Abstract
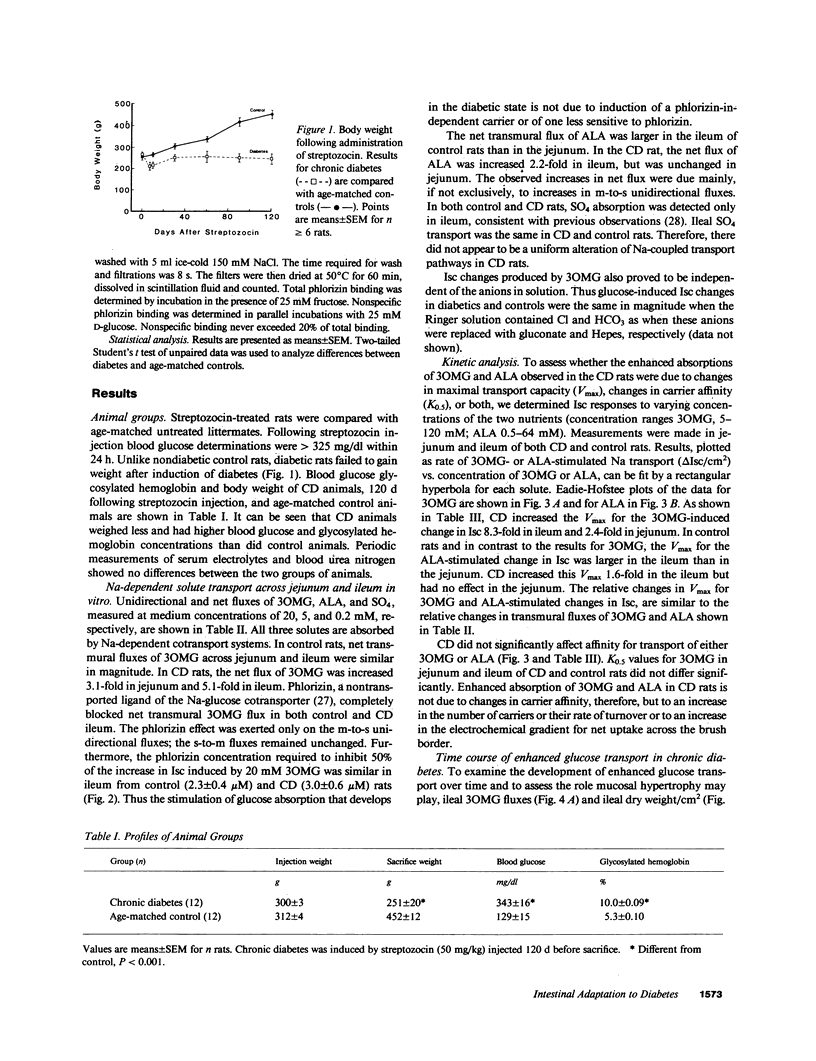
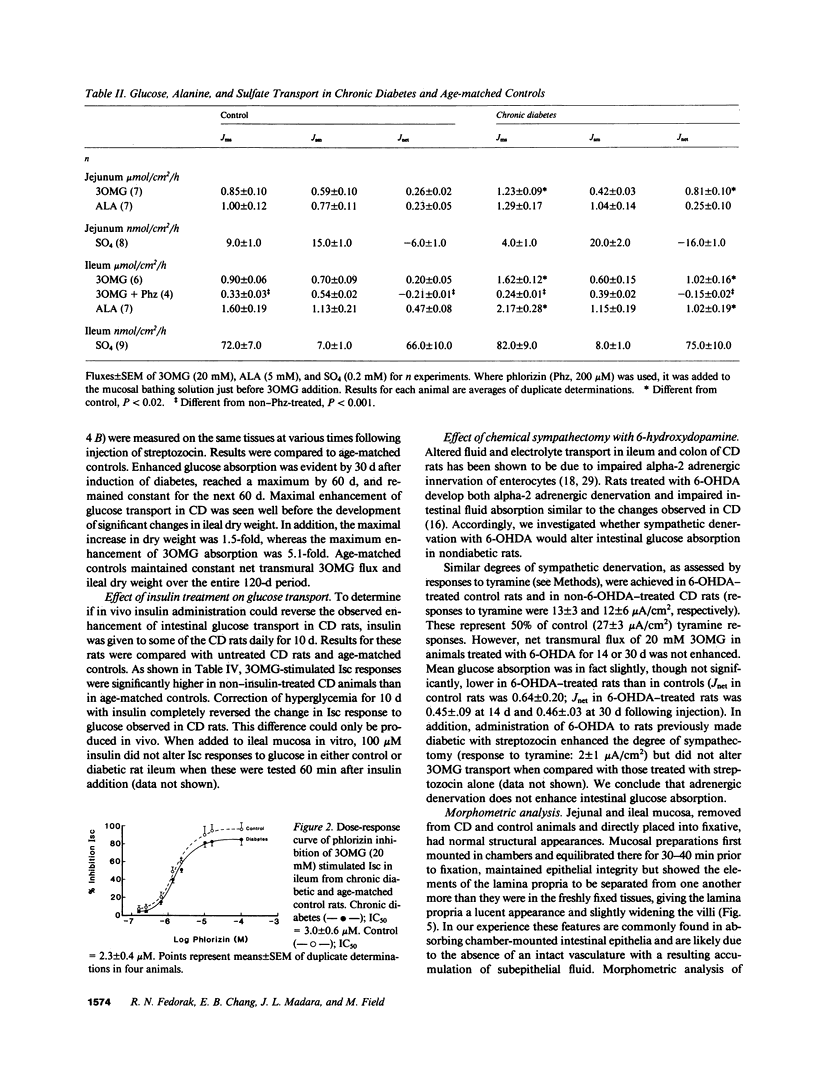
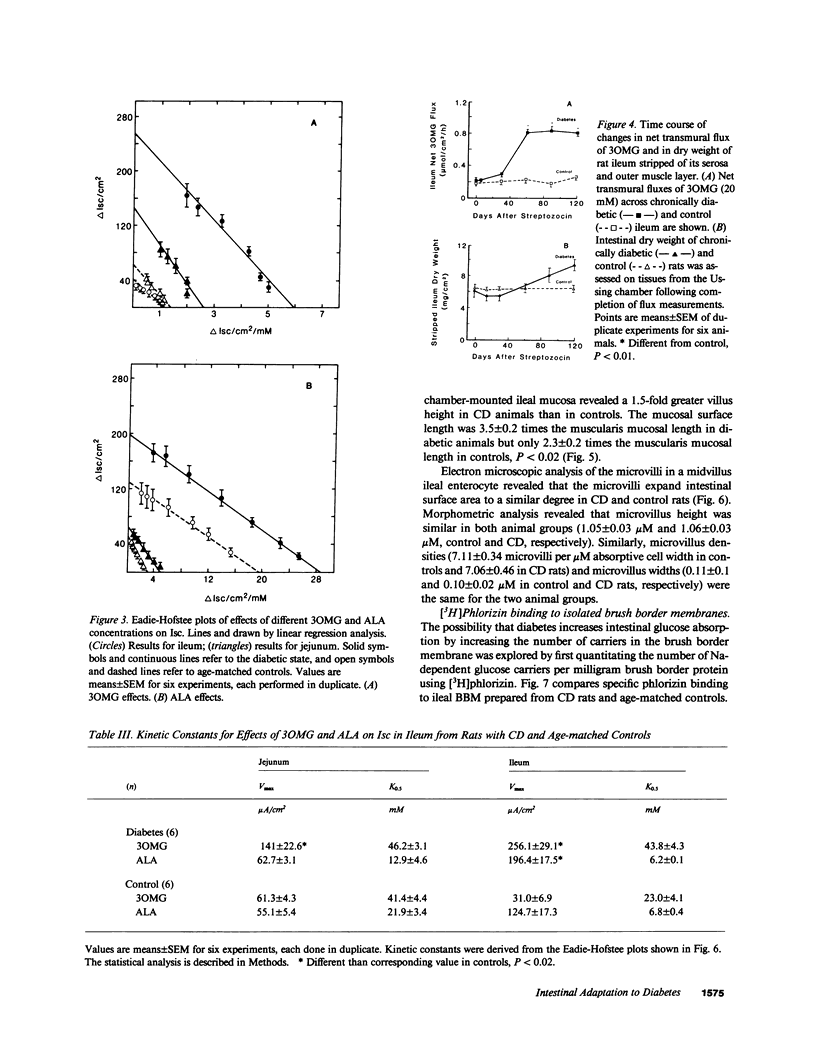
To examine the pattern and mechanisms of enhanced intestinal nutrient absorption in diabetes, we measured intestinal transport of 3-O-methylglucose (3OMG), l-alanine (ALA), and SO4 in male Lewis rats made diabetic with streptozocin. Diabetes enhanced 3OMG absorption fivefold in ileum and threefold in jejunum; ALA absorption increased twofold in ileum but not at all in jejunum; ileal SO4 transport was unaffected. Increases in 3OMG and ALA transport were due solely to increases in maximum velocity. The enhancement of ileal glucose absorption was half-maximal in 40-45 d, could be reversed by 10 d of treatment with insulin and did not result from adrenergic denervation. The density of glucose carriers per milligram brush border protein (measured as [3H]phlorizin binding sites) was not altered but there was a sixfold increase in the number of glucose-inhibitable [3H]phlorizin-binding sites in the intact epithelium. Generalized mucosal hypertrophy accounted for less than 30% of this increase. We conclude that the intestine adapts to streptozocin-induced diabetes through recruitment of additional brush border carriers for sugar, probably in the midvillus-to-crypt region.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brasitus T. A., Dudeja P. K. Correction of abnormal lipid fluidity and composition of rat ileal microvillus membranes in chronic streptozotocin-induced diabetes by insulin therapy. J Biol Chem. 1985 Oct 15;260(23):12405–12409. [PubMed] [Google Scholar]
- Brasitus T. A., Schachter D. Cholesterol biosynthesis and modulation of membrane cholesterol and lipid dynamics in rat intestinal microvillus membranes. Biochemistry. 1982 Apr 27;21(9):2241–2246. doi: 10.1021/bi00538a037. [DOI] [PubMed] [Google Scholar]
- Brasitus T. A., Schachter D., Mamouneas T. G. Functional interactions of lipids and proteins in rat intestinal microvillus membranes. Biochemistry. 1979 Sep 18;18(19):4136–4144. doi: 10.1021/bi00586a013. [DOI] [PubMed] [Google Scholar]
- CRANE R. K. An effect of alloxan-diabetes on the active transport of sugars by rat small intestine, in vitro. Biochem Biophys Res Commun. 1961 Apr 28;4:436–440. doi: 10.1016/0006-291x(61)90304-7. [DOI] [PubMed] [Google Scholar]
- Caspary W. F. Increase of active transport of conjugated bile salts in streptozotocin-diabetic rat small intestine. Gut. 1973 Dec;14(12):949–955. doi: 10.1136/gut.14.12.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E. B., Bergenstal R. M., Field M. Diarrhea in streptozocin-treated rats. Loss of adrenergic regulation of intestinal fluid and electrolyte transport. J Clin Invest. 1985 May;75(5):1666–1670. doi: 10.1172/JCI111874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E. B., Fedorak R. N., Field M. Experimental diabetic diarrhea in rats. Intestinal mucosal denervation hypersensitivity and treatment with clonidine. Gastroenterology. 1986 Sep;91(3):564–569. [PubMed] [Google Scholar]
- Csáky T. Z., Fischer E. Intestinal sugar transport in experimental diabetes. Diabetes. 1981 Jul;30(7):568–574. doi: 10.2337/diab.30.7.568. [DOI] [PubMed] [Google Scholar]
- Field M., Fromm D., McColl I. Ion transport in rabbit ileal mucosa. I. Na and Cl fluxes and short-circuit current. Am J Physiol. 1971 May;220(5):1388–1396. doi: 10.1152/ajplegacy.1971.220.5.1388. [DOI] [PubMed] [Google Scholar]
- Field M., McColl I. Ion transport in rabbit ileal mucosa. 3. Effects of catecholamines. Am J Physiol. 1973 Oct;225(4):852–857. doi: 10.1152/ajplegacy.1973.225.4.852. [DOI] [PubMed] [Google Scholar]
- Flores P., Schedl H. P. Intestinal transport of 3-O-methyl-D-glucose in the normal and alloxan-diabetic rat. Am J Physiol. 1968 Apr;214(4):725–729. doi: 10.1152/ajplegacy.1968.214.4.725. [DOI] [PubMed] [Google Scholar]
- Gabbay K. H., Sosenko J. M., Banuchi G. A., Mininsohn M. J., Flückiger R. Glycosylated hemoglobins: increased glycosylation of hemoglobin A in diabetic patients. Diabetes. 1979 Apr;28(4):337–340. doi: 10.2337/diab.28.4.337. [DOI] [PubMed] [Google Scholar]
- Genel M., London D., Holtzapple P. G., Segal S. Uptake of alpha-methylglucoside by normal and diabetic human jejunal mucosa. J Lab Clin Med. 1971 May;77(5):743–750. [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- Kimmich G. A., Carter-Su C., Randles J. Energetics of Na+-dependent sugar transport by isolated intestinal cells: evidence for a major role for membrane potentials. Am J Physiol. 1977 Nov;233(5):E357–E362. doi: 10.1152/ajpendo.1977.233.5.E357. [DOI] [PubMed] [Google Scholar]
- Kostrzewa R. M., Jacobowitz D. M. Pharmacological actions of 6-hydroxydopamine. Pharmacol Rev. 1974 Sep;26(3):199–288. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lal D., Schedl H. P. Intestinal adaptation in diabetes: amino acid absorption. Am J Physiol. 1974 Oct;227(4):827–831. doi: 10.1152/ajplegacy.1974.227.4.827. [DOI] [PubMed] [Google Scholar]
- Leese H. J., Mansford K. R. The effect of insulin and insulin deficiency on the transport and metabolism of glucose by rat small intestine. J Physiol. 1971 Feb;212(3):819–838. doi: 10.1113/jphysiol.1971.sp009358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lücke H., Stange G., Murer H. Sulfate-sodium cotransport by brush-border membrane vesicles isolated from rat ileum. Gastroenterology. 1981 Jan;80(1):22–30. [PubMed] [Google Scholar]
- Madara J. L., Wolf J. L., Trier J. S. Structural features of the rat small intestinal microvillus membrane in acute experimental diabetes. Dig Dis Sci. 1982 Sep;27(9):801–806. doi: 10.1007/BF01391373. [DOI] [PubMed] [Google Scholar]
- Miazza B. M., Al-Mukhtar M. Y., Salmeron M., Ghatei M. A., Felce-Dachez M., Filali A., Villet R., Wright N. A., Bloom S. R., Crambaud J. C. Hyperenteroglucagonaemia and small intestinal mucosal growth after colonic perfusion of glucose in rats. Gut. 1985 May;26(5):518–524. doi: 10.1136/gut.26.5.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. L., Hanson W., Schedl H. P., Osborne J. W. Proliferation rate and transit time of mucosal cells in small intestine of the diabetic rat. Gastroenterology. 1977 Dec;73(6):1326–1332. [PubMed] [Google Scholar]
- Miller D. L., Schedl H. P. Effects of experimental diabetes on intestinal strontium absorption in the rat. Proc Soc Exp Biol Med. 1976 Sep;152(4):589–592. doi: 10.3181/00379727-152-39446. [DOI] [PubMed] [Google Scholar]
- O'Grady S. M., Musch M. W., Field M. Stoichiometry and ion affinities of the Na-K-Cl cotransport system in the intestine of the winter flounder (Pseudopleuronectes americanus). J Membr Biol. 1986;91(1):33–41. doi: 10.1007/BF01870212. [DOI] [PubMed] [Google Scholar]
- Olsen W. A., Rosenberg I. H. Intestinal transport of sugars and amino acids in diabetic rats. J Clin Invest. 1970 Jan;49(1):96–105. doi: 10.1172/JCI106227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl H. P., Wilson H. D. Effects of diabetes on intestinal growth and hexose transport in the rat. Am J Physiol. 1971 Jun;220(6):1739–1745. doi: 10.1152/ajplegacy.1971.220.6.1739. [DOI] [PubMed] [Google Scholar]
- Schedl H. P., Wilson H. D. Effects of diabetes on intestinal growth in the rat. J Exp Zool. 1971 Apr;176(4):487–495. doi: 10.1002/jez.1401760410. [DOI] [PubMed] [Google Scholar]
- Semenza G., Kessler M., Hosang M., Weber J., Schmidt U. Biochemistry of the Na+, D-glucose cotransporter of the small-intestinal brush-border membrane. The state of the art in 1984. Biochim Biophys Acta. 1984 Sep 3;779(3):343–379. doi: 10.1016/0304-4157(84)90016-9. [DOI] [PubMed] [Google Scholar]
- Smith P. L., Orellana S. A., Field M. Active sulfate absorption in rabbit ileum: dependence on sodium and chloride and effects of agents that alter chloride transport. J Membr Biol. 1981;63(3):199–206. doi: 10.1007/BF01870981. [DOI] [PubMed] [Google Scholar]
- Tannenbaum C., Toggenburger G., Kessler M., Rothstein A., Semenza G. High-affinity phlorizin binding to brush border membranes from small intestine: identity with (a part of) the glucose transport system, dependence on Na +-gradient, partial purification. J Supramol Struct. 1977;6(4):519–533. doi: 10.1002/jss.400060406. [DOI] [PubMed] [Google Scholar]
- Thompson S. M., Dawson D. C. Sodium uptake across the apical border of the isolated turtle colon: confirmation of the two-barrier model. J Membr Biol. 1978 Sep 25;42(4):357–374. doi: 10.1007/BF01870356. [DOI] [PubMed] [Google Scholar]
- Thomson A. B., Rajotte R. Effect of dietary modification on the uptake of glucose, fatty acids, and alcohols in diabetic rats. Am J Clin Nutr. 1983 Sep;38(3):394–403. doi: 10.1093/ajcn/38.3.394. [DOI] [PubMed] [Google Scholar]
- Thomson A. B. Uptake of glucose into the intestine of diabetic rats: effects of variations in the effective resistance of the unstirred water layer. Diabetes. 1981 Mar;30(3):247–255. doi: 10.2337/diab.30.3.247. [DOI] [PubMed] [Google Scholar]
- Toggenburger G., Kessler M., Rothstein A., Semenza G., Tannenbaum C. Similarity in effects of Na+ gradients and membrane potentials on D-glucose transport by, and phlorizin binding to, vesicles derived from brush borders of rattit intestinal mucosal cells. J Membr Biol. 1978 May 3;40(3):269–290. doi: 10.1007/BF02002972. [DOI] [PubMed] [Google Scholar]
- Toggenburger G., Kessler M., Semenza G. Phlorizin as a probe of the small-intestinal Na+,D-glucose cotransporter. A model. Biochim Biophys Acta. 1982 Jun 14;688(2):557–571. doi: 10.1016/0005-2736(82)90367-4. [DOI] [PubMed] [Google Scholar]





