Driven by the limitations of traditional approaches to treating depression, there has recently been a surge of studies examining the utility of various noninvasive neuromodulation technologies in the treatment of depression. In this issue, Rohan et al [1] report substantial improvement in mood immediately following one twenty-minute treatment application of low-field magnetic stimulation (LFMS), performed with a novel portable tabletop device. The stimulation paradigm they utilize consists of a 1 kHz oscillating magnetic field, adapted from the component of the MRI protocol that they previously serendipitously found to have beneficial mood effects [2]. In the current study, LFMS was applied in a double-blind, sham-controlled design to a heterogeneous group of 63 patients with either bipolar depression or major depressive disorder, and effects on mood were assessed primarily using a self-rated visual analog scales (VAS) and observer-rated Hamilton depression rating scale (HDRS-17). The authors found that real LFMS produced an immediate improvement on several scales across the combined population of depressed patients as compared to sham. Although they must be interpreted with caution and much additional work is necessary before the clinical utility of the approach can be determined, these results are highly intriguing.
A particularly striking aspect of the LFMS effect is that a mood-elevation was found immediately after one brief treatment. Psychiatric treatments, including the neuromodulatory gold standard of electroconvulsive therapy [3], generally show much slower onset of effect, typically requiring weeks before separating from placebo in sham-controlled clinical trials. While ketamine has been shown to have a rapid antidepressant effect within twenty-four hours [4], durability and clinical utility need further elucidation. Rohan et al. [1] were able to demonstrate improvement in mood ten to fifteen minutes after completion of the intervention, although whether these effects had any durability could not be determined by their study design. Rapidity of onset can be an essential factor in the clinical realm, where there are few effective treatment options available to rapidly assist the high-risk acutely suicidal patient. The LFMS approach features other notable strengths, including a completely non-invasive approach with no known adverse effects. The absence of any physical sensation with stimulation enables fairly robust blinding, of benefit for future trials. The device is also small and portable, thus enabling potential future home use, and utilizes technology and physical properties that are relatively well known.
However, there are a number of unanswered questions that cloud an assessment of the clinical significance of the present results. Most importantly, the study was not designed to measure the durability of mood improvement. Mood effects were evaluated immediately (10 to 15 minutes) after the intervention, but there were no subsequent assessments to see if the effects persisted for any meaningful period of time. A related concern is the unclear validity and reliability of the outcome measures over such short periods of time. For example, one of the two primary outcome measures, the HDRS-17, requires the clinician to assess the patient’s symptoms of depression over the past week; it is difficult to know what reported changes in these measures mean when they are assessed less than an hour apart.
Another concern is the relatively small size and heterogeneity of the tested population. The authors included patients with bipolar disorder as well as major depressive disorder. Given that the underlying psychopathologies and the pharmacologic treatments in these two populations are distinct, combining these populations into a single sample may confound the data in unclear ways. A related concern is the heterogeneity of the results between the different subpopulations; a significant benefit over sham was seen in the VAS in the MDD but not the BPD subjects, whereas the opposite was observed on the PANAS-PA, and significant effects were not present in either subpopulation (but were present across all subjects) in the HDRS. This variability underscores the need for reliable assessments in larger, more homogenous populations.
Neuromodulation based on electromagnetic induction has become broadly accepted in psychiatric therapeutics thanks to the Food and Drug Administration’s approval in 2008 of the Neuronetics device, and more recently in 2013 of the Brainsway device for the treatment of medication-resistant depression. In the meantime, over 500 TMS devices are in operation in the US alone. Alternative approaches are also being explored including EEG-synchronized transcranial magnetic stimulation (sTMS). A pilot study of this intervention showed that subjects with either fixed or random frequency sTMS had significantly greater reductions in depression severity than those receiving sham [5]. Like LFMS, sTMS is delivered with a portable device and the tolerability was outstanding. In addition, TMS is being actively investigated for a growing number of neurologic disorders [6].
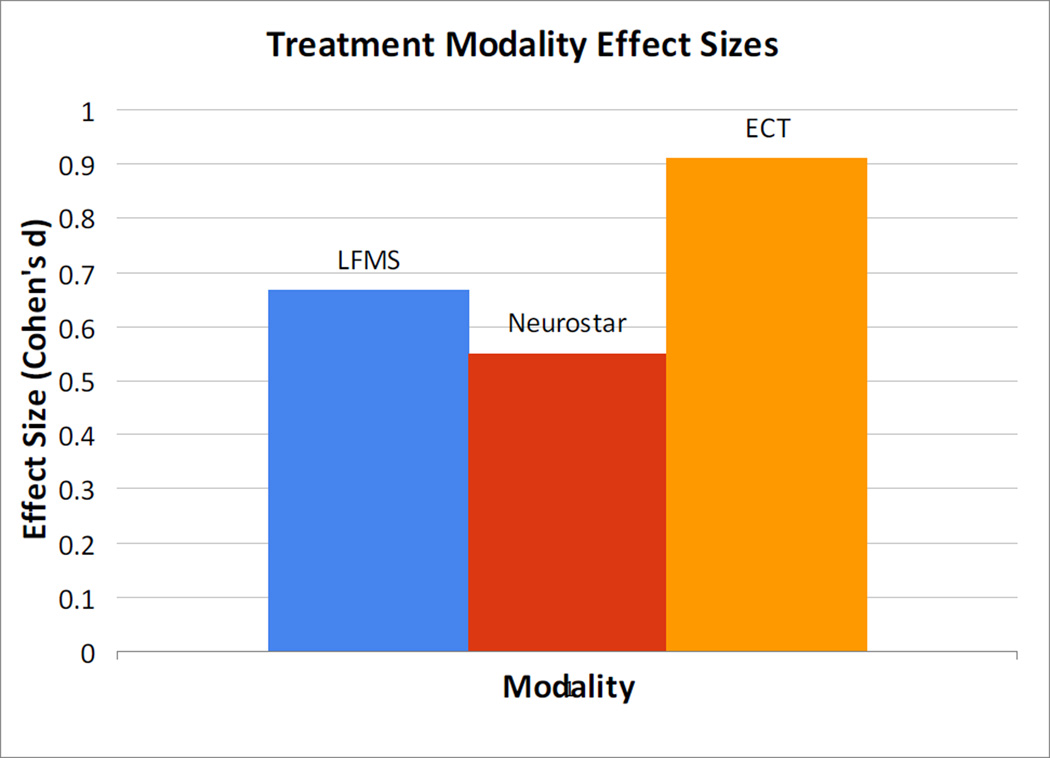
How can we think about the LFMS findings in the larger context of the expanding field of noninvasive neuromodulation? LFMS induces electric fields that are of significantly lower strength (<1 V/m) as compared to more established forms of electromagnetic stimulation (≥100 V/M in electroconvulsive therapy (ECT), deep brain stimulation (DBS) and rTMS, in which the electric field at the target site is of sufficient magnitude to directly induce neuronal depolarization [7]. In contrast to LFMS, DBS and TMS have a more focal field of stimulation and are aiming to target specific neural networks. As a result, the mechanism by which LFMS could be exerting a behaviorally relevant effect is highly undefined. The authors suggest that the effect seen from LFMS may stem from changes in membrane potential in the dendritic cortex in layers 5 and 6, which project to limbic and other subcortical regions. What is evident, however, is that the device produces a global electric field that is likely affecting a wide array of cortical brain structures. With this widespread approach, it is also unclear if specific neuroanatomical structures or functional neural networks may be implicated in the observed behavioral effect. In the absence of a putative neural substrate for the observed effect, moving directly to large-scale clinical trials may be risky, and in conflict with recent NIMH directives calling for assessments of engagement of a defined neurobiologic target or mediator. Further investigation into the potential mechanisms of action of this modality could better inform the approach, and also potentially allow for experimental optimization of parameters to maximize any potential behavioral effect.
The consideration of low strength magnetic fields for modulation of biological activity is not new. A recent Cochrane review of electromagnetic fields used in the treatment of osteoarthritis showed that electromagnetic field treatment may provide moderate benefit with regard to pain relief [8]. Low frequency electromagnetic fields have also been shown to have anti-inflammatory and neo-angiogenic effects which can contribute to wound healing [9]. The biological effects of magnetic fields are diverse, and the potential applications for this approach are therefore quite broad and largely undefined.
Despite all of the uncertainties, the results described by Rohan et al [1] -- in particular the rapidity of the response -- are highly intriguing and add a novel paradigm to the repertoire of neuromodulation techniques that may have therapeutic utility in neuropsychiatric diseases. It is unclear at this time if these approaches achieve their antidepressant effects through a common mechanism, or whether their approaches may in some ways be complementary. The benefit from combining neuromodulation techniques with conventional behavioral and/or pharmacologic therapy is also an area that needs further exploration. Future research should further also be directed towards an evaluation of the neural substrates and functional networks modulated by these different techniques, optimization of the stimulation parameters to maximize the clinical effect, and an assessment of how these various therapeutic modalities can be integrated together.
If the results described in this study are replicated in larger studies, and the effects are shown to be durable, LFMS would be a welcome addition to the clinical armamentarium in the treatment of depression, may find application in other psychiatric and neurologic diseases, and may help to inform and guide us toward future directions in neuromodulation.
Figure 1.
Acknowledgments
Dr. Shafi reports funding support from Harvard Catalyst KL2 (1KL2 TR001100-01).
Footnotes
Financial Disclosures: Dr. Stern reports no biomedical disclosures or potential conflicts of interest. Dr. Pascual-Leone reports that he serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Neuroelectris, and Neosync. He is also listed as an inventor on several issued and pending patents on the real-time integration of TMS with electroencephalography and magnetic resonance imaging.
References
- 1.Rohan ML, Yamamoto RT, Ravichandran CT, et al. Rapid mood-elevating effects of low field magnetic stimulation in depression. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2013.10.024. X:XX-XX. [DOI] [PubMed] [Google Scholar]
- 2.Rohan M, Parow A, Stoll AL, et al. Low-field magnetic stimulation in bipolar depression using an MRI-based stimulator. Am J Psychiatry. 1994;161:93–98. doi: 10.1176/appi.ajp.161.1.93. [DOI] [PubMed] [Google Scholar]
- 3.UK ECT Review Group. Efficacy and Safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361:799–808. doi: 10.1016/S0140-6736(03)12705-5. [DOI] [PubMed] [Google Scholar]
- 4.Lapitus KAB, Levitch CF, Perez AM, et al. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.03.026. In Press; Published online 4/2/2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin Y, Phillips B. A pilot study of the use of EEG-based synchronized Transcranial Magnetic Stimulation (sTMS) for treatment of major depression. BMC Psychiatry. 2014;18:14–13. doi: 10.1186/1471-244X-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shafi MM, Liu A, Fox MD, et al. Transcranial magnetic stimulation in the treatment of neurological disease. Psychiatric Annals. (in press) [Google Scholar]
- 7.Silva S, Basser PJ, Miranda PC. Elucidating the mechanisms and loci of neuronal excitation by transcranial magnetic stimulation using a fine element model of a cortical sulcus. Clin Neurophysiol. 2008;119:2405–2413. doi: 10.1016/j.clinph.2008.07.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li S, Yu B, Zhou D, et al. Electromagnetic fields for treating osteoarthritis. Cochrane Database Syst Rev. 2013;14:12. doi: 10.1002/14651858.CD003523.pub2. CD003523. [DOI] [PubMed] [Google Scholar]
- 9.Costin GE, Birlea SA, Norris DA. Trends in wound repair: cellular and molecular basis of regenerative therapy using electromagnetic fields. Curr Mol Med. 2012;12:14–26. doi: 10.2174/156652412798376143. [DOI] [PubMed] [Google Scholar]
- 10.Slotema CW, Blom JD, Hoek HW, et al. Should we expand the toolbox of psychiatric treatment methods to include Repetitive Transcranial Magnetic Stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry. 2010;71:873–874. doi: 10.4088/JCP.08m04872gre. [DOI] [PubMed] [Google Scholar]