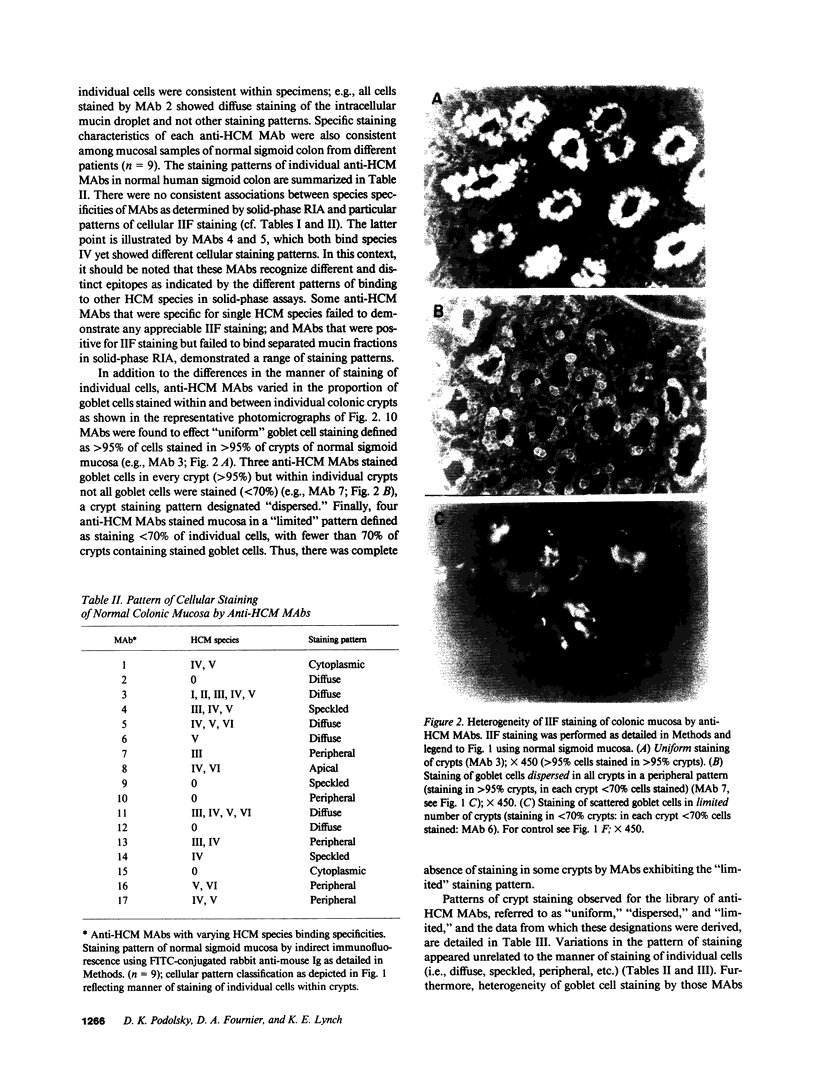
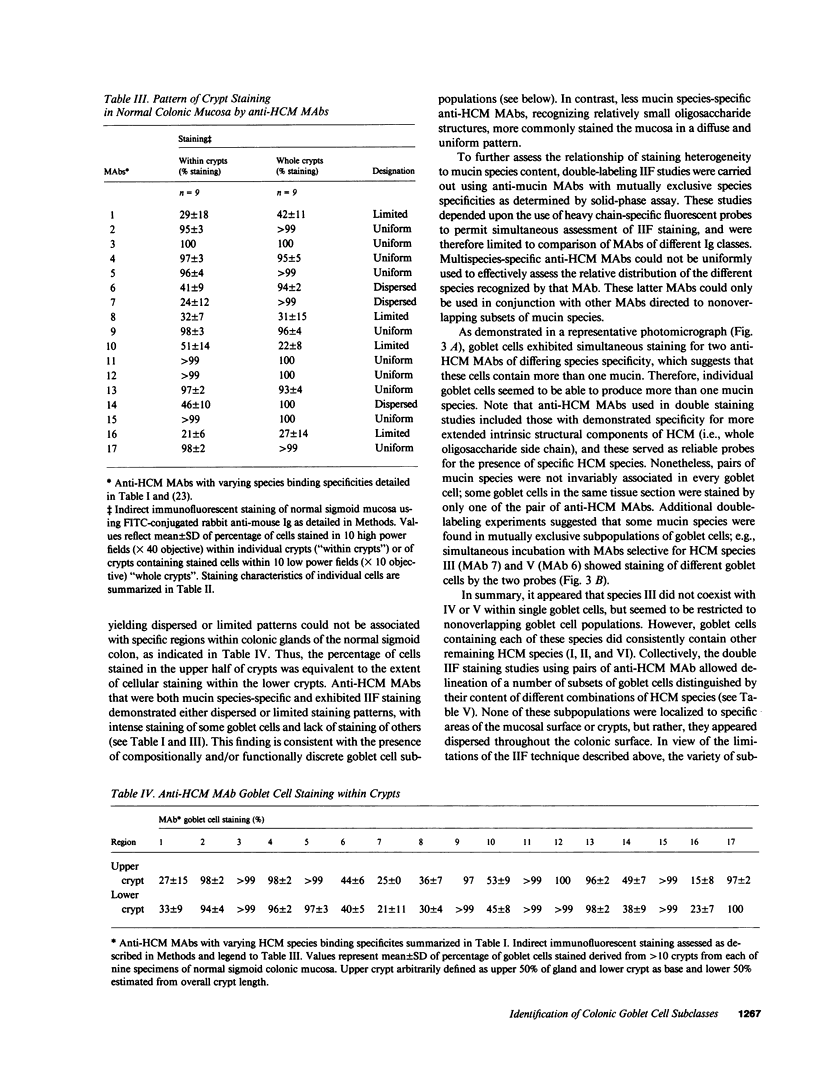
Abstract
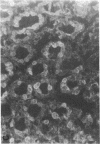
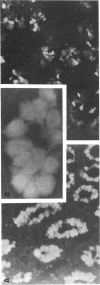
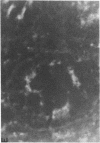
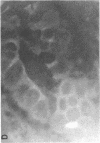
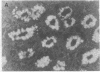
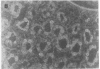
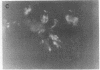
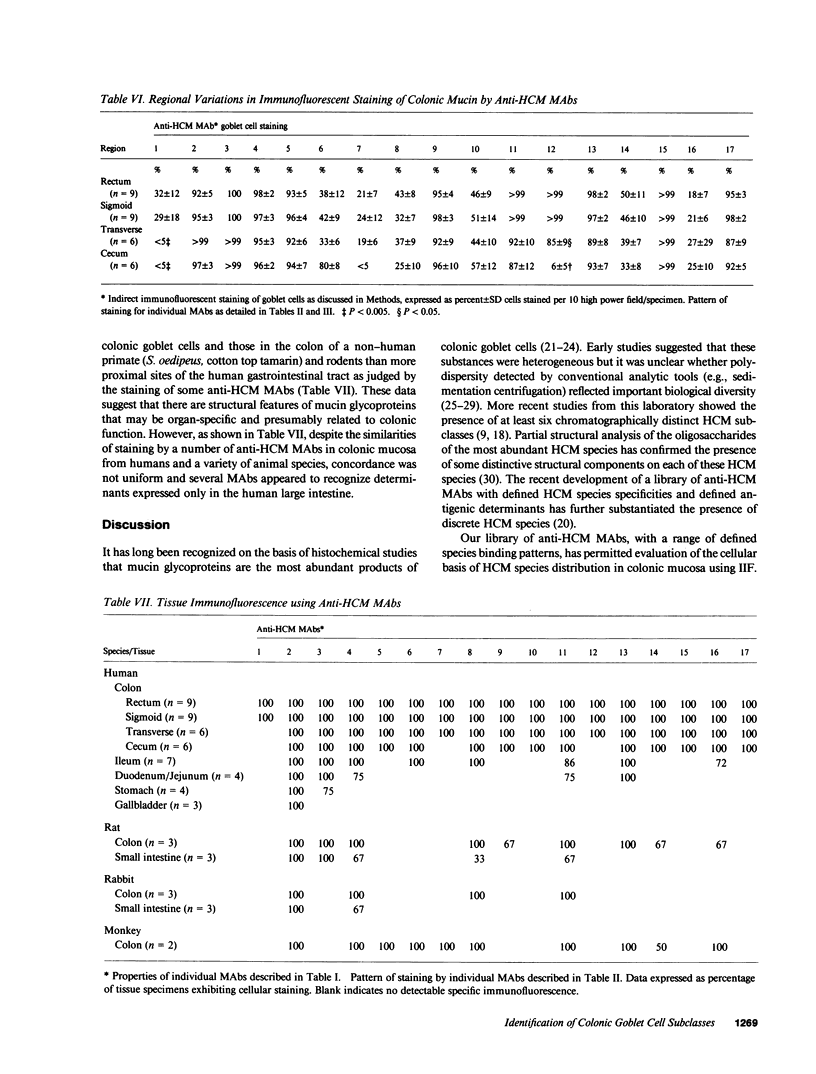
We studied glycoprotein content of human colonic goblet cells, using a library of monoclonal antibodies (MAbs) directed against purified human colonic mucin (HCM). Using indirect immunofluorescence (IIF), we found that 17 of 23 anti-HCM MAbs stained some or all goblet cells of normal human colonic mucosa. We observed a variety of cellular staining patterns, including (a) diffuse (homogeneous) staining of intracellular mucin, (b) speckled (inhomogeneous) staining of mucin droplets, (c) peripheral staining of intracellular droplets, (d) cytoplasmic staining of goblet cells, and (e) apical (luminal) surface staining. Staining patterns were not associated with particular HCM species. In addition to variable patterns of IIF within individual cells, anti-HCM MAbs varied in the proportion of goblet cells stained. Some MAbs stained all goblet cells, while others stained a limited number of goblet cells. Although each goblet cell contained more than one type mucin, HCM species III, and IV and V appeared to exist in mutually exclusive goblet cell populations and it was possible to define at least seven subpopulations of goblet cells in colonic mucosa by their content of various combinations of HCM species. Anti-HCM MAbs stained goblet cells from other sites within the gastrointestinal tract to a varying extent. Anti-HCM MAbs also showed extensive cross-reactivity with rodent, rabbit, and monkey colonic mucosa. However, several anti-HCM MAbs stained only human colonic mucosa. These data show that human colonic mucosa contains discrete subpopulations of goblet cells that produce distinctive combinations of specific mucin glycoprotein species.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A., Bell A., Mantle M., Pearson J. P. The structure and physiology of gastrointestinal mucus. Adv Exp Med Biol. 1982;144:115–133. doi: 10.1007/978-1-4615-9254-9_15. [DOI] [PubMed] [Google Scholar]
- Bara J., Loisillier F., Burtin P. Antigens of gastric and intestinal mucous cells in human colonic tumours. Br J Cancer. 1980 Feb;41(2):209–221. doi: 10.1038/bjc.1980.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland C. R., Lance P., Levin B., Riddell R. H., Kim Y. S. Abnormal goblet cell glycoconjugates in rectal biopsies associated with an increased risk of neoplasia in patients with ulcerative colitis: early results of a prospective study. Gut. 1984 Dec;25(12):1364–1371. doi: 10.1136/gut.25.12.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland C. R., Montgomery C. K., Kim Y. S. Alterations in human colonic mucin occurring with cellular differentiation and malignant transformation. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2051–2055. doi: 10.1073/pnas.79.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra D. P., Yeh K., Brockman R. W. Isolation and characterization of epithelial cell types from the normal rat colon. Cancer Res. 1981 Jan;41(1):168–175. [PubMed] [Google Scholar]
- Dawson P. A., Patel J., Filipe M. I. Variations in sialomucins in the mucosa of the large intestine in malignancy: a quantimet and statistical analysis. Histochem J. 1978 Sep;10(5):559–572. doi: 10.1007/BF01003137. [DOI] [PubMed] [Google Scholar]
- Etzler M. E. Lectins as probes in studies of intestinal glycoproteins and glycolipids. Am J Clin Nutr. 1979 Jan;32(1):133–138. doi: 10.1093/ajcn/32.1.133. [DOI] [PubMed] [Google Scholar]
- Fahim R. E., Forstner G. G., Forstner J. F. Heterogeneity of rat goblet-cell mucin before and after reduction. Biochem J. 1983 Jan 1;209(1):117–124. doi: 10.1042/bj2090117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipe M. I., Dawson I. The diagnostic value of mucosubstances in rectal biopsies from patients with ulcerative colitis and Crohn's disease. Gut. 1970 Mar;11(3):229–234. doi: 10.1136/gut.11.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipe M. I. Mucins in the human gastrointestinal epithelium: a review. Invest Cell Pathol. 1979 Jul-Sep;2(3):195–216. [PubMed] [Google Scholar]
- Forstner G., Wesley A., Forstner J. Clinical aspects of gastrointestinal mucus. Adv Exp Med Biol. 1982;144:199–224. doi: 10.1007/978-1-4615-9254-9_32. [DOI] [PubMed] [Google Scholar]
- Gad A. A histochemical study of human alimentary tract mucosubstances in health and disease. II. Inflammatory conditions. Br J Cancer. 1969 Mar;23(1):64–68. doi: 10.1038/bjc.1969.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold D. V. Immunoperoxidase localization of colonic mucoprotein antigen in neoplastic tissues. Cancer Res. 1981 Mar;41(3):767–772. [PubMed] [Google Scholar]
- Gold D. V., Shochat D., Miller F. Protease digestion of colonic mucin. Evidence for the existence of two immunochemically distinct mucins. J Biol Chem. 1981 Jun 25;256(12):6354–6358. [PubMed] [Google Scholar]
- Jacobs L. R., De Fontes D., Cox K. L. Cytochemical localization of small intestinal glycoconjugates by lectin histochemistry in controls and subjects with cystic fibrosis. Dig Dis Sci. 1983 May;28(5):422–428. doi: 10.1007/BF02430531. [DOI] [PubMed] [Google Scholar]
- Jacobs L. R., Huber P. W. Regional distribution and alterations of lectin binding to colorectal mucin in mucosal biopsies from controls and subjects with inflammatory bowel diseases. J Clin Invest. 1985 Jan;75(1):112–118. doi: 10.1172/JCI111662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. S., Isaacs R. Glycoprotein metabolism in inflammatory and neoplastic diseases of the human colon. Cancer Res. 1975 Aug;35(8):2092–2097. [PubMed] [Google Scholar]
- Lev R., Orlic D. Histochemical and radioautographic studies of normal human fetal colon. Histochemistry. 1974 Jun 5;39(4):301–311. doi: 10.1007/BF00495681. [DOI] [PubMed] [Google Scholar]
- Listinsky C. M., Riddell R. H. Patterns of mucin secretion in neoplastic and non-neoplastic diseases of the colon. Hum Pathol. 1981 Oct;12(10):923–929. doi: 10.1016/s0046-8177(81)80198-0. [DOI] [PubMed] [Google Scholar]
- MacDermott R. P., Donaldson R. M., Jr, Trier J. S. Glycoprotein synthesis and secretion by mucosal biopsies of rabbit colon and human rectum. J Clin Invest. 1974 Sep;54(3):545–554. doi: 10.1172/JCI107791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall T., Allen A. The isolation and characterization of the high-molecular-weight glycoprotein from pig colonic mucus. Biochem J. 1978 Aug 1;173(2):569–578. doi: 10.1042/bj1730569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménard D., Arsenault P. Explant culture of human fetal small intestine. Gastroenterology. 1985 Mar;88(3):691–700. doi: 10.1016/0016-5085(85)90139-8. [DOI] [PubMed] [Google Scholar]
- Neutra M. R., Grand R. J., Trier J. S. Glycoprotein synthesis, transport, and secretion by epithelial cells of human rectal mucosa: normal and cystic fibrosis. Lab Invest. 1977 May;36(5):535–546. [PubMed] [Google Scholar]
- Neutra M. R., O'Malley L. J., Specian R. D. Regulation of intestinal goblet cell secretion. II. A survey of potential secretagogues. Am J Physiol. 1982 Apr;242(4):G380–G387. doi: 10.1152/ajpgi.1982.242.4.G380. [DOI] [PubMed] [Google Scholar]
- O'Gorman T. A., LaMont J. T. Glycoprotein synthesis and secretion in human colon cancers and normal colonic mucosa. Cancer Res. 1978 Sep;38(9):2784–2789. [PubMed] [Google Scholar]
- Phillips T. E., Phillips T. H., Neutra M. R. Regulation of intestinal goblet cell secretion. III. Isolated intestinal epithelium. Am J Physiol. 1984 Dec;247(6 Pt 1):G674–G681. doi: 10.1152/ajpgi.1984.247.6.G674. [DOI] [PubMed] [Google Scholar]
- Phillips T. E., Phillips T. H., Neutra M. R. Regulation of intestinal goblet cell secretion. IV. Electrical field stimulation in vitro. Am J Physiol. 1984 Dec;247(6 Pt 1):G682–G687. doi: 10.1152/ajpgi.1984.247.6.G682. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Fournier D. A., Lynch K. E. Development of anti-human colonic mucin monoclonal antibodies. Characterization of multiple colonic mucin species. J Clin Invest. 1986 Apr;77(4):1251–1262. doi: 10.1172/JCI112428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky D. K., Isselbacher K. J. Characterization of monoclonal antibodies to serum galactosyltransferase. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2529–2533. doi: 10.1073/pnas.81.8.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky D. K., Isselbacher K. J. Glycoprotein composition of colonic mucosa. Specific alterations in ulcerative colitis. Gastroenterology. 1984 Nov;87(5):991–998. [PubMed] [Google Scholar]
- Podolsky D. K. Oligosaccharide structures of human colonic mucin. J Biol Chem. 1985 Jul 15;260(14):8262–8271. [PubMed] [Google Scholar]
- Shamsuddin A. K., Trump B. F. Colon epithelium. I. Light microscopic, histochemical, and ultrastructural features of normal colon epithelium of male Fischer 344 rats. J Natl Cancer Inst. 1981 Feb;66(2):375–388. [PubMed] [Google Scholar]
- Silberberg A., Meyer F. A. Structure and function of mucus. Adv Exp Med Biol. 1982;144:53–74. doi: 10.1007/978-1-4615-9254-9_6. [DOI] [PubMed] [Google Scholar]
- Specian R. D., Neutra M. R. Regulation of intestinal goblet cell secretion. I. Role of parasympathetic stimulation. Am J Physiol. 1982 Apr;242(4):G370–G379. doi: 10.1152/ajpgi.1982.242.4.G370. [DOI] [PubMed] [Google Scholar]
- Yonezawa S., Nakamura T., Tanaka S., Sato E. Glycoconjugate with Ulex europaeus agglutinin-I-binding sites in normal mucosa, adenoma, and carcinoma of the human large bowel. J Natl Cancer Inst. 1982 Oct;69(4):777–785. [PubMed] [Google Scholar]