Abstract
Diabetes mellitus is a common cause of pyelonephritis. Both emphysematous pyelonephritis (EPN) and non-EPN (NEPN) are associated with poor outcome. This study was aimed at analyzing the clinical features, microbiological profile, prognostic factors, and treatment outcome of pyelonephritis in diabetic patients. A total of 105 diabetic patients with pyelonephritis were admitted from July 2010 to June 2012. Patients were treated with appropriate antibiotics and percutaneous drainage (PCD) as indicated. Nephrectomy was carried out in patients of EPN who were refractory to conservative measures. NEPN and EPN were seen in 79 (75.2%) and 26 (24.7%) patients, respectively. Escherichia coli was the most common organism. Pyelonephritis was associated with renal abscess and papillary necrosis in 13 (12.4%) and 4 (3.8%) patients with EPN and NEPN, respectively. Worsening of renal functions were seen in 92 and 93% of patients with EPN and NEPN, respectively. Class 1 EPN was seen in 2 (7.7%), Class II in 8 (30.7%), IIIa in 7 (27%), IIIb in 5 (19.3), and IV in 4 (15.4%) patients. Antibiotics alone were sufficient in 38.5% of EPN versus 62% in NEPN; additional PCD was required in 42.3% in EPN and 21.4% in NEPN. Nephrectomy was required in 5 (19.2%) EPN patients with Class IIIB or IV. A total of 13 patients (12.4%) expired, 4 (15.4%) in EPN, and 9 (11.4%) in NEPN group. Patients with EPN had a higher incidence of shock (6% vs. 0; P < 0.05) and poorly controlled blood sugar (26% vs. 50%; P < 0.05) compared with NEPN. Presence of shock and altered sensorium were associated with poor outcome in patients with EPN. Diabetics with pyelonephritis have severe disease. Patients of EPN have poorer treatment outcome compared with those with NEPN. However, there is no difference in the mortality, but a greater need of nephrectomy in EPN compared with NEPN patients. Presence of shock and altered sensorium at presentation were poor prognostic factors in EPN.
Keywords: Diabetes mellitus, emphysematous pyelonephritis, nonemphysematous pyelonephritis
Introduction
In a recent community-based estimate, urinary tract infections (UTIs) were found to be second only to lower respiratory tract infections among older diabetics with incidence of 51.4 and 147.9/1000 years for men and women, respectively.[1] The extent of involvement ranges from inconsequential lower urinary tract colonization to cystitis, pyelonephritis and renal or perirenal abscess. Patients with diabetes mellitus (DM) have increased incidence of acute pyelonephritis compared with nondiabetics; however, there are no studies to address this issue.
Emphysematous pyelonephritis (EPN) is the necrotizing infection of renal parenchyma with the presence of gas in the renal parenchyma, collecting system or perinephric tissue. EPN is an uncommon life-threatening condition, precipitated mainly by poorly controlled blood sugars and urinary tract obstruction. Prevalence of diabetes in patients with EPN ranges from 53%-90%, respectively. Conventional treatment of EPN is parenteral antibiotics with percutaneous or open surgical drainage and/or nephrectomy. There is no current consensus on management of EPN as to whether present day antibiotics alone good enough or is surgical intervention necessary and if surgical intervention required when should one go for nephrectomy.
NonEPN (NEPN) is a common UTI encountered in diabetic patients. Rollino et al. had analyzed 223 patients of NEPN, but it had only 14 patients with DM. The authors observed that the risk factor for NEPN was seen in 26% of cases and renal failure during the course of illness was seen in 9.4% of cases.[2] In addition, bilateral pyelonephritis is more common in diabetics, which predisposes them to more severe infection and greater complications. Diabetic patients are more likely to suffer from acute kidney injury due to UTI compared with nondiabetics.[3]
However, there have been no large studies, which have selectively looked into the clinical, microbial profile and treatment outcome of diabetic patients with pyelonephritis both NEPN and EPN. Hence to address this issue, this prospective observational study was undertaken to evaluate clinical, microbial profile, and treatment outcome of both NEPN and EPN in diabetic patients.
Materials and Methods
We prospectively followed all patients hospitalized at this hospital from July 2010 to June 2012 with a diagnosis of type 2 DM with pyelonephritis. The clinical features and laboratory data at the initial presentation, management and outcomes were collected prospectively. The laboratory data included hemoglobin (HbA1c), total leukocyte count, differential count, platelet count, serum creatinine, fasting and post prandial blood glucose, urine routine examination with culture sensitivity, blood culture sensitivity, glycosylated HbA1c, and ultrasonography of urinary tract was performed at baseline. Contrast enhanced computerized tomography (CECT) was performed in case of suspected renal abscess and nonrecovering pyelonephritis.
Definitions
Acute pyelonephritis was said to be present when patient complained of fever with chills and rigors, flank pain, nausea, and vomiting. Ultrasound imaging studies were done and was considered to be suggestive of pyelonephritis if there was a combination of enlarged kidney, presence of collection and/or perinephric stranding
Emphysematous pyelonephritis was defined based on the presence of gas in the renal parenchyma, collecting system or perinephric tissue. On the basis of CT scan patients were classified into the following classes: (1) Class 1: Gas in the collecting system only (2) Class 2: Gas in the renal parenchyma without extension to the extrarenal space (3) Class 3A; Extension of gas or abscess to the perinephric space; Class 3B: Extension of gas or abscess to the pararenal space (4) Class 4: Bilateral EPN or solitary kidney with EPN
Renal abscess: Clinical manifestations of renal and perinephric abscess were similar to those of acute pyelonephritis. Imaging studies was done to localize abscess
Papillary necrosis was said to be present when tissueuria on histopathology showed papilla or CECT depicted contrast material-filled clefts in the renal medulla, and nonenhanced lesions surrounded by rings of excreted contrast material
Renal dysfunction: Presenting serum creatinine >1.5 mg/dl
Urine culture positive: >103 colony-forming units/ml of bacteria were found
Computed tomography was diagnostic of pyelonephritis if single or multiple hypodense areas were evidenced after contrast medium injection along with above-mentioned clinical features
Glycemic control: Defined as good if HbA1c <7%, moderate if HbA1c 7-7.5% and poor if HbA1c >7.5%.
Management
Patients were treated with antibiotic as per culture sensitivity reports. Patients with NEPN were treated with parenteral antibiotics for 1 week followed by oral antibiotics for 2 weeks and EPN patients received antibiotics for at least 3 weeks. Patients with fungal UTI were initially treated with fluconazole for Candida Sp. and amphotericin for noncandida sp. and changed as per culture sensitivity and continued for 2 weeks.
Percutaneous drainage (PCD) with pigtail or percutaneous nephrostomy tube was inserted into pelvis or perirenal space to drain out fluid collection/gas in addition to antibiotics. Nephrectomy was carried out in patients refractory to antibiotics, PCD and/or clinical deterioration.
Patients were divided into “good” and “poor” outcome groups to elucidate the risk factors. The patients who were successfully treated with antibiotics alone or with PCD were assigned to “good” outcome group. Those who had nephrectomy or died were classified as “poor” outcome group. The two groups were compared for clinical features, and laboratory data at initial presentation.
Statistical methods
Values are expressed as mean ± standard deviation. The differences two groups were compared using Fischer exact test (two tailed) for categorical variables and Wilcoxon rank sum test for continuous variables. Univariate analysis was used to assess the outcomes in patients with EPN and NEPN. P < 0.05 was taken as an upper limit of statistical significance.
Results
During the study period, a total of 105 patients with type 2 DM had pyelonephritis. Of these, 26 (24.8%) had EPN and 79 (75.2%) had NEPN. Renal papillary necrosis and renal abscess was seen in 4 (3.8%) and 13 (12.3%) patients, respectively. The mean age of the patients was 57.4 ± 8.5 years (age range 20-75 years). Pyelonephritis was more common in males compared with female sex (62:43). Duration of symptoms prior to hospitalization ranged from 18.34 ± 6.33 (range 5-30) days. Renal dysfunction at presentation was seen in 98 (93.3%) patients. Bilateral involvement was seen in 35 (33.3%) patients. Fever was the most common presenting symptom followed by dysuria. Urine and blood cultures were positive in 93 (88.5%) and 41 (39%) patients respectively. Gram-negative bacilli were the most frequent organisms isolated, Escherichia coli in 81 (77.1%), Klebsiella sp. in 5 (4.7%), Pseudomonas in 8 (7.6%), polymicrobial and fungal UTI were seen in 12 (11.4%) and 7 (6.6%) respectively. The fungus included Candida albicans in five patients (all managed with fluconazole), Candida glabrata in one (initially managed with fluconazole and changed to amphotericin once the species identification and sensitivity was available) and aspergillus sp. (managed with amphotericin) in one patient. Good, moderate, and poor glycemic control was seen in 13 (12.3%), 16 (15.2%) and 76 (72.3%), respectively.
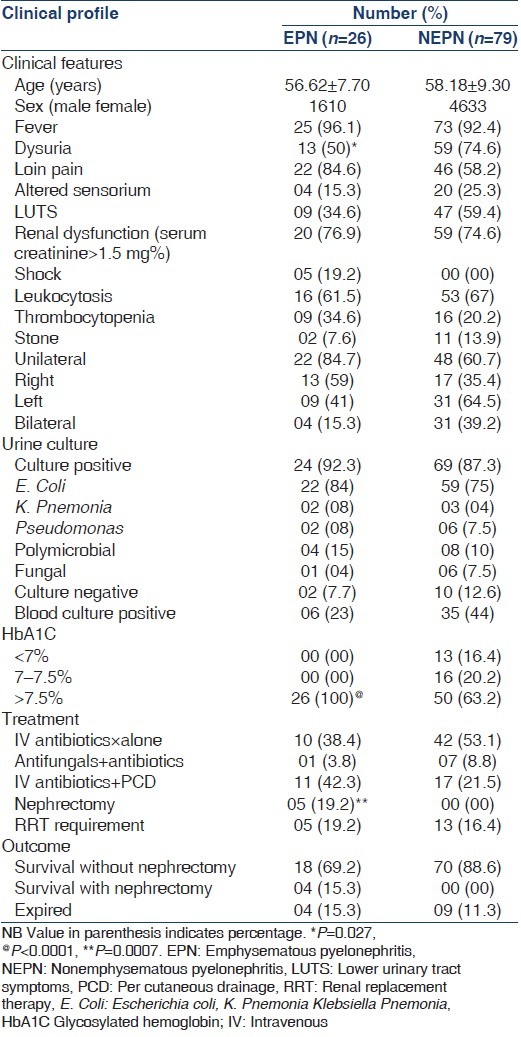
Comparison of the clinical features, microbiological details, laboratory data, management and outcome of EPN and NEPN are given in Table 1. Patients with EPN had poorer sugar control and higher rates of nephrectomy compared with NEPN patients (P < 0.05). Other clinical features were statistically similar in the two groups. Majority of patients with EPN and NEPN responded to antibiotics with or without PCD. Of 26 EPN patients, 22 (84.5%) survived and 4 (15.3%) expired. While in NEPN, 70 (88.6%) survived and 9 (11.3%) expired. In none of the NEPN patients, nephrectomy was required, while in EPN five patients underwent nephrectomy of these four survived.
Table 1.
Characteristics of EPN and NEPN
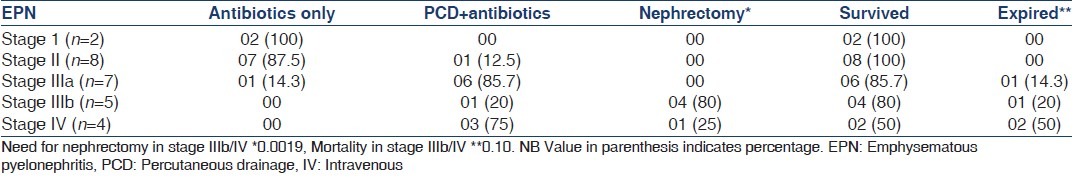
The nature of treatment and clinical outcome of patients with EPN is depicted in Table 2. All patients with Stage I, II and IIIa EPN (n = 17) were managed with antibiotics with or without PCD. One patient (5.9%) expired in this subgroup. In EPN Stage IIIb/IV (n = 9), 4 (44.4%) patients were managed with antibiotics and PCD and 5 (55.6%) needed nephrectomy. The need of nephrectomy was significantly higher in Stage IIIb/IV; however, the mortality in these stages were higher when compared to I/II/IIIa but not statistically significant.
Table 2.
Treatment and outcome of EPN
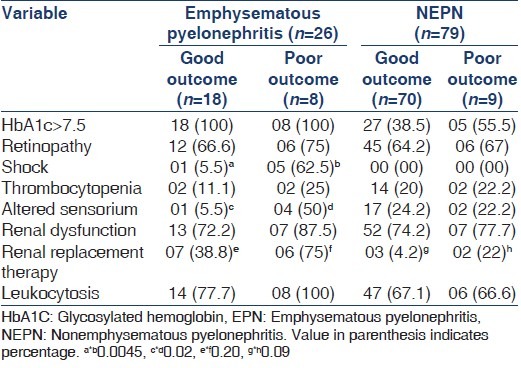
In EPN good outcome (survival without nephrectomy) was seen in 18 (69.2%), while the poor outcome (mortality/nephrectomy) in 8 (30.8%) patients. In NEPN patients, good outcome was observed in 70 (88.6%) and poor outcome in 9 (11.4%). Of different variable viz. HbA1c, retinopathy, shock, thrombocytopenia, altered sensorium, renal dysfunction and leukocytosis, only altered sensorium and shock at presentation were associated with poor outcome in EPN patients only (P < 0.05) [Table 3].
Table 3.
Comparison of prognostic indicators of poor and good outcome in EPN and NEPN
Discussion
DM is a common predisposing factor for UTI. In comparison to nondiabetics, epidemiological studies have shown that the relative risk of UTI in diabetics increases by a factor of 1.2-2.2.[4,5] Among hospitalized patients with acute pyelonephritis, DM has been shown to be the single most common predisposing cause.[3] The severity of UTIs is also increased in DM; the mean hospitalization rate in patients with acute pyelonephritis was found to be 3.4-24. One times higher in diabetics than nondiabetics.[6] Pyelonephritis in DM tends to be more frequently bilateral and is associated with greater complications. This fact is also corroborated by the present study, which is the largest study of pyelonephritis in diabetics, one-third of patients with acute pyelonephritis had bilateral disease, 39% had bacteremia and 17% had renal failure requiring renal replacement therapy at presentation. Patients with suspected complications underwent CECT irrespective of their renal functions, as the majority of the patients had renal dysfunction even before contrast exposure, it would be very difficult to judge the contribution of contrast to persistent renal failure. Although, UTIs in diabetics tend to be more severe, the spectrum of causative organisms for acute pyelonephritis is similar when compared to nondiabetics. In this study, E. coli was isolated in urine culture in 77% of patients.
Emphysematous pyelonephritis is a severe, necrotizing renal infection with potential to cause high morbidity and mortality, particularly if the diagnosis (and subsequent percutaneous/surgical intervention) is delayed. In this study, there were no many statistically significant differences in the presenting clinical features between nonEPN and EPN. The diagnosis in the majority of our patients was made incidentally by performing imaging studies in all patients of suspected pyelonephritis. Since there is a significant difference in the management approach between nonEPN and EPN, the results of the present study emphasize the importance of maintaining a high degree of suspicion and performing imaging studies early during the course of illness in diabetics with suspected acute pyelonephritis. All cases of EPN were confirmed on CT scans. In literature, the reported sensitivity of plain X-ray kidneys, ureters, and bladder, ultrasound and CT scan for picking up EPN is 65%, 69% and 100%, respectively.[7]
In the present study, all patients with EPN had poorly controlled blood sugars (HbA1c >7.5%). Hyperglycemia has been postulated as an important factor for the formation of gas in the renal parenchyma, probably because gas formation requires anaerobic metabolism of glucose.[8,9,10] This is in contrast to NEPN, which commonly occurs in the nondiabetic setting and can occur in the presence of well-controlled sugars in diabetic. Even in the present study, about one third of patients with NEPN had good or moderate control of blood sugars.
The optimal management of EPN is a matter of debate. The initial management consists of fluid and electrolyte resuscitation, antibiotics, glycemic control, and relief of obstruction. This is followed by either continuation of medical management alone, or PCD or surgical nephrectomy. Although medical therapy alone has been shown to be successful in a few reports,[11] this approach is generally associated with the highest mortality.[8,12] Thus, surgical therapy was thought to be the gold standard for treating EPN until the early 1990s. PCD along with antibiotics has been increasingly recognized over the last two decades for treating EPN.[7,13] Whether antibiotics along with drainage is enough, or there is a need of urgent nephrectomy? This study provides an answer to this dilemma faced by the clinicians. In the present study, majority (94.1%) of patients with Class I, II or IIIa disease on CT scan improved after antibiotic therapy either alone (used in Class I only) or combined with PCD. In contrast, 80% of patients with Class IIIb disease underwent nephrectomy and improved subsequently. The results of our study support the prognostic significance of the CT scan based classification proposed by Huang and Tseng,[8] and suggest that antibiotics along with PCD can be successfully used to manage Class I, II, IIIa EPN, whereas for IIIb and IV, a strict vigil is required as nephrectomy may have to be contemplated.
In contrast to EPN, NEPN is much more frequently seen in clinical practice and commonly occurs in the absence of diabetes and usually responds to antibiotics with or without aspiration of any abscesses. When compared to largest series of acute pyelonephritis by Rollino et al., patients in the present series had higher blood and urine culture positivity, renal dysfunction and mortality. The poor outcomes of the present study could be explained by the presence of diabetes, bilateral pyelonephritis and delayed institution of antibiotics. Nephrectomy was required in 19% of patients with EPN and none of patients with NEPN required nephrectomy (P = 0.0007). Mortality was reported in 15% and 11% of patients with EPN and NEPN respectively (P = 0.73). Poor outcomes was reported more often in EPN compared to NEPN (P = 0.03); however, when nephrectomy and mortality were analyzed as two different variables, only nephrectomy was found to be significantly higher in patients of EPN as compared to NEPN.
Conclusion
A high index of suspicion and early imaging studies are required to diagnose EPN in diabetics presenting with features of pyelonephritis, especially if blood sugars are poorly controlled. EPN patients with Class I, II and IIIa can be managed successfully with either antibiotics or with additional PCD. Class IIIb and IV may need nephrectomy. EPN with thrombocytopenia and altered sensorium at presentation portends poor prognosis. NEPN is diabetics may be associated with renal failure and a high mortality rate.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.McDonald HI, Nitsch D, Millett ER, Sinclair A, Thomas SL. New estimates of the burden of acute community-acquired infections among older people with diabetes mellitus: A retrospective cohort study using linked electronic health records. Diabet Med. 2014;31:606–14. doi: 10.1111/dme.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rollino C, Beltrame G, Ferro M, Quattrocchio G, Sandrone M, Quarello F. Acute pyelonephritis in adults: A case series of 223 patients. Nephrol Dial Transplant. 2012;27:3488–93. doi: 10.1093/ndt/gfr810. [DOI] [PubMed] [Google Scholar]
- 3.Chiu PF, Huang CH, Liou HH, Wu CL, Wang SC, Chang CC. Long-term renal outcomes of episodic urinary tract infection in diabetic patients. J Diabetes Complications. 2013;27:41–3. doi: 10.1016/j.jdiacomp.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Boyko EJ, Fihn SD, Scholes D, Abraham L, Monsey B. Risk of urinary tract infection and asymptomatic bacteriuria among diabetic and nondiabetic postmenopausal women. Am J Epidemiol. 2005;161:557–64. doi: 10.1093/aje/kwi078. [DOI] [PubMed] [Google Scholar]
- 5.Boyko EJ, Fihn SD, Scholes D, Chen CL, Normand EH, Yarbro P. Diabetes and the risk of acute urinary tract infection among postmenopausal women. Diabetes Care. 2002;25:1778–83. doi: 10.2337/diacare.25.10.1778. [DOI] [PubMed] [Google Scholar]
- 6.Nicolle LE, Friesen D, Harding GK, Roos LL. Hospitalization for acute pyelonephritis in Manitoba, Canada, during the period from 1989 to 1992; impact of diabetes, pregnancy, and aboriginal origin. Clin Infect Dis. 1996;22:1051–6. doi: 10.1093/clinids/22.6.1051. [DOI] [PubMed] [Google Scholar]
- 7.Somani BK, Nabi G, Thorpe P, Hussey J, Cook J, N'Dow J, et al. Is percutaneous drainage the new gold standard in the management of emphysematous pyelonephritis. Evidence from a systematic review? J Urol. 2008;179:1844–9. doi: 10.1016/j.juro.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Huang JJ, Tseng CC. Emphysematous pyelonephritis: Clinicoradiological classification, management, prognosis, and pathogenesis. Arch Intern Med. 2000;160:797–805. doi: 10.1001/archinte.160.6.797. [DOI] [PubMed] [Google Scholar]
- 9.Schainuck LI, Fouty R, Cutler RE. Emphysematous pyelonephritis. A new case and review of previous observations. Am J Med. 1968;44:134–9. doi: 10.1016/0002-9343(68)90245-3. [DOI] [PubMed] [Google Scholar]
- 10.Piccoli GB, Consiglio V, Deagostini MC, Serra M, Biolcati M, Ragni F, et al. The clinical and imaging presentation of acute “noncomplicated” pyelonephritis: A new profile for an ancient disease. BMC Nephrol. 2011;12:68. doi: 10.1186/1471-2369-12-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flores G, Nellen H, Magaña F, Calleja J. Acute bilateral emphysematous pyelonephritis successfully managed by medical therapy alone: A case report and review of the literature. BMC Nephrol. 2002;3:4. doi: 10.1186/1471-2369-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michaeli J, Mogle P, Perlberg S, Heiman S, Caine M. Emphysematous pyelonephritis. J Urol. 1984;131:203–8. doi: 10.1016/s0022-5347(17)50309-2. [DOI] [PubMed] [Google Scholar]
- 13.Sharma PK, Sharma R, Vijay MK, Tiwari P, Goel A, Kundu AK. Emphysematous pyelonephritis: Our experience with conservative management in 14 cases. Urol Ann. 2013;5:157–62. doi: 10.4103/0974-7796.115734. [DOI] [PMC free article] [PubMed] [Google Scholar]


