Abstract
Endometrial carcinomas show frequent PTEN-PI3K pathway abnormalities, and there are currently multiple trials focused on PI3K pathway inhibitors in endometrial carcinoma patients. PTEN immunohistochemistry may help select patients with potential for response to targeted therapy making it important to develop and validate this stain in formalin-fixed, paraffin embedded tissue. Immunohistochemistry for PTEN was performed and scored independently on 118 cases of endometrial carcinomas from 2 cancer centers using monoclonal DAKO 6H2.1antibody. Cases were scored as positive, negative or heterogeneous; reproducibility of PTEN staining and interpretation was assessed.
Overall interobserver agreement was good (weighted κ = 0.80), with 82% concordance, similar for non endometrioid (81%) and endometrioid carcinomas (85%). 21 of 118 cases showed discrepant results (17%); that resulted from differences in interpretation and not staining.
Our study shows that evaluation of PTEN loss by immunohistochemistry is highly reproducible with the application of standard immunohistochemical techniques and simple scoring criteria.
Keywords: Endometrial carcinoma, PI3K pathway, PTEN immunohistochemistry
INTRODUCTION
Endometrial carcinoma is the most common malignancy of the female genital tract. It is a heterogeneous disease that has traditionally been divided into type 1 and type 2 cancers (1). Type 1 tumors are low grade endometrioid carcinomas that frequently arise in a setting of estrogen excess and are generally associated with good clinical outcomes. In contrast, type 2 tumors are high grade, serous, clear cell, undifferentiated or poorly differentiated endometrioid carcinomas which have an aggressive clinical course and hormone-independent pathogenesis (1). Type 1 tumors are typically associated with microsatellite instability and mutations in PTEN, K-RAS and CTNNB1. Type 2 endometrial carcinomas often show p53 mutations. Both type 1 and type 2 endometrial carcinomas can have PIK3CA mutations, and these can coexist with mutations in PTEN and p53 respectively (2, 3, 4).
PTEN is a tumor suppressor gene located on chromosome 10q23. PTEN plays a key role in the regulation of PTEN- PI3K -AKT pathway. Loss of PTEN results in AKT activation which initiates a signal transduction pathway that leads to increased growth, inappropriate survival and altered metabolism of cancer cells. MTOR is one of the major downstream effectors of AKT. Abnormalities in the PTEN-PI3K pathway have been detected in many human tumors, including endometrial carcinomas (5, 6). PTEN knock-out mice develop proliferative endometrial lesions and germline PTEN mutations (Cowden syndrome) are associated with increased risk for endometrial carcinoma (7).
PTEN loss is also detected in 30–50% of sporadic endometrial cancers (8, 9, 10, 11, 12) and has been shown to occur through both genetic and epigenetic mechanisms. Many of these molecular alterations appear to be an early event in endometrial carcinogenesis, as they are frequently seen in endometrial hyperplasia (9).
While patients with early stage endometrial type 1 tumors typically have a good prognosis, those with type 2 tumors, or advanced stage or recurrent type 1 tumors, have poor outcomes. Furthermore, there is no uniform standard of care therapy for these patients, so they are typically treated with combinations of surgery, chemotherapy and radiation, with limited success. Targeted therapy offers a new avenue of treatment for this group of patients.
Due to frequent PTEN loss in endometrial tumors, the PTEN- PI3K –AKT pathway is a rational target for the treatment of endometrial cancer. Inhibitors to MTOR, PI3K and AKT, all of which target this pathway, are currently in development (13). Presence of PIK3CA mutations in various advanced carcinomas, including endometrial carcinoma, has been shown to correlate with response to PI3K/AKT/MTOR inhibitors (14).
Some mouse model studies have shown increased sensitivity of PTEN-deficient tumors to inhibition of MTOR (15, 16), and suppression of PTEN function in breast cancer cell lines was shown to correlate with resistance to chemotherapy and susceptibility to MTOR inhibitors (17). Thus far, phase II clinical trials with MTOR inhibitors for pretreated recurrent/advanced endometrial carcinoma have shown promising results (18, 19). Slomovitz et al demonstrated in an abstract that loss of PTEN correlated with clinical response to everolimus in recurrent endometrial carcinoma (19). Similarly, Garrido-Laguna and colleagues also showed in an abstract that PTEN loss by immunohistochemistry correlates with response to drugs targeting the PI3K-AKT-MTOR pathway in patients with various advanced cancers, including endometrial carcinoma (20).
PTEN immunohistochemistry (IHC) has been suggested as potentially the most accurate reflection of functional PTEN status. PTEN mutational analysis is difficult on a clinical basis, because there are no “hot-spot” mutations. Moreover, mutational analysis will not detect PTEN loss by epigenetic mechanisms, such as promoter methylation and increased protein degradation (21). In order to identify endometrial cancer patients who may be eligible for targeted PTEN- PI3K –AKT pathway inhibitor therapy, reliable methods of detecting and scoring PTEN protein expression in formalin-fixed, paraffin embedded tissues must be developed and validated.
To date, PTEN antibodies in endometrial carcinoma have been difficult to work with and have had limited success (22, 23). Pallares et al tested 4 different PTEN antibodies (10PO3, 28H6, polyclonal and 6H2.1) in an endometrial carcinoma tissue microarray, and observed no concordance between the 4 antibodies, with wide variability in staining. They found that the 6H2.1 antibody was the only antibody that showed an inverse correlation with the phosporylated AKT staining pattern (an activated form of AKT) and some correlation with molecular alterations in PTEN (22).
Another problematic aspect of PTEN immunohistochemistry is its interpretation. There are no uniform scoring criteria for PTEN immunohistochemistry, and most of the proposed scoring systems are complex and tedious (22). For advanced endometrial cancer patients being considered for targeted therapy, accurate scoring and interpretation of PTEN immunohistochemistry is critical for several reasons. One, targeted therapy is typically very expensive, so the pathologic assays determining enrollment must be accurate and reproducible to ensure maximum patient benefit. Second, and more importantly, patients with recurrent/metastatic endometrial cancer have a finite, limited life expectancy. Thus, the laboratory tools used to guage potential eligibility of targeted therapy must be accurate.
In this study, two different groups performed and interpreted immunohistochemistry for PTEN in endometrial carcinoma. The aim of this study was to propose a useful scoring system for PTEN immunohistochemistry expression in endometrial carcinoma and then cross-validate this scoring system between two major cancer centers, Memorial Sloan Kettering Cancer Center (MSKCC) and MD Anderson Cancer Center (MDACC).
MATERIALS AND METHODS
A total of one hundred and twenty two cases (n=122) of endometrial carcinoma from 2 institutions (62 from MSKCC and 60 from MDACC) were subjected to PTEN immunohistochemistry. Both endometrioid and non-endometrioid histologies were included. Of the 122 cases, 4 cases from MSKCC were excluded due to technical issues (tissue falling off the glass slides). The remaining 118 cases constituted the study group (n=118).
Whole tissue sections, rather than tissue microarray, were used for all immunohistochemistry analyses.
Immunohistochemistry for PTEN was performed on all 118 cases at each institution. Each institution exchanged a set of unstained slides in order for both institutions to achieve a full complement of cases included in the study. At each institution, 2 pathologists derived consensus scores for PTEN immunohistochemistry. Reproducibility of PTEN interpretation between the two institutions was then assessed.
Immunohistochemistry for PTEN was performed using the monoclonal DAKO antibody (clone 6H2.1) at both institutions, and technical details are provided below.
Memorial Sloan Kettering Cancer Center (MSKCC)
PTEN immunohistochemistry was validated using endometrial carcinoma cases with confirmed PTEN mutations and the monoclonal PTEN antibody from DAKO (clone 6H2.1, catalog # M3627). Antigen retrieval was accomplished using EDTA buffer (pH 9.0) with steaming for 30 minutes, followed by PTEN antibody (dilution 1:50). DAB was used as the chromogen. For tumors with mutant PTEN and loss of PTEN protein expression, positive staining blood vessels and stromal cells were used as an internal positive control.
MD Anderson Cancer Center (MDACC)
PTEN immunohistochemistry was validated using breast cancer cell lines with known wild type or mutant PTEN. These cell lines were xenografted into nude mice, and sections of excised tumor were subsequently subjected to PTEN immunohistochemistry. Immunohistochemistry was performed using the monoclonal PTEN antibody from DAKO (clone 6H2.1). Antigen retrieval was accomplished using Tris-EDTA buffer for 20 minutes at 100°C, followed by application of the PTEN antibody (dilution 1:100). DAB was used as the chromogen. For tumors with mutant PTEN and loss of PTEN protein expression, positive staining of blood vessels and stromal cells were used as an internal positive control.
Immunohistochemistry for PTEN was interpreted by both institutions as follows:
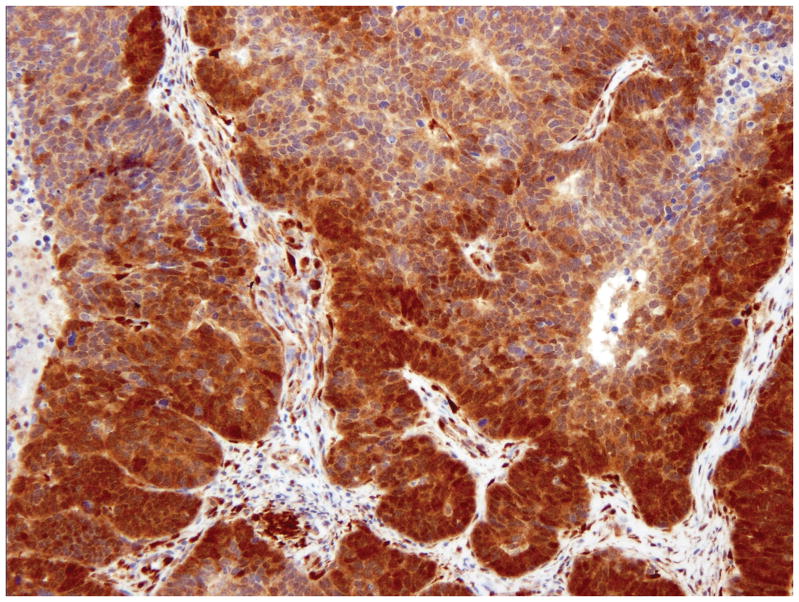
Positive (pos) - Strong positive staining in the entire tumor or vast majority of the tumor (figure 1A).
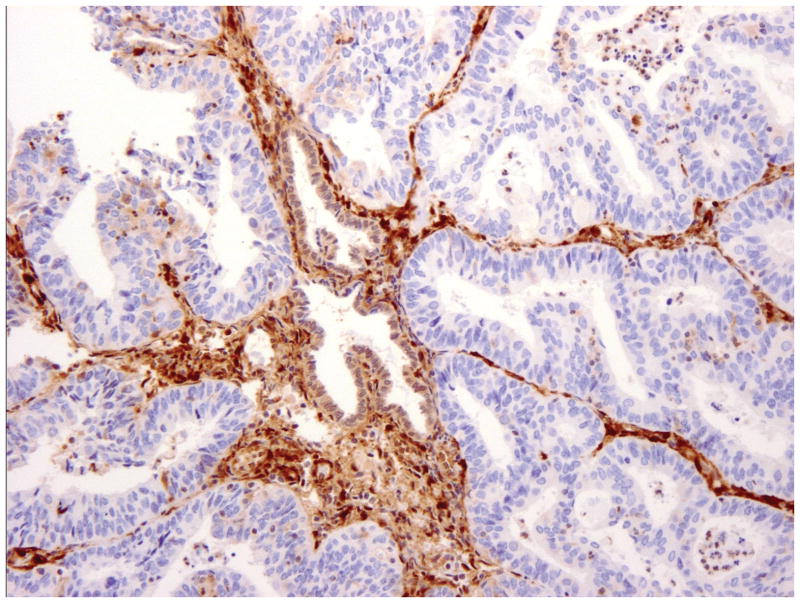
Negative (neg) -No staining in entire/most tumor, with strong positive staining of adjacent normal endometrium or stromal cells (figure 1B).
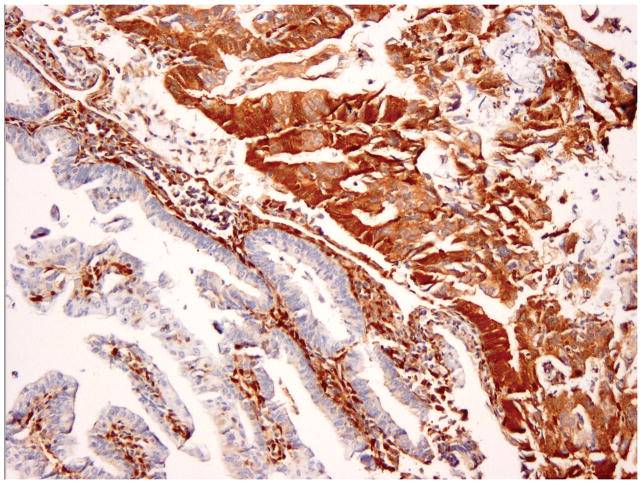
Heterogeneous (het) – Tumor with convincingly positive staining and convincingly negative staining (figure 1C).
Figure 1.
PTEN Immunohistochemistry in endometrial carcinoma. Strong staining in the entire tumor or vast majority of the tumor was interpreted as positive (A). No staining in entire/most tumor, with strong positive staining of adjacent normal endometrium or stromal cells, was interpreted as negative (B). Tumors with convincingly positive staining and convincingly negative staining were interpreted as heterogeneous (C).
The histologic score (H-score) was then calculated for cases with heterogeneous staining according to previously reported methods (24). H-score ranged from 0 (no staining) to 300 (maximum staining) and was calculated by application of the following formula:
Statistical analyses
Staining was evaluated in three categories as negative, positive, and heterogenous. Interobserver agreement between MDACC and MSKCC was assessed by calculating weighted κ (with its 95% confidence intervals) and percent agreement (with its 95% confidence intervals). Weighted κ (as opposed to just κ) was calculated in order to account for different levels of disagreement, positive vs. negative being a higher level of disagreement than heterogeneous vs. either positive or negative.
This same analysis was then repeated controlling for the site of sample origin (MDACC versus MSKCC) and for histology (endometrioid versus non-endometrioid). Hypothesis testing was conducted to determine if weighted κ differed by originating site or histology.
The final set of analyses involved reclassification of cases that were scored as heterogenous to negative, in order to differentiate cases with no PTEN loss (positive) from cases with any type of PTEN loss (heterogeneous and negative). Because the categories in this analysis were binary, κ was not weighted.
The κ value was interpreted according to Landis et al (25). A κ <0.20 was regarded as poor agreement; κ=0.21 – 0.40 as fair agreement; κ=0.41 – 0.60 as moderate agreement; κ=0.61 – 0.80 as good agreement; and κ= 0.81 – 1.00 as very good agreement.
RESULTS
PTEN scoring results for all cases are listed in table 1. The overall interobserver agreement between MDACC and MSKCC for all cases was good (weighted κ = 0.80), with 82% concordance between MDACC and MSKCC interpretations (table 2).
Table 1.
PTEN scoring results for all cases in the study set following independent performance of immunohistochemistry and scoring at MSKCC and MDACC.
| Histology | IHC Performance and Interpretation at MSKCC | IHC Performance and Interpretation at MDACC | Source | Number of Cases |
|---|---|---|---|---|
| Endometrioid | Negative | Negative | MDACC | 21 |
| Endometrioid | Negative | Negative | MSKCC | 18 |
| Endometrioid | Negative | Heterogeneous | MDACC | 1 |
| Endometrioid | Negative | Heterogeneous | MSKCC | 7 |
| Endometrioid | Negative | Positive | MDACC | 1 |
| Endometrioid | Heterogeneous | Negative | MDACC | 1 |
| Endometrioid | Heterogeneous | Heterogeneous | MDACC | 4 |
| Endometrioid | Heterogeneous | Heterogeneous | MSKCC | 5 |
| Endometrioid | Heterogeneous | Positive | MSKCC | 2 |
| Endometrioid | Positive | Heterogeneous | MDACC | 1 |
| Endometrioid | Positive | Heterogeneous | MSKCC | 3 |
| Endometrioid | Positive | Positive | MDACC | 8 |
| Endometrioid | Positive | Positive | MSKCC | 12 |
| Non-endometrioid | Negative | Negative | MDACC | 3 |
| Non-endometrioid | Negative | Negative | MSKCC | 1 |
| Non-endometrioid | Negative | Heterogeneous | MSKCC | 1 |
| Non-endometrioid | Negative | Positive | MDACC | 1 |
| Non-endometrioid | Heterogeneous | Heterogeneous | MDACC | 4 |
| Non-endometrioid | Heterogeneous | Positive | MDACC | 1 |
| Non-endometrioid | Positive | Heterogeneous | MSKCC | 2 |
| Non-endometrioid | Positive | Positive | MDACC | 14 |
| Non-endometrioid | Positive | Positive | MDACC | 7 |
Table 2.
PTEN scoring agreement for all cases in the study set (n=118) following independent performance of immunohistochemistry and scoring at MSKCC and MDACC.
| MSKCC | MDACC | ||
|---|---|---|---|
| Negative | Heterogeneous | Positive | |
| Negative | 43 | 9 | 2 |
| Heterogeneous | 1 | 13 | 3 |
| Positive | 0 | 6 | 19 |
| Weighted κ (95% CI) | 0.80 (0.72 – 0.88) | ||
| Percent Agreement (95% CI) | 82% (72–88%) | ||
In table 3, interobserver agreement by histology was good for both non endometrioid and endometrioid carcinomas (weighted κ = 0.74 and 0.78, respectively). The weighted κ was not statistically different between the histotypes (p >0.05). Percent agreement was similar for non endometrioid (81%) and endometrioid carcinomas (85%).
Table 3.
PTEN scoring agreement for endometrioid (n=84) vs non-endometrioid (n=34) cases in the study set following independent performance of immunohistochemistry and scoring at MSKCC and MDACC.
| Non-endometrioid | Endometrioid | |||||
|---|---|---|---|---|---|---|
| MDACC | ||||||
| MSKCC | Neg. | Het. | Pos. | Neg. | Het. | Pos. |
| Negative | 4 | 1 | 1 | 39 | 8 | 1 |
| Heterogeneous | 0 | 4 | 1 | 1 | 9 | 2 |
| Positive | 0 | 2 | 21 | 0 | 4 | 20 |
| Weighted κ (95% CI) | 0.74 (0.52–0.96) | 0.78 (0.67–0.88) | ||||
| Percent Agreement (95% CI) | 81% (71–89%) | 85% (69%–95%) | ||||
Considering cases in which agreement was achieved, negative PTEN staining was seen more frequently in endometrioid carcinomas (57% of cases), compared to non- endometrioid carcinomas (13% of cases). Positive PTEN staining was observed more often in non- endometrioid carcinomas (72%) compared to endometrioid carcinomas (29%).
The nature of the heterogeneous cases (figure 1C) is somewhat uncertain. There are no cell lines or animal models that recapitulate this tissue expression pattern. These cases displayed varying amount and intensity of staining, and the H-score ranged from 15 to 230 (mean=121, median=127). Because at least a portion of these tumors show definite loss of PTEN protein expression by immunohistochemistry, we reasoned that such tumors might demonstrate activation of the PI3K-AKT pathway and therefore be susceptible to drugs that inhibit this pathway. Therefore, the weighted κ and percent agreement values were re-calculated in which a heterogeneous score was considered to be equivalent to a negative score. Using this approach, the overall interobserver agreement between MDACC and MSKCC for all cases was good (weighted κ = 0.82), with 91 % concordance between MDACC and MSKCC interpretations (table 4).
Table 4.
PTEN scoring agreement for all cases in the study set (n=118) following independent performance of immunohistochemistry and scoring at MSKCC and MDACC. In this analysis, negative and heterogeneous categories have been combined into one category.
| MSKCC | MDACC | |
|---|---|---|
| Negative and Heterogeneous | Positive | |
| Negative and Heterogeneous | 66 | 5 |
| Positive | 6 | 41 |
| Weighted κ (95% CI) | 0.82 (0.72 – 0.92) | |
| Percent Agreement (95% CI) | 91% (84–95%) | |
Using this approach of combining negative and heterogeneous cases, interobserver agreement was good for both non-endometrioid and endometrioid carcinomas (weighted κ = 0.73 and 0.79; respectively) (table 5). The weighted κ was not statistically different between the histotypes (p >0.05). Percent agreement was similar for non-endometrioid (88%) and endometrioid carcinomas (92%) and was slightly higher than the corresponding percent agreements in the non-grouped analysis (table 3).
Table 5.
PTEN scoring agreement for endometrioid (n=84) vs non-endometrioid (n=34) cases in the study set following independent performance of immunohistochemistry and scoring at MSKCC and MDACC. In this analysis, negative and heterogeneous categories have been combined into one category.
| MSKCC Interpretation | MDACC Interpretation | |||
|---|---|---|---|---|
| Non-Endometrioid | Endometrioid | |||
| Negative and Heterogeneous | Positive | Negative and Heterogeneous | Positive | |
| Negative | 9 | 2 | 57 | 3 |
| Positive | 2 | 21 | 4 | 20 |
| Weighted κ (95% CI) | 0.73 (0.49 – 0.98) | 0.79 (0.65 – 0.94) | ||
| Percent Agreement (95% CI) | 88% (73% – 97%) | 92% (84% – 97%) | ||
When controlling for the site of sample origin, interobserver agreement was very good for MDACC cases and good for MSKCC cases (weighted κ = 0.86 and 0.73, respectively). The weighted κ was not statistically different between the two sites (p >0.05). Percent agreement was also better for MDACC cases (90%) than for MSKCC cases (74%). The percent agreement for the MSKCC cases was appreciably greater (88%) when the heterogenous and negative scores are grouped than when they are analyzed separately (74%).
Using the 3-tiered scoring system of positive, negative, and heterogeneous, of the 118 cases from both institutions, 21 showed discrepant IHC results (17%). The discrepancies typically consisted of cases interpreted as heterogeneous at one center versus positive/negative at the other center (9 and 10 cases scored as heterogeneous at one institution were scored as positive and negative respectively at the second institution). There were only 2 cases that were differentially interpreted as positive vs. negative. When heterogeneous cases were included with negative cases, the number of discrepant cases between the 2 institutions decreased to only 11 (9%).
DISCUSSION
New therapies including inhibitors of the AKT pathway may be active in patients with recurrent/metastatic endometrial carcinoma. Loss of PTEN by immunohistochemistry is indicative of potential PI3K/AKT pathway activation and may correlate with response to agents that block this pathway. It is therefore important to standardize PTEN immunohistochemical techniques and interpretation to enable detection of tumors with PTEN loss across different CLIA certified laboratories. Our study shows that immunohistochemistry for PTEN in endometrial carcinoma is highly reproducible through the use of standard immunohistochemical techniques and simple scoring criteria.
Prior studies of PTEN immunohistochemistry in endometrial carcinoma have shown inconsistent results (22, 23). In our study, both institutions used the same monoclonal antibody with a good internal positive control in the form of blood vessels, stromal cells and lymphocytes. There are currently no uniform guidelines for PTEN immunohistochemistry interpretation, and prior studies have used complex semi-quantitative scoring criteria like the histologic score (H-score) which entail substantial time and effort (22). We used a simple approach, dividing our cases into positive (all or most tumor is staining), negative (none or most of tumor shows no staining) and heterogeneous (discrete positive and negative areas). Application of these scoring criteria was relatively easy and yielded good reproducibility between the two centers. This type of three-tiered scoring has been shown to yield significantly higher reproducibility rates compared to the more complex semi-quantitative H-score for PTEN staining in endometrial carcinomas (23). The immunohistochemistry was interpretable in all cases. Although a few cases were slightly problematic, including negative cases with a faint background blush and some cases that showed variable staining intensity, they could still be scored. This is reflected in the high concordance rates seen between the two institutions (kappa of 0.8). This kappa value is close to the target kappa values for ER and HER-2 staining in large quality assurance Canadian studies (26, 27). It also appears that PTEN immunohistochemistry performs equally well in both histologic subtypes of endometrial carcinoma, as there was no difference in kappa for endometrioid versus non-endometrioid carcinomas. Similar to prior studies, we noted loss of PTEN staining more frequently in endometrioid compared to non-endometrioid tumors (8, 9, 10, 12, 28).
While this simple scoring system appears to be effective for interpretation of PTEN immunohistochemistry in endometrial carcinoma, it is not clear if this scoring scheme is applicable in other tumor types. Endometrial carcinoma has frequent mutations in PTEN; the immunohistochemical expression of PTEN may be very different in cancer types with lower mutation frequencies or with alternative mechanisms of PTEN protein regulation. As with endometrial carcinoma, there are currently no guidelines for PTEN interpretation in other cancer types.
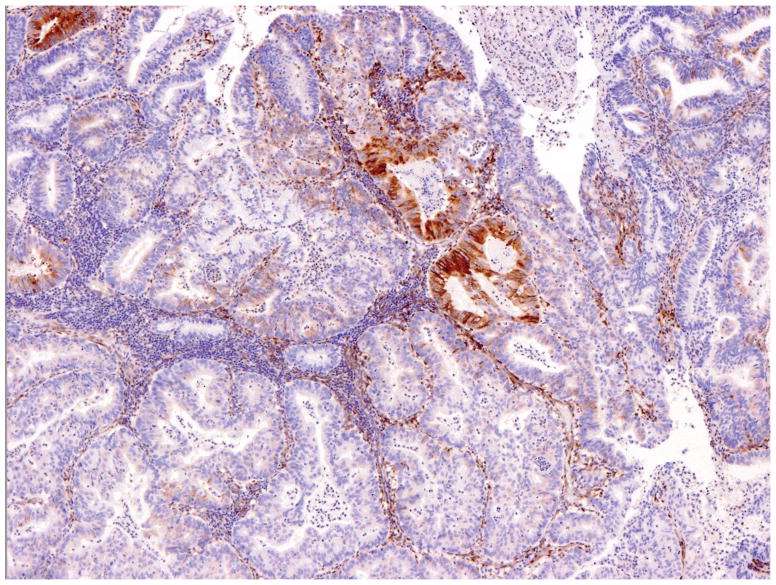
Overall, 17% of cases yielded discrepant results between the two institutions. These cases were re-evaluated, and most actually showed identical PTEN staining patterns; the discrepancies resulted from different interpretations. This typically occurred in tumors with very focal staining or very focal absence of staining; cases such as these were interpreted as negative and positive respectively at one institution and heterogeneous at the second center (figure 2A). Incorporation of more rigid criteria to define negative and positive staining would substantially reduce this problem. We preliminarily propose using a cut-off of 5% as follows: negative (tumor with lack of staining in 95% or more tumor cells), positive (presence of staining in 95% or more of tumor cells) and heterogeneous. Application of these criteria should further increase inter-observer agreement rates. These numbers can be modified in future studies based on correlation with genotyping data and response to targeted therapy. The 2 cases interpreted as positive versus negative showed faint staining throughout, a pattern that was interpreted as positive at one institution and negative at the other (figure 2B).
Figure 2.
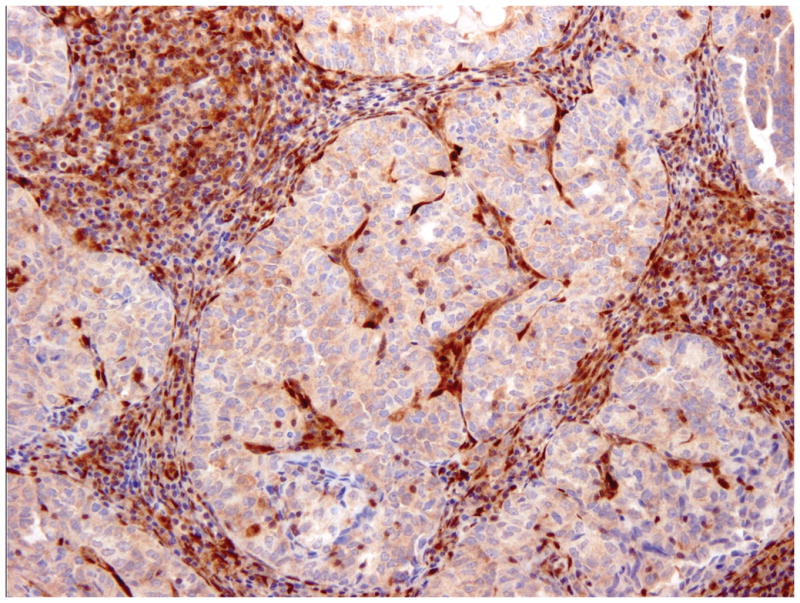
Discrepant PTEN immunohistochemistry cases. Tumors with very focal staining (arrow) were interpreted as negative at one institution and heterogeneous at the second (A). Cases with faint staining were typically problematic and interpreted as positive at one institution and negative at the second (B).
Heterogeneous PTEN protein expression was noted in 13% of our cases with agreement. Tumors with heterogeneous staining typically show discrete foci with absence of staining while adjacent foci are clearly positive. Often, these foci are scattered throughout the tumor. When a semi-quantitative scoring system (H-score) was applied to these cases, they showed a wide range in amount and intensity of staining, reflected in the wide variation in H-score (15 to 230) (24). The significance of heterogeneous staining is not clear and whether it is indicative of different tumor clones or presence of multiple mutations, including other mutations along the PI3K pathway, is not currently known. However, the absence of PTEN staining, even in a proportion of the tumor, may indicate activation of PI3K-AKT pathway at least in this tumor subpopulation. Correlation of H-score values with molecular analyses and response to PI3K pathway inhibitors, when these data become available in the future, may help clarify the significance of these results. Pallares et al found that lower H-score correlated with presence of PTEN molecular alterations, although this was not statistically significant (22). Given this uncertainty as to the nature of the heterogeneous group, we repeated our statistical analyses to differentiate cases with no PTEN loss from those with any amount of PTEN loss, by grouping the heterogeneous cases with the negative cases. The kappa values remained comparable to the original analysis.
It is interesting to note that concordance rates were higher for cases originating from MDACC compared to cases from MSKCC. This difference may be attributable to the fact that some of the MSKCC cases sent to MDACC were older slides that had been obtained more than one year ago, and may thus have contributed to staining differences. Therefore, it is possible that PTEN immunohistochemistry is best obtained on freshly cut tissue sections as noted previously by Mutter (8). It is also interesting to note that the subset of endometrial carcinomas scored as heterogeneous might have been under-represented or missed if tissue microarrays, which sample a very small core of tumor, had been used for this study. Also, tissue microarray cores tend to sample very little stroma; as we have shown, such stroma is crucial in ascertaining that the immunohistochemistry test is working optimally.
With the goal of integrating PTEN testing for clinical purposes, additional studies are needed to examine the correlation of PTEN staining patterns with response to PI3K-AKT pathway inhibitors. There are other immunohistochemical stains that may indicate activation of this pathway, including p-AKT, pS6, MTOR, INPP4B and 4EB-P1 amongst others. Antibodies for p-AKT have been particularly difficult to work with. While some studies have shown correlation between PTEN and p-AKT staining, others have failed to demonstrate good p-AKT staining or an inverse relationship between the two (22, 29, 30). We have had no success with p-AKT antibody and are currently working on some of the other antibodies along this pathway.
In conclusion, PTEN inactivation is frequently seen in endometrial carcinomas, particularly of the endometrioid type, suggestive of PI3K-AKT pathway dysregulation. Therefore these patients may potentially respond to inhibitors to this pathway. PTEN immunohistochemistry is an effective and easy method to detect PTEN inactivation and may provide a method to select patients with potential for response to these inhibitors. For the first time, our study demonstrates that evaluation of PTEN loss by immunohistochemistry in endometrial carcinomas is highly reproducible with the application of standard immunohistochemical techniques and simple scoring criteria.
Acknowledgments
Research support:
Stand Up to Cancer/American Association for Cancer Research Dream Team Translational Cancer Research Grant, Grant No. SU2C-AACR- DT0209 (KG, RRB, RAS, DAL).
Uterine Cancer SPORE NIH P50 CA098258 (RRB)
The authors would like to acknowledge the contributions of Alexia Iasonos and Christine Zhou, Department of Biostatistics, Memorial Sloan Kettering Cancer Center.
References
- 1.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15(1):10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 2.Oda K, Stokoe D, Taketani Y, et al. High frequency of coexistent mutations of PIK3CA and PTEN genes in endometrial carcinoma. Cancer Res. 2005;65:10669–10673. doi: 10.1158/0008-5472.CAN-05-2620. [DOI] [PubMed] [Google Scholar]
- 3.Velasco A, Bussaglia E, Pallares J, et al. PIK3CA gene mutations in endometrial carcinoma. Correlation with PTEN and K-RAS alterations. Hum Pathol. 2006;37:1465–1472. doi: 10.1016/j.humpath.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Catasus L, Gallardo A, Cuatrecasas M, et al. Concomitant PI3K-AKT and p53 alterations in endometrial carcinomas are associated with poor prognosis. Mod Pathol. 2009;22:522–529. doi: 10.1038/modpathol.2009.5. [DOI] [PubMed] [Google Scholar]
- 5.Engelman JA. Targeting PI3K signaling in cancer: opportunities, challenges and limitations. Nature reviews. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 6.Risinger JL, Hayes AK, Berchuck A, et al. PTEN/MMAC1 mutations in endometrial cancers. Cancer Res. 1997;57:4736–8. [PubMed] [Google Scholar]
- 7.Stambolic V, Tsao MS, Macpherson D, et al. High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in PTEN +/− mice. Cancer Res. 2000;60:3605–11. [PubMed] [Google Scholar]
- 8.Mutter GL, Lin MC, Fitzgerald JT, et al. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst. 200;92:924–931. doi: 10.1093/jnci/92.11.924. [DOI] [PubMed] [Google Scholar]
- 9.Mutter GL, Ince TA, Baak JPA, et al. Molecular identification of latent precancers in histologically normal endometrium. Cancer Res. 2001;61:4311–4314. [PubMed] [Google Scholar]
- 10.Bussaglia E, DelRio E, Matias-Guiu X, et al. PTEN mutations in endometrial carcinomas: A molecular and clinicopathologic analysis of 38 cases. Hum Pathol. 2000;31:312–317. doi: 10.1016/s0046-8177(00)80244-0. [DOI] [PubMed] [Google Scholar]
- 11.Tashiro H, Blazes MS, Wu R, et al. Mutations in PTEN are frequent in endometrial carcinoma but rare in other common gynecological malignancies. Cancer Res. 1997;57:3935–3940. [PubMed] [Google Scholar]
- 12.Risinger JI, Hayes K, Maxwell GL, et al. PTEN mutation in endometrial cancers is associated with favorable clinical and pathologic characteristics. Clin Cancer Res. 1998;4:3005–3010. [PubMed] [Google Scholar]
- 13.Kong D, Yamori T. Phosphatidylinositol 3-kinase inhibitors: promising drug candidates for cancer therapy. Cancer Sci. 2008;99(9):1734–1740. doi: 10.1111/j.1349-7006.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janku F, Tsimberidou AM, Garrido-Laguna I, et al. PK3CA mutations in patients with advanced cancers treated with PI3K/AKTMTOR axis inhibitors. Mol Cancer Ther. 2011;10(3):558–565. doi: 10.1158/1535-7163.MCT-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neshat MS, Mellinghoff IK, Tran C, et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. PNAS. 2001;98(18):10314–10319. doi: 10.1073/pnas.171076798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Podsypanina K, Lee RT, Politis C, et al. An inhibitor of MTOR reduces neoplasia and normalizes p70/S6 kinase activity in PTEN +/− mice. PNAS. 2001;98(18):10320–10325. doi: 10.1073/pnas.171060098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steelman LS, Navolanic PM, Sokolosky ML, et al. Suppression of PTEN function increased breast cancer chemotherapeutic resistance while conferring sensitivity to MTOR inhibitors. Oncogene. 2008;27:4086–4095. doi: 10.1038/onc.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oza AM, Elit L, Provechner D, et al. A phase II study of temsirolimus (CCI-779) in patients with metastatic and/or locally advanced recurrent endometrial cancer previously treated with chemotherapy. J Clin Oncol. 2008;26:296s. [Google Scholar]
- 19.Slomovitz BM, Lu KH, Johnston T, et al. A phase II study of oral mammalian target of rapamycin (Mtor) inhibitor RAD001 (everolimus) in patients with recurrent endometrial carcinoma (EC) J Clin Oncol. 2008;26(15S):5502. [Google Scholar]
- 20.Garrido-Laguna I, Janku F, Tsimberidou A, et al. Phosphatase and tensin homologue (PTEN) loss and response to phase I trials targeting PI3K/AKT/MTOR pathway in patients with advanced cancer. J Clin Oncol. 2010;28(suppl):abstr e13018. [Google Scholar]
- 21.Zhang S, Yu D. PI(3)King apart PTEN’s role in Cancer. Clin Cancer Res. 2010;16(17):4325–4330. doi: 10.1158/1078-0432.CCR-09-2990. [DOI] [PubMed] [Google Scholar]
- 22.Pallares J, Bussaglia E, Martinez-Guitarte JL, et al. Immunohistochemical analysis of PTEN in endometrial carcinoma: a tissue microarray study with a comparison of four commercial antibodies in correlation with molecular abnormalities. Mod Pathol. 2005;18:719–727. doi: 10.1038/modpathol.3800347. [DOI] [PubMed] [Google Scholar]
- 23.Sangale Z, Prass C, Carlson A, et al. A robust immunohistochemical assay for detecting PTEN expression in human tumors. Appl Immunohistochem Mol Morphol. 2011;19:173–183. doi: 10.1097/PAI.0b013e3181f1da13. [DOI] [PubMed] [Google Scholar]
- 24.McCarty KS, Miller LS, Cox EB, et al. Estrogen receptor analyses. Correlation of biochemical and immunohistochemical;, ethods using monoclonal antireceptor antibodies. Arch Pathol Lab Med. 1985;109(8):716–721. [PubMed] [Google Scholar]
- 25.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 26.Terry J, Torlakovik EE, Garratt J, et al. Implementation of a Canadian quality assurance program for breast cancer biomarkers: An initiative of Canadian quality control in immunohistochemistry and Canadian Association of Pathologists national standards committee/immunohistochemistry. Appl Immunohistochem Mol Morph. 2009;17(5):375–382. doi: 10.1097/PAI.0b013e31819adacf. [DOI] [PubMed] [Google Scholar]
- 27.Parker RL, Huntsman SG, Lesack DW, et al. Assessment of interlaboratory variation in the immunohistochemical determination of estrogen receptor status using a breast cancer tissue microarray. Am J Clin Pathol. 2002;117:723–728. doi: 10.1309/PEF8-GL6F-YWMC-AG56. [DOI] [PubMed] [Google Scholar]
- 28.Dellas A, Jundt G, Sartorius G, et al. Combined PTEN and p27 protein expression patterns are associated with obesity and prognosis in endometrial carcinomas. Clin Cancer Res. 2009;15:2456–62. doi: 10.1158/1078-0432.CCR-08-1732. [DOI] [PubMed] [Google Scholar]
- 29.Soslow RA, Saunders N, Dupont J. PTEN and AKT expression in endometrial and ovarian carcinomas. Mod Pathol. 2004;17(suppl 1):214A. [Google Scholar]
- 30.Kanamori Y, Kigawa J, Itamochi h, et al. Correlation between loss of PTEN expression and Akt phosphorylation in endometrial carcinoma. Clin Cancer Res. 2001;7:892–895. [PubMed] [Google Scholar]