Abstract
The vomeronasal organ (VNO) is a sensory organ that is found in most terrestrial vertebrates and that is principally implicated in the detection of pheromones. The VNO contains specialized sensory neurons organized in a pseudostratified neuroepithelium that recognize chemical signals involved in initiating innate behavioral responses. In rodents, the VNO neuroepithelium is segregated into two distinct zones, apical and basal. The molecular mechanisms involved in ligand detection by apical and basal VNO sensory neurons differ extensively. These two VNO subsystems express different subfamilies of vomeronasal receptors and signaling molecules, detect distinct chemosignals, and project to separate regions of the accessory olfactory bulb (AOB). The roles that these olfactory subdivisions play in the control of specific olfactory-mediated behaviors are largely unclear. However, analysis of mutant mouse lines for signal transduction components together with identification of defined chemosensory ligands has revealed a fundamental role of the basal part of the mouse VNO in mediating a wide range of instinctive behaviors, such as aggression, predator avoidance, and sexual attraction. Here we will compare the divergent functions and synergies between the olfactory subsystems and consider new insights in how higher neural circuits are defined for the initiation of instinctive behaviors.
Keywords: vomeronasal organ, olfaction, Gαo signaling, V2R, peptides, pheromone, behavior
Introduction
The mammalian olfactory system is composed of multiple chemosensory subsystems that differ in anatomical location, receptor types, and innervation within the central nervous system (Munger et al., 2009). The vomeronasal organ (VNO) is the sensory substructure of the accessory olfactory system that is specialized in the identification of specific chemosensory cues important for the display of socio-sexual behaviors. The VNO detects a range of molecules that can be both volatile and non-volatile, including peptides and small proteins. These molecules may be either pheromones secreted externally by conspecifics in urine, tears and saliva, as well as non-pheromones such as those from preys and predators (Wyatt, 2014). During olfactory investigation, chemosignals entering the nasal cavity are pumped into the VNO lumen, where they are detected by vomeronasal sensory neurons (VSNs). The mechanism of pumping consists in a distension-contraction of vascularized erectile tissue located in the lateral side of the VNO lumen (Trotier, 2011). Sympathetic stimulation triggers this vascular pump (Ben-Shaul et al., 2010) during exploratory behaviors, probably as a result of detection of other volatile stimuli by the main olfactory system (Martínez-García et al., 2009). Therefore, vomeronasal activity is unlikely to remain as an autonomous olfactory unit but instead requires intense interaction with other sensory inputs to transduce the stimulus information to downstream targets. Mature VSNs reside in the medial side within a pseudostratified sensory neuroepithelium formed by bipolar neurons, directed to the aqueous lumen, with an extended dendrite and cilia in which detection takes place. The VNO emerges in evolution on amphibians during adaptation to life on land (Trotier, 2011), and is present in many but not all mammals. It is missing in cetaceans, some bats and some primates (Mucignat-Caretta, 2010). In humans it appears to be vestigial (Trotier et al., 2000; Meredith, 2001). In the mouse, the VNO neuroepithelium is divided in non-overlapping apical and basal layers that express two different families of receptors, vomeronasal receptors 1 and 2 (V1Rs and V2Rs), respectively (Dulac and Axel, 1995; Herrada and Dulac, 1997; Matsunami and Buck, 1997; Ryba and Tirindelli, 1997). Furthermore, a subset of neurons in the VNO express five members of the formyl peptide receptor (FPR) family, four of them apical and one basal (Liberles et al., 2009; Riviere et al., 2009). The wiring logic of the VSN projection is maintained at the level of the accessory olfactory bulb (AOB): Apical VSNs connect with glomeruli in the rostral half of the AOB, whereas basal VSNs axons synapse in the caudal AOB. The implications of this neuronal segregation in the accessory olfactory system for the processing of chemosensory information are largely unknown. Moreover, certain species such as the goat, marmoset and tammar wallaby lack this anatomical segregation and display uniform-type vomeronasal epitheliums (Takigami et al., 2000, 2004; Schneider et al., 2012). Recent advances in the identification of subzone-specific ligands and ablation of sensory transduction components have now enabled a more detailed analysis of the molecular mechanisms controlled by each neural VNO pathway to initiate olfactory-mediated behaviors.
Molecular and functional organization of the basal VNO
The spatial segregation in the VNO correlates with the differential expression of two G-protein subunits, Gαi2 and Gαo (Berghard and Buck, 1996; Jia and Halpern, 1996). These G-proteins are the initial step of a phospholipase C (PLC)-mediated signaling cascade to transduce sensory signals detected by V1Rs and V2Rs (Chamero et al., 2012). In the VNO, G-proteins form complexes identified as Gαoβ2γ8 in the basal and Gαi2β2γ2 in the apical neurons (Montani et al., 2013). The functional importance of the G-protein subunits in mediating sensory transduction responses was established by ablating genes in mice. VSNs from Gαo mutants display severe deficits to transduce chemosensory signals that result in a number of behavioral alterations including reduced olfactory-mediated aggression (Chamero et al., 2011). Furthermore, Gαo seems to be critical for the maintenance of the cellular homeostasis in the postnatal sensory neuroepithelium as Gαo mutant mice show a remarkable reduction in the size of the basal neuronal layer (Tanaka et al., 1999). Likewise, mutant mice lacking Gγ8 subunit display a similar cell loss in the VNO epithelium and a diminished aggressive response (Montani et al., 2013). Thus, Gαo and subsequent coupling with Gβ2γ8 represent the key candidate molecules to control PLC activation through specific olfactory stimuli in the basal VNO (Rünnenburger et al., 2002).
PLC activation produces inositol 1,4,5-trisphosphate and diacylglycerol, the only known activator of a member of the transient receptor potential family of ion channels, Trpc2. Trpc2 expressed in both apical and basal VNO layer is another key player in VNO signal transduction (Liman et al., 1999). Genetic ablation of Trpc2 results in dramatic consequences in vomeronasal function in terms of VSNs responsiveness to urinary signals, cell survival, and socio-sexual behavior (Leypold et al., 2002; Stowers et al., 2002; Kimchi et al., 2007; Ferrero et al., 2013; Wu et al., 2014). The Trpc2 gene, initially assumed to be exclusively expressed in the VNO, has been abundantly detected in the main olfactory epithelium (MOE) as well (Omura and Mombaerts, 2014). Therefore, the contribution of MOE-specific Trpc2 signaling to the described behavioral Trpc2 null phenotype remains to be dissected. This may help to explain observed phenotypic discrepancies of Trpc2 deletion (Leypold et al., 2002; Stowers et al., 2002; Kimchi et al., 2007) and surgical VNO removal (Clancy et al., 1984; Wysocki and Lepri, 1991; Pankevich et al., 2004; Martel and Baum, 2009) on ultrasonic vocalizations and sex-specific behaviors in both male and female mice. Additional signaling components expressed in both basal and apical VSNs are the discovered calcium-activated chloride and potassium channels, which seem to participate in the VNO sensory responses (Dibattista et al., 2008; Billig et al., 2011; Kim et al., 2011, 2012). A recent study using deep RNA sequencing identified nearly 800 novel, putative protein-coding, multi-exonic genes expressed in the whole VNO (Ibarra-Soria et al., 2014). Thus, new vomeronasal signaling components are expected to emerge in the near future.
The range and specificity of chemosignals detected by the VNO depend on the expression of particular vomeronasal receptors. Three families of vomeronasal receptor genes have been identified in the mouse VNO: V1Rs, V2Rs (also known as Vmn1rs and Vmn2rs) and Fprs (Tirindelli et al., 2009). VSNs in the basal layer of the VNO express V2Rs as well as a single FPR member, Fpr-rs1. The mouse genome contains 121 functional V2R genes and—curiously—even a larger number (158) of pseudogenes (Young and Trask, 2007). V2Rs evolved independently from V1Rs and differ in the type of chemosignals they detect, to date: peptides/proteins by V2Rs and small organic molecules by V1Rs, and in the expression logic: VSNs expressing V1Rs show a single-receptor type expression whereas basal VSNs expresses one V2R member of the subfamily C, along with an additional V2R gene from subfamily A, B or D in a non-random manner (Figure 1; Martini et al., 2001; Silvotti et al., 2007; Ishii and Mombaerts, 2011). Until now, only a handful of V2Rs have been deorphanized (Table 1) and all of them belong to the subfamily A, which represents nearly 85% of the V2R genes. Furthermore, V2R sequences of inbred mouse strains show high variation in subfamily A1, A5 and A8 while subfamilies B, C and D are highly conserved (Wynn et al., 2012). The importance of V2R subfamily expression for VSN pheromone specificity and detection still needs to be resolved. However, recent evidences suggest that expression of multiple receptors may have a role in the combinatorial activation logic of VSNs by overlapping specificities and concentrations (Leinders-Zufall et al., 2009; Kaur et al., 2014).
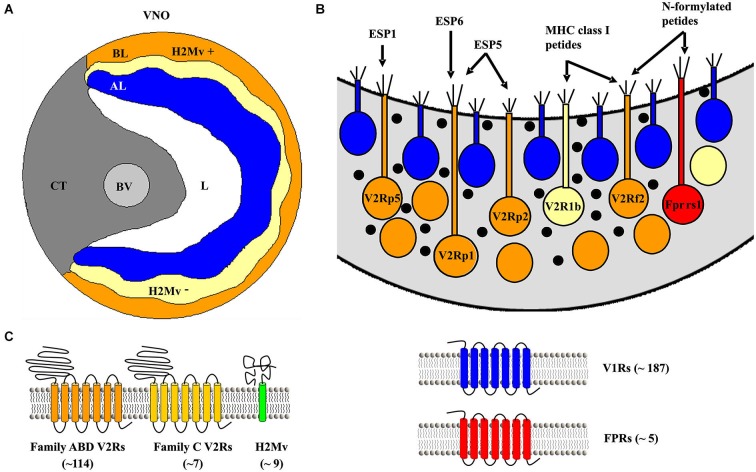
Figure 1.
The mouse vomeronasal organ (VNO) and established receptors located in the epithelium. (A) Schematic coronal section of the VNO. AL, apical layer of the sensory epithelium (blue); BL, basal layer (yellow/orange); BV, blood vessel; CT, cavernous tissue; H2Mv+, sensory epithelium cells lacking one of the nine known H2-Mv genes; H2Mv−, sensory neurons not expressing any of the nine H2-Mv genes; L, lumen. (B) Schematic drawing of sensory neurons showing their location in the basal sensory epithelium with their proposed ligands. (C) Schematic picture of the proposed receptors expressed in the basal part of the VNO sensory epithelium.
Table 1.
List of signaling molecules with proposed receptors located in the basal sensory epithelium.
| Chemosignal | Source | Receptor | Gαo need | Behavioral effects | References |
|---|---|---|---|---|---|
| ESP1 | Male mouse tears | V2Rp5 (Vmn2r116) | ✔ | -Lordosis | Kimoto et al. (2007), Haga et al. (2010) |
| ESP5 | Mouse tears | V2Rp1 (Vmn2r112), V2Rp2 (Vmn2r111) | ? | ? | Kimoto et al. (2007), Dey and Matsunami (2011) |
| ESP6 | Mouse tears | V2Rp1 (Vmn2r112) | ? | ? | Kimoto et al. (2007), Dey and Matsunami (2011) |
| ESP22 | Juvenile mouse tears | ? | ? | -Inhibition of male sexual behavior | Ferrero et al. (2013) |
| HMW/MUPs | Mouse urine | ? | ✔ | -Male-male aggression | Chamero et al. (2007, 2011) |
| -Maternal aggression | Martín-Sánchez et al. (2014) | ||||
| ? | ✔ | -Preference in females | Hurst et al. (2001), Cheetham et al. (2007), Sherborne et al. (2007), Roberts et al. (2010) | ||
| -Urine countermarking behavior | Kaur et al. (2014) | ||||
| -Puberty acceleration | Mucignat-Caretta et al. (1995) | ||||
| -Ovulation | Morè (2006) | ||||
| MUP3 | ? | ✔ | -Male-male aggression -Countermarking |
Kaur et al. (2014) | |
| MUP20 | ? | ✔ | -Attraction in females -Conditioned place preference -Countermarking behavior -Maternal aggression |
Roberts et al. (2010, 2012), Kaur et al. (2014), Martín-Sánchez et al. (2014) | |
| LMW | Mouse urine | ? | * | -Male-male aggression -Maternal aggression |
Chamero et al. (2007, 2011) |
| MHC class I peptides | Mouse urine | V2R1b (Vmn2r26) V2Rf2 (Vmn2r81) | ✔ | -Bruce effect | Leinders-Zufall et al. (2009, 2014) |
| N-formylated peptides | Bacteria or mitochondria | Fpr-rs1, V2Rf2 (Vmn2r81) | ✔ | ? | Liberles et al. (2009), Riviere et al. (2009), Bufe et al. (2012), Leinders-Zufall et al. (2014) |
✔: Gαo expression needed to detect the chemosignal; *: partial detection by both apical and basal layers; ?: not determined; Brackets: alternative receptor names.
In addition to V2R expression, a subset of basal VSNs have been shown to express genes of the major histocompatibility complex (MHC) class 1b, also known as H2Mv molecules (Ishii et al., 2003; Loconto et al., 2003). This family comprises nine genes—M1, M9, M11 and six members of the M10 family—clustered in the genome. Most of the neurons express a single gene, but some seem to be able to express two or three. The proteins localize to the dendritic tips and microvilli of VSNs predicting a potential role in pheromone detection or signal transduction. H2Mv molecules have been proposed to form a protein complex together with V2Rs and β2-microglobulin necessary for the transport of the receptor to the plasma membrane (Loconto et al., 2003). Certainly, H2Mv molecules are dispensable for chemosignal detection but seem to be required to show high sensitivity to peptide ligands necessary for the display of aggressive and sexual behaviors (Leinders-Zufall et al., 2014). A fraction of Gαo-expressing VSNs do not co-express H2-Mv genes, for example sensory neurons expressing the Vmn2r26 receptor (also known as V2r1b), which are localized to the upper sublayer of the basal VNO. This spatial segregation is also maintained at the level of the AOB, defining a tripartite organization of the mouse vomeronasal system (Figure 1; Ishii and Mombaerts, 2008).
A third population of Gαo-expressing VSNs expresses Fpr-rs1 (Figure 1), an additional chemosensory G-protein coupled receptor (GPCR) that belongs to the FPR family (Liberles et al., 2009; Riviere et al., 2009). Fpr-rs1 neurons do not co-express V2Rs or other FPR members. Fpr-rs1 was found to display stereo-selectivity for peptides with a D-amino acid in the C-terminal position, which are contained in pathogenic microorganisms (Bufe et al., 2012). This ligand detection profile raises the possibility of a pathogenic sensing role of the vomeronasal system to assess the health status of conspecifics during social communication.
Sensory ligands detected by the basal VNO
Early functional experiments in the rat vomeronasal sensory epithelium described urine profile activation differences between the apical and basal VNSs (Inamura et al., 1999). Up to now, considerable evidence has shown that sensory neurons located in the basal VNO detect several families of nonvolatile peptide and protein chemosignals. The family of MHC class I peptides were the first identified sensory stimuli for V2R-positive VSNs (Leinders-Zufall et al., 2004). MHC peptides, that have been identified in mouse urine together with some other interesting species-specific peptide ligands (Sturm et al., 2013), are detected by VSNs at ultralow concentrations and require Gαo, but not Trpc2 (Leinders-Zufall et al., 2004; Kelliher et al., 2006; Chamero et al., 2011). Detection of MHC peptide ligands does not require or correlate with the expression of H2Mv molecules. Instead, vomeronasal receptors seem to be essential: genetic deletion experiments showed two V2Rs—Vmn2r26 (V2R1b) and Vmn2r81 (V2Rf2)—which are needed by their VSNs to respond to specific MHC peptides (Leinders-Zufall et al., 2009, 2014). Interestingly, MHC-independent peptides as well as formylated and non-formylated versions of mitochondrial peptides can also activate V2R positive VSNs (Sturm et al., 2013; Leinders-Zufall et al., 2014).
A second family of peptides—the exocrine-gland-secreting peptide (ESP) family—has been identified to be detected by V2R-expressing VSNs. The mouse genome contains 24 members of this family of 5–15 kDa peptides expressed in extraorbital, lacrimal, Harderian, and submaxillary glands (exocrine glands) in a sex- and strain-specific manner (Kimoto et al., 2007). Field potential recordings have shown that at least 16 ESPs elicit electrical responses in the VNO (Kimoto et al., 2007; Haga et al., 2010; Ferrero et al., 2013). Responses to ESP1, 5 and 6 have been linked with expression of a specific V2R subfamily (V2Rp) either by c-Fos activity measures, or by heterologous expression (Haga et al., 2010; Dey and Matsunami, 2011).
A third group of nonvolatile chemosignals functioning as stimuli of the basal VNO layer consist of the major urinary proteins (MUPs) and other related lipocalins. MUPs are abundantly expressed in urine, but are also found in other secretions, including saliva, milk, and even the olfactory epithelium (Ibarra-Soria et al., 2014). In the mouse, MUPs are encoded by a multigene family of 21 homologous, highly identical genes which are expressed in a sex- and strain-dependent manner (Logan et al., 2008; Mudge et al., 2008). MUPs evoke Ca2+ and electrophysiological responses on Gαo- and V2R-expressing VSNs using Trpc2/Gαo signaling (Chamero et al., 2007, 2011) and benefit of the presence of H2Mv molecules (Leinders-Zufall et al., 2014), but specific MUP receptors are yet to be described. Mouse VSNs detect conspecific MUPs utilizing a combinatorial strategy (Kaur et al., 2014) in addition to being activated by orthologous MUP proteins secreted by cats and rats (Papes et al., 2010), adopting a new chemosensory role as interspecific genetically encoded signals.
Behavioral responses
Odor-driven behaviors are reported to depend on the basal VNO layer largely relying on two main criteria: First, as result of gene knockout studies of specific signal transduction molecules or receptors from basal VSNs, and/or second, from experiments using chemosignals shown to (specifically) activate basal VSNs. Aggressive behavior toward intruder males was identified to require sensory transduction from basal VSNs. Ablation of either Gαo, Gγ8, and H2Mv genes severely reduced or eliminated male-male and maternal aggression (Chamero et al., 2011; Montani et al., 2013; Leinders-Zufall et al., 2014), both types of aggression shown to be partially elicited by MUPs (Chamero et al., 2007, 2011; Kaur et al., 2014). Gαo gene removal also resulted in a wide range of deficient reproductive behaviors in female mice, including defective puberty acceleration (Vandenbergh effect) and estrus induction (Whitten effect) in adult mice (Oboti et al., 2014). The identities of the pheromones that underlie the Vandenbergh and Whitten effects are still controversial. Molecules that activate either apical (Jemiolo et al., 1986; Novotny et al., 1999) and basal (Nishimura et al., 1989; Mucignat-Caretta et al., 1995; Morè, 2006) VSNs have been described to participate in these estrus-modulating effects. Nonetheless, it cannot be excluded that multiple olfactory subsystems are required to evoke certain behavioral responses triggered by odorant blends. Consistent with this view, apical and basal VNO subsystems are necessary and seem to interact in the generation of male and female aggression (Del Punta et al., 2002; Norlin et al., 2003; Chamero et al., 2011). In contrast, other pheromone-induced behavioral responses are controlled by single VNO receptor-ligand interactions. The sexual stance lordosis is enhanced by the tear peptide ESP1 that activates Vmn2r116 receptor, and mutant animals lacking this receptor display a striking lordosis deficit (Haga et al., 2010). Consistent with these experiments, surgical lesions on the VNO and AOB (Keller et al., 2006; Martel and Baum, 2009) as well as deletion of Gαo and H2Mv genes (Leinders-Zufall et al., 2014; Oboti et al., 2014) also resulted in a drastic reduction of lordosis. Another member of the ESP peptide family has been implicated in the control of a different type of sexual behavior: ESP22, expressed in tears of prepubertal mice, was found to elicit a Trpc2-dependent inhibitory effect on adult male mating behavior (Ferrero et al., 2013).
MHC peptides have been shown to alter female reproductive function as detected in the Bruce effect test (Leinders-Zufall et al., 2004). Here, pregnancy is terminated in a recently mated female by the odor of a strange male. This test is frequently used as paradigm to assess genetic compatibility and individual recognition. MUPs are also proposed to operate as olfactory cues governing individual recognition, as they are genetically encoded and highly polymorphic (Cheetham et al., 2007). Hence, MUPs have been reported to mediate inbreeding avoidance, countermarking and female sexual attraction (Hurst et al., 2001; Sherborne et al., 2007; Roberts et al., 2010; Kaur et al., 2014). Related to this recognition capacity, MUP detection also plays an important role on aggression, conditioned learned spatial preference and detection of predators (Chamero et al., 2007, 2011; Papes et al., 2010; Roberts et al., 2012). Remarkably, single MUP ligands are able to evoke multiple behavioral responses depending on the gender and reproductive status of the receiving individual; MUP20—also known as darcin—may elicit sexual attraction and spatial learning in estrous females, maternal aggression in lactating females, and countermarking and aggression in adult males (Roberts et al., 2010, 2012; Kaur et al., 2014; Martín-Sánchez et al., 2014). Whether these responses are mediated by a single or multiple sensory neurons or receptor types remain to be elucidated.
These recent advances in the identification of specialized receptors, neural pathways and sensory ligands from the basal VNO layer provide the tools to stimulate, study, and determine the molecular mechanisms that trigger specific behavioral responses.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (CH 920/2-1), HOMFORexzellent (Pablo Chamero) and the VolkswagenFoundation (Trese Leinders-Zufall). Trese Leinders-Zufall is a Lichtenberg Professor of the Volkswagen Foundation.
References
- Ben-Shaul Y., Katz L. C., Mooney R., Dulac C. (2010). In vivo vomeronasal stimulation reveals sensory encoding of conspecific and allospecific cues by the mouse accessory olfactory bulb. Proc. Natl. Acad. Sci. U S A 107, 5172–5177. 10.1073/pnas.0915147107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghard A., Buck L. B. (1996). Sensory transduction in vomeronasal neurons: evidence for G alpha o, G alpha i2 and adenylyl cyclase II as major components of a pheromone signaling cascade. J. Neurosci. 16, 909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billig G. M., Pál B., Fidzinski P., Jentsch T. J. (2011). Ca2+-activated Cl− currents are dispensable for olfaction. Nat. Neurosci. 14, 763–769. 10.1038/nn.2821 [DOI] [PubMed] [Google Scholar]
- Bufe B., Schumann T., Zufall F. (2012). Formyl peptide receptors from immune and vomeronasal system exhibit distinct agonist properties. J. Biol. Chem. 287, 33644–33655. 10.1074/jbc.m112.375774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamero P., Katsoulidou V., Hendrix P., Bufe B., Roberts R., Matsunami H., et al. (2011). G protein G(alpha)o is essential for vomeronasal function and aggressive behavior in mice. Proc. Natl. Acad. Sci. U S A 108, 12898–12903. 10.1073/pnas.1107770108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamero P., Leinders-Zufall T., Zufall F. (2012). From genes to social communication: molecular sensing by the vomeronasal organ. Trends Neurosci. 35, 597–606. 10.1016/j.tins.2012.04.011 [DOI] [PubMed] [Google Scholar]
- Chamero P., Marton T. F., Logan D. W., Flanagan K., Cruz J. R., Saghatelian A., et al. (2007). Identification of protein pheromones that promote aggressive behaviour. Nature 450, 899–902. 10.1038/nature05997 [DOI] [PubMed] [Google Scholar]
- Cheetham S. A., Thom M. D., Jury F., Ollier W. E., Beynon R. J., Hurst J. L. (2007). The genetic basis of individual-recognition signals in the mouse. Curr. Biol. 17, 1771–1777. 10.1016/j.cub.2007.10.007 [DOI] [PubMed] [Google Scholar]
- Clancy A. N., Coquelin A., Macrides F., Gorski R. A., Noble E. P. (1984). Sexual behavior and aggression in male mice: involvement of the vomeronasal system. J. Neurosci. 4, 2222–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Punta K., Leinders-Zufall T., Rodriguez I., Jukam D., Wysocki C. J., Ogawa S., et al. (2002). Deficient pheromone responses in mice lacking a cluster of vomeronasal receptor genes. Nature 419, 70–74. 10.1038/nature00955 [DOI] [PubMed] [Google Scholar]
- Dey S., Matsunami H. (2011). Calreticulin chaperones regulate functional expression of vomeronasal type 2 pheromone receptors. Proc. Natl. Acad. Sci. U S A 108, 16651–16656. 10.1073/pnas.1018140108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibattista M., Mazzatenta A., Grassi F., Tirindelli R., Menini A. (2008). Hyperpolarization-activated cyclic nucleotide-gated channels in mouse vomeronasal sensory neurons. J. Neurophysiol. 100, 576–586. 10.1152/jn.90263.2008 [DOI] [PubMed] [Google Scholar]
- Dulac C., Axel R. (1995). A novel family of genes encoding putative pheromone receptors in mammals. Cell 83, 195–206. 10.1016/0092-8674(95)90161-2 [DOI] [PubMed] [Google Scholar]
- Ferrero D. M., Moeller L. M., Osakada T., Horio N., Li Q., Roy D. S., et al. (2013). A juvenile mouse pheromone inhibits sexual behaviour through the vomeronasal system. Nature 502, 368–371. 10.1038/nature12579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga S., Hattori T., Sato T., Sato K., Matsuda S., Kobayakawa R., et al. (2010). The male mouse pheromone ESP1 enhances female sexual receptive behaviour through a specific vomeronasal receptor. Nature 466, 118–122. 10.1038/nature09142 [DOI] [PubMed] [Google Scholar]
- Herrada G., Dulac C. (1997). A novel family of putative pheromone receptors in mammals with a topographically organized and sexually dimorphic distribution. Cell 90, 763–773. 10.1016/s0092-8674(00)80536-x [DOI] [PubMed] [Google Scholar]
- Hurst J. L., Payne C. E., Nevison C. M., Marie A. D., Humphries R. E., Robertson D. H., et al. (2001). Individual recognition in mice mediated by major urinary proteins. Nature 414, 631–634. 10.1038/414631a [DOI] [PubMed] [Google Scholar]
- Ibarra-Soria X., Levitin M. O., Saraiva L. R., Logan D. W. (2014). The olfactory transcriptomes of mice. PLoS Genet. 10:e1004593. 10.1371/journal.pgen.1004593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamura K., Matsumoto Y., Kashiwayanagi M., Kurihara K. (1999). Laminar distribution of pheromone-receptive neurons in rat vomeronasal epithelium. J. Physiol. 517(Pt. 3), 731–739. 10.1111/j.1469-7793.1999.0731s.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T., Hirota J., Mombaerts P. (2003). Combinatorial coexpression of neural and immune multigene families in mouse vomeronasal sensory neurons. Curr. Biol. 13, 394–400. 10.1016/s0960-9822(03)00092-7 [DOI] [PubMed] [Google Scholar]
- Ishii T., Mombaerts P. (2008). Expression of nonclassical class I major histocompatibility genes defines a tripartite organization of the mouse vomeronasal system. J. Neurosci. 28, 2332–2341. 10.1523/jneurosci.4807-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T., Mombaerts P. (2011). Coordinated coexpression of two vomeronasal receptor V2R genes per neuron in the mouse. Mol. Cell. Neurosci. 46, 397–408. 10.1016/j.mcn.2010.11.002 [DOI] [PubMed] [Google Scholar]
- Jemiolo B., Harvey S., Novotny M. (1986). Promotion of the Whitten effect in female mice by synthetic analogs of male urinary constituents. Proc. Natl. Acad. Sci. U S A 83, 4576–4579. 10.1073/pnas.83.12.4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia C., Halpern M. (1996). Subclasses of vomeronasal receptor neurons: differential expression of G proteins (Gi alpha 2 and G(o alpha)) and segregated projections to the accessory olfactory bulb. Brain Res. 719, 117–128. 10.1016/0006-8993(96)00110-2 [DOI] [PubMed] [Google Scholar]
- Kaur A. W., Ackels T., Kuo T.-H., Cichy A., Dey S., Hays C., et al. (2014). Murine pheromone proteins constitute a context-dependent combinatorial code governing multiple social behaviors. Cell 157, 676–688. 10.1016/j.cell.2014.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M., Pierman S., Douhard Q., Baum M. J., Bakker J. (2006). The vomeronasal organ is required for the expression of lordosis behaviour, but not sex discrimination in female mice. Eur. J. Neurosci. 23, 521–530. 10.1111/j.1460-9568.2005.04589.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher K. R., Spehr M., Li X. H., Zufall F., Leinders-Zufall T. (2006). Pheromonal recognition memory induced by TRPC2-independent vomeronasal sensing. Eur. J. Neurosci. 23, 3385–3390. 10.1111/j.1460-9568.2006.04866.x [DOI] [PubMed] [Google Scholar]
- Kim S., Ma L., Jensen K. L., Kim M. M., Bond C. T., Adelman J. P., et al. (2012). Paradoxical contribution of SK3 and GIRK channels to the activation of mouse vomeronasal organ. Nat. Neurosci. 15, 1236–1244. 10.1038/nn.3173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Ma L., Yu C. R. (2011). Requirement of calcium-activated chloride channels in the activation of mouse vomeronasal neurons. Nat. Commun. 2:365. 10.1038/ncomms1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi T., Xu J., Dulac C. (2007). A functional circuit underlying male sexual behaviour in the female mouse brain. Nature 448, 1009–1014. 10.1038/nature06089 [DOI] [PubMed] [Google Scholar]
- Kimoto H., Sato K., Nodari F., Haga S., Holy T. E., Touhara K. (2007). Sex- and strain-specific expression and vomeronasal activity of mouse ESP family peptides. Curr. Biol. 17, 1879–1884. 10.1016/j.cub.2007.09.042 [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T., Brennan P., Widmayer P., S P. C., Maul-Pavicic A., Jager M., et al. (2004). MHC class I peptides as chemosensory signals in the vomeronasal organ. Science 306, 1033–1037. 10.1126/science.1102818 [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T., Ishii T., Chamero P., Hendrix P., Oboti L., Schmid A., et al. (2014). A family of nonclassical class I MHC genes contributes to ultrasensitive chemodetection by mouse vomeronasal sensory neurons. J. Neurosci. 34, 5121–5133. 10.1523/jneurosci.0186-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinders-Zufall T., Ishii T., Mombaerts P., Zufall F., Boehm T. (2009). Structural requirements for the activation of vomeronasal sensory neurons by MHC peptides. Nat. Neurosci. 12, 1551–1558. 10.1038/nn.2452 [DOI] [PubMed] [Google Scholar]
- Leypold B. G., Yu C. R., Leinders-Zufall T., Kim M. M., Zufall F., Axel R. (2002). Altered sexual and social behaviors in trp2 mutant mice. Proc. Natl. Acad. Sci. U S A 99, 6376–6381. 10.1073/pnas.082127599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberles S. D., Horowitz L. F., Kuang D., Contos J. J., Wilson K. L., Siltberg-Liberles J., et al. (2009). Formyl peptide receptors are candidate chemosensory receptors in the vomeronasal organ. Proc. Natl. Acad. Sci. U S A 106, 9842–9847. 10.1073/pnas.0904464106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman E. R., Corey D. P., Dulac C. (1999). TRP2: a candidate transduction channel for mammalian pheromone sensory signaling. Proc. Natl. Acad. Sci. U S A 96, 5791–5796. 10.1073/pnas.96.10.5791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loconto J., Papes F., Chang E., Stowers L., Jones E. P., Takada T., et al. (2003). Functional expression of murine V2R pheromone receptors involves selective association with the M10 and M1 families of MHC class Ib molecules. Cell 112, 607–618. 10.1016/s0092-8674(03)00153-3 [DOI] [PubMed] [Google Scholar]
- Logan D. W., Marton T. F., Stowers L. (2008). Species specificity in major urinary proteins by parallel evolution. PLoS One 3:e3280. 10.1371/journal.pone.0003280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel K. L., Baum M. J. (2009). Adult testosterone treatment but not surgical disruption of vomeronasal function augments male-typical sexual behavior in female mice. J. Neurosci. 29, 7658–7666. 10.1523/jneurosci.1311-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-García F., Martínez-Ricós J., Agustín-Pavón C., Martínez-Hernández J., Novejarque A., Lanuza E. (2009). Refining the dual olfactory hypothesis: pheromone reward and odour experience. Behav. Brain Res. 200, 277–286. 10.1016/j.bbr.2008.10.002 [DOI] [PubMed] [Google Scholar]
- Martini S., Silvotti L., Shirazi A., Ryba N. J., Tirindelli R. (2001). Co-expression of putative pheromone receptors in the sensory neurons of the vomeronasal organ. J. Neurosci. 21, 843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Sánchez A., McLean L., Beynon R. L., Hurst J. L., Ayala G., Lanuza E., et al. (2014). From sexual attraction to maternal aggression: when pheromones change their behavioural significance. Horm. Behav. [Epub ahead of print]. 10.1016/j.yhbeh.2014.08.007 [DOI] [PubMed] [Google Scholar]
- Matsunami H., Buck L. B. (1997). A multigene family encoding a diverse array of putative pheromone receptors in mammals. Cell 90, 775–784. 10.1016/s0092-8674(00)80537-1 [DOI] [PubMed] [Google Scholar]
- Meredith M. (2001). Human vomeronasal organ function: a critical review of best and worst cases. Chem. Senses 26, 433–445. 10.1093/chemse/26.4.433 [DOI] [PubMed] [Google Scholar]
- Montani G., Tonelli S., Sanghez V., Ferrari P. F., Palanza P., Zimmer A., et al. (2013). Aggressive behaviour and physiological responses to pheromones are strongly impaired in mice deficient for the olfactory G-protein -subunit G8. J. Physiol. 591, 3949–3962. 10.1113/jphysiol.2012.247528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morè L. (2006). Mouse major urinary proteins trigger ovulation via the vomeronasal organ. Chem. Senses 31, 393–401. 10.1093/chemse/bjj043 [DOI] [PubMed] [Google Scholar]
- Mucignat-Caretta C. (2010). The rodent accessory olfactory system. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 196, 767–777. 10.1007/s00359-010-0555-z [DOI] [PubMed] [Google Scholar]
- Mucignat-Caretta C., Caretta A., Cavaggioni A. (1995). Acceleration of puberty onset in female mice by male urinary proteins. J. Physiol. 486(Pt. 2), 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudge J. M., Armstrong S. D., McLaren K., Beynon R. J., Hurst J. L., Nicholson C., et al. (2008). Dynamic instability of the major urinary protein gene family revealed by genomic and phenotypic comparisons between C57 and 129 strain mice. Genome Biol. 9:R91. 10.1186/gb-2008-9-5-r91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger S. D., Leinders-Zufall T., Zufall F. (2009). Subsystem organization of the mammalian sense of smell. Annu. Rev. Physiol. 71, 115–140. 10.1146/annurev.physiol.70.113006.100608 [DOI] [PubMed] [Google Scholar]
- Nishimura K., Utsumi K., Yuhara M., Fujitani Y., Iritani A. (1989). Identification of puberty-accelerating pheromones in male mouse urine. J. Exp. Zool. 251, 300–305. 10.1002/jez.1402510306 [DOI] [PubMed] [Google Scholar]
- Norlin E. M., Gussing F., Berghard A. (2003). Vomeronasal phenotype and behavioral alterations in G alpha i2 mutant mice. Curr. Biol. 13, 1214–1219. 10.1016/s0960-9822(03)00452-4 [DOI] [PubMed] [Google Scholar]
- Novotny M. V., Ma W., Wiesler D., Zidek L. (1999). Positive identification of the puberty-accelerating pheromone of the house mouse: the volatile ligands associating with the major urinary protein. Proc. Biol. Sci. 266, 2017–2022. 10.1098/rspb.1999.0880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oboti L., Pérez-Gómez A., Keller M., Jacobi E., Birnbaumer L., Leinders-Zufall T., et al. (2014). A wide range of pheromone-stimulated sexual and reproductive behaviors in female mice depend on G protein Galphao. BMC Biol. 12:31. 10.1186/1741-7007-12-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura M., Mombaerts P. (2014). Trpc2-expressing sensory neurons in the main olfactory epithelium of the mouse. Cell Rep. 8, 583–595. 10.1016/j.celrep.2014.06.010 [DOI] [PubMed] [Google Scholar]
- Pankevich D. E., Baum M. J., Cherry J. A. (2004). Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. J. Neurosci. 24, 9451–9457. 10.1523/jneurosci.2376-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papes F., Logan D. W., Stowers L. (2010). The vomeronasal organ mediates interspecies defensive behaviors through detection of protein pheromone homologs. Cell 141, 692–703. 10.1016/j.cell.2010.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere S., Challet L., Fluegge D., Spehr M., Rodriguez I. (2009). Formyl peptide receptor-like proteins are a novel family of vomeronasal chemosensors. Nature 459, 574–577. 10.1038/nature08029 [DOI] [PubMed] [Google Scholar]
- Roberts S. A., Davidson A. J., McLean L., Beynon R. J., Hurst J. L. (2012). Pheromonal induction of spatial learning in mice. Science 338, 1462–1465. 10.1126/science.1225638 [DOI] [PubMed] [Google Scholar]
- Roberts S. A., Simpson D. M., Armstrong S. D., Davidson A. J., Robertson D. H., McLean L., et al. (2010). Darcin: a male pheromone that stimulates female memory and sexual attraction to an individual male’s odour. BMC Biol. 8:75. 10.1186/1741-7007-8-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rünnenburger K., Breer H., Boekhoff I. (2002). Selective G protein beta gamma-subunit compositions mediate phospholipase C activation in the vomeronasal organ. Eur. J. Cell Biol. 81, 539–547. 10.1078/0171-9335-00277 [DOI] [PubMed] [Google Scholar]
- Ryba N. J., Tirindelli R. (1997). A new multigene family of putative pheromone receptors. Neuron 19, 371–379. 10.1016/s0896-6273(00)80946-0 [DOI] [PubMed] [Google Scholar]
- Schneider N. Y., Fletcher T. P., Shaw G., Renfree M. B. (2012). Goalpha expression in the vomeronasal organ and olfactory bulb of the tammar wallaby. Chem. Senses 37, 567–577. 10.1093/chemse/bjs040 [DOI] [PubMed] [Google Scholar]
- Sherborne A. L., Thom M. D., Paterson S., Jury F., Ollier W. E. R., Stockley P., et al. (2007). The genetic basis of inbreeding avoidance in house mice. Curr. Biol. 17, 2061–2066. 10.1016/j.cub.2007.10.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvotti L., Moiani A., Gatti R., Tirindelli R. (2007). Combinatorial co-expression of pheromone receptors, V2Rs. J. Neurochem. 103, 1753–1763. 10.1111/j.1471-4159.2007.04877.x [DOI] [PubMed] [Google Scholar]
- Stowers L., Holy T. E., Meister M., Dulac C., Koentges G. (2002). Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science 295, 1493–1500. 10.1126/science.1069259 [DOI] [PubMed] [Google Scholar]
- Sturm T., Leinders-Zufall T., Maček B., Walzer M., Jung S., Pömmerl B., et al. (2013). Mouse urinary peptides provide a molecular basis for genotype discrimination by nasal sensory neurons. Nat. Commun. 4:1616. 10.1038/ncomms2610 [DOI] [PubMed] [Google Scholar]
- Takigami S., Mori Y., Ichikawa M. (2000). Projection pattern of vomeronasal neurons to the accessory olfactory bulb in goats. Chem. Senses 25, 387–393. 10.1093/chemse/25.4.387 [DOI] [PubMed] [Google Scholar]
- Takigami S., Mori Y., Tanioka Y., Ichikawa M. (2004). Morphological evidence for two types of mammalian vomeronasal system. Chem. Senses 29, 301–310. 10.1093/chemse/bjh032 [DOI] [PubMed] [Google Scholar]
- Tanaka M., Treloar H., Kalb R. G., Greer C. A., Strittmatter S. M. (1999). G(o) protein-dependent survival of primary accessory olfactory neurons. Proc. Natl. Acad. Sci. U S A 96, 14106–14111. 10.1073/pnas.96.24.14106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirindelli R., Dibattista M., Pifferi S., Menini A. (2009). From pheromones to behavior. Physiol. Rev. 89, 921–956. 10.1152/physrev.00037.2008 [DOI] [PubMed] [Google Scholar]
- Trotier D. (2011). Vomeronasal organ and human pheromones. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 128, 184–190. 10.1016/j.anorl.2010.11.008 [DOI] [PubMed] [Google Scholar]
- Trotier D., Eloit C., Wassef M., Talmain G., Bensimon J. L., Døving K. B., et al. (2000). The vomeronasal cavity in adult humans. Chem. Senses 25, 369–380. 10.1093/chemse/25.4.369 [DOI] [PubMed] [Google Scholar]
- Wu Z., Autry A. E., Bergan J. F., Watabe-Uchida M., Dulac C. G. (2014). Galanin neurons in the medial preoptic area govern parental behaviour. Nature 509, 325–330. 10.1038/nature13307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt T. D. (2014). Pheromones and Animal Behavior: Chemical Signals and Signature Mixes. 2nd Edn. Cambridge: Cambridge University Press. [Google Scholar]
- Wynn E. H., Sánchez-Andrade G., Carss K. J., Logan D. W. (2012). Genomic variation in the vomeronasal receptor gene repertoires of inbred mice. BMC Genomics 13:415. 10.1186/1471-2164-13-415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki C. J., Lepri J. J. (1991). Consequences of removing the vomeronasal organ. J. Steroid Biochem. Mol. Biol. 39, 661–669. 10.1016/0960-0760(91)90265-7 [DOI] [PubMed] [Google Scholar]
- Young J. M., Trask B. J. (2007). V2R gene families degenerated in primates, dog and cow, but expanded in opossum. Trends Genet. 23, 212–215. 10.1016/j.tig.2007.03.004 [DOI] [PubMed] [Google Scholar]