 molecules, and one p59 subunit, for which the molecular mass adds up to approximately 300 kDa. Thus, the nonactivated estrogen receptor has a molecular architecture homologous to those of glucocorticoid and progesterone receptors, even though phylogenetically the estrogen receptor gene forms a distinct subgroup within the gene family of nuclear hormone receptors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdella P. M., Smith P. K., Royer G. P. A new cleavable reagent for cross-linking and reversible immobilization of proteins. Biochem Biophys Res Commun. 1979 Apr 13;87(3):734–742. doi: 10.1016/0006-291x(79)92020-5. [DOI] [PubMed] [Google Scholar]
- Ali S., Lutz Y., Bellocq J. P., Chenard-Neu M. P., Rouyer N., Metzger D. Production and characterization of monoclonal antibodies recognising defined regions of the human oestrogen receptor. Hybridoma. 1993 Aug;12(4):391–405. doi: 10.1089/hyb.1993.12.391. [DOI] [PubMed] [Google Scholar]
- Amero S. A., Kretsinger R. H., Moncrief N. D., Yamamoto K. R., Pearson W. R. The origin of nuclear receptor proteins: a single precursor distinct from other transcription factors. Mol Endocrinol. 1992 Jan;6(1):3–7. doi: 10.1210/mend.6.1.1738368. [DOI] [PubMed] [Google Scholar]
- Atrache V., Ratajczak T., Senafi S., Hähnel R. Purification of the molybdate-stabilized 9-10 S estradiol receptor from calf uterus. J Biol Chem. 1985 May 25;260(10):5936–5941. [PubMed] [Google Scholar]
- Berthois Y., Katzenellenbogen J. A., Katzenellenbogen B. S. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czar M. J., Owens-Grillo J. K., Dittmar K. D., Hutchison K. A., Zacharek A. M., Leach K. L., Deibel M. R., Jr, Pratt W. B. Characterization of the protein-protein interactions determining the heat shock protein (hsp90.hsp70.hsp56) heterocomplex. J Biol Chem. 1994 Apr 15;269(15):11155–11161. [PubMed] [Google Scholar]
- Eckert R. L., Katzenellenbogen B. S. Physical properties of estrogen receptor complexes in MCF-7 human breast cancer cells. Differences with anti-estrogen and estrogen. J Biol Chem. 1982 Aug 10;257(15):8840–8846. [PubMed] [Google Scholar]
- Fawell S. E., Lees J. A., White R., Parker M. G. Characterization and colocalization of steroid binding and dimerization activities in the mouse estrogen receptor. Cell. 1990 Mar 23;60(6):953–962. doi: 10.1016/0092-8674(90)90343-d. [DOI] [PubMed] [Google Scholar]
- Gehring U., Mugele K., Arndt H., Busch W. Subunit dissociation and activation of wild-type and mutant glucocorticoid receptors. Mol Cell Endocrinol. 1987 Sep;53(1-2):33–44. doi: 10.1016/0303-7207(87)90189-4. [DOI] [PubMed] [Google Scholar]
- Hearn M. T., Bethell G. S., Ayers J. S., Hancock W. S. Application of 1,1'-carbonyldiimidazole-activated agarose for the purification of proteins. II. The use of an activated matrix devoid of additional charged groups for the purification of thyroid proteins. J Chromatogr. 1979 Dec 20;185:463–470. doi: 10.1016/s0021-9673(00)85622-8. [DOI] [PubMed] [Google Scholar]
- Hutchison K. A., Dittmar K. D., Czar M. J., Pratt W. B. Proof that hsp70 is required for assembly of the glucocorticoid receptor into a heterocomplex with hsp90. J Biol Chem. 1994 Feb 18;269(7):5043–5049. [PubMed] [Google Scholar]
- Katzenellenbogen J. A., Carlson K. E., Heiman D. F., Robertson D. W., Wei L. L., Katzenellenbogen B. S. Efficient and highly selective covalent labeling of the estrogen receptor with [3H]tamoxifen aziridine. J Biol Chem. 1983 Mar 25;258(6):3487–3495. [PubMed] [Google Scholar]
- King W. J., Greene G. L. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984 Feb 23;307(5953):745–747. doi: 10.1038/307745a0. [DOI] [PubMed] [Google Scholar]
- Laudet V., Hänni C., Coll J., Catzeflis F., Stéhelin D. Evolution of the nuclear receptor gene superfamily. EMBO J. 1992 Mar;11(3):1003–1013. doi: 10.1002/j.1460-2075.1992.tb05139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncharmont B., Anderson W. L., Rosenberg B., Parikh I. Interaction of estrogen receptor of calf uterus with a monoclonal antibody: probing of various molecular forms. Biochemistry. 1984 Aug 14;23(17):3907–3912. doi: 10.1021/bi00312a018. [DOI] [PubMed] [Google Scholar]
- Murayama A., Fukai F., Yamamoto T. Estrogen receptor of cow uterus. III. Molecular constitution of estrogen receptor-binding factors. J Biochem. 1980 Oct;88(4):969–976. doi: 10.1093/oxfordjournals.jbchem.a133085. [DOI] [PubMed] [Google Scholar]
- Parker M. G. Steroid and related receptors. Curr Opin Cell Biol. 1993 Jun;5(3):499–504. doi: 10.1016/0955-0674(93)90016-j. [DOI] [PubMed] [Google Scholar]
- Pratt W. B., Czar M. J., Stancato L. F., Owens J. K. The hsp56 immunophilin component of steroid receptor heterocomplexes: could this be the elusive nuclear localization signal-binding protein? J Steroid Biochem Mol Biol. 1993 Sep;46(3):269–279. doi: 10.1016/0960-0760(93)90216-j. [DOI] [PubMed] [Google Scholar]
- Pratt W. B. The role of heat shock proteins in regulating the function, folding, and trafficking of the glucocorticoid receptor. J Biol Chem. 1993 Oct 15;268(29):21455–21458. [PubMed] [Google Scholar]
- Ratajczak T., Carrello A., Mark P. J., Warner B. J., Simpson R. J., Moritz R. L., House A. K. The cyclophilin component of the unactivated estrogen receptor contains a tetratricopeptide repeat domain and shares identity with p59 (FKBP59). J Biol Chem. 1993 Jun 25;268(18):13187–13192. [PubMed] [Google Scholar]
- Ratajczak T., Hlaing J., Brockway M. J., Hähnel R. Isolation of untransformed bovine estrogen receptor without molybdate stabilization. J Steroid Biochem. 1990 Apr;35(5):543–553. doi: 10.1016/0022-4731(90)90197-z. [DOI] [PubMed] [Google Scholar]
- Redeuilh G., Moncharmont B., Secco C., Baulieu E. E. Subunit composition of the molybdate-stabilized "8-9 S" nontransformed estradiol receptor purified from calf uterus. J Biol Chem. 1987 May 25;262(15):6969–6975. [PubMed] [Google Scholar]
- Rehberger P., Rexin M., Gehring U. Heterotetrameric structure of the human progesterone receptor. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8001–8005. doi: 10.1073/pnas.89.17.8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renoir J. M., Radanyi C., Faber L. E., Baulieu E. E. The non-DNA-binding heterooligomeric form of mammalian steroid hormone receptors contains a hsp90-bound 59-kilodalton protein. J Biol Chem. 1990 Jun 25;265(18):10740–10745. [PubMed] [Google Scholar]
- Rexin M., Busch W., Gehring U. Chemical cross-linking of heteromeric glucocorticoid receptors. Biochemistry. 1988 Jul 26;27(15):5593–5601. doi: 10.1021/bi00415a030. [DOI] [PubMed] [Google Scholar]
- Rexin M., Busch W., Gehring U. Protein components of the nonactivated glucocorticoid receptor. J Biol Chem. 1991 Dec 25;266(36):24601–24605. [PubMed] [Google Scholar]
- Rexin M., Busch W., Segnitz B., Gehring U. Structure of the glucocorticoid receptor in intact cells in the absence of hormone. J Biol Chem. 1992 May 15;267(14):9619–9621. [PubMed] [Google Scholar]
- Rexin M., Busch W., Segnitz B., Gehring U. Tetrameric structure of the nonactivated glucocorticoid receptor in cell extracts and intact cells. FEBS Lett. 1988 Dec 5;241(1-2):234–238. doi: 10.1016/0014-5793(88)81068-8. [DOI] [PubMed] [Google Scholar]
- Riehl R. M., Sullivan W. P., Vroman B. T., Bauer V. J., Pearson G. R., Toft D. O. Immunological evidence that the nonhormone binding component of avian steroid receptors exists in a wide range of tissues and species. Biochemistry. 1985 Nov 5;24(23):6586–6591. doi: 10.1021/bi00344a042. [DOI] [PubMed] [Google Scholar]
- Rossini G. P., Camellini L. Oligomeric structures of cytosoluble estrogen-receptor complexes as studied by anti-estrogen receptor antibodies and chemical crosslinking of intact cells. J Steroid Biochem Mol Biol. 1994 Sep;50(5-6):241–252. doi: 10.1016/0960-0760(94)90128-7. [DOI] [PubMed] [Google Scholar]
- Rossini G. P. The quaternary structures of untransformed steroid hormone receptors: an open issue. J Theor Biol. 1994 Feb 7;166(3):339–353. doi: 10.1006/jtbi.1994.1031. [DOI] [PubMed] [Google Scholar]
- Salomonsson M., Häggblad J., O'Malley B. W., Sitbon G. M. The human estrogen receptor hormone binding domain dimerizes independently of ligand activation. J Steroid Biochem Mol Biol. 1994 Apr;48(5-6):447–452. doi: 10.1016/0960-0760(94)90192-9. [DOI] [PubMed] [Google Scholar]
- Sherman M. R. Physical-chemical analysis of steroid hormone receptors. Methods Enzymol. 1975;36:211–234. doi: 10.1016/s0076-6879(75)36021-7. [DOI] [PubMed] [Google Scholar]
- Smith D. F., Stensgard B. A., Welch W. J., Toft D. O. Assembly of progesterone receptor with heat shock proteins and receptor activation are ATP mediated events. J Biol Chem. 1992 Jan 15;267(2):1350–1356. [PubMed] [Google Scholar]
- Smith D. F., Toft D. O. Steroid receptors and their associated proteins. Mol Endocrinol. 1993 Jan;7(1):4–11. doi: 10.1210/mend.7.1.8446107. [DOI] [PubMed] [Google Scholar]
- Tai P. K., Faber L. E. Isolation of dissimilar components of the 8.5S nonactivated uterine progestin receptor. Can J Biochem Cell Biol. 1985 Jan;63(1):41–49. doi: 10.1139/o85-006. [DOI] [PubMed] [Google Scholar]
- Tai P. K., Maeda Y., Nakao K., Wakim N. G., Duhring J. L., Faber L. E. A 59-kilodalton protein associated with progestin, estrogen, androgen, and glucocorticoid receptors. Biochemistry. 1986 Sep 9;25(18):5269–5275. doi: 10.1021/bi00366a043. [DOI] [PubMed] [Google Scholar]
- Veldscholte J., Berrevoets C. A., Brinkmann A. O., Grootegoed J. A., Mulder E. Anti-androgens and the mutated androgen receptor of LNCaP cells: differential effects on binding affinity, heat-shock protein interaction, and transcription activation. Biochemistry. 1992 Mar 3;31(8):2393–2399. doi: 10.1021/bi00123a026. [DOI] [PubMed] [Google Scholar]
- Welshons W. V., Lieberman M. E., Gorski J. Nuclear localization of unoccupied oestrogen receptors. Nature. 1984 Feb 23;307(5953):747–749. doi: 10.1038/307747a0. [DOI] [PubMed] [Google Scholar]