Abstract
The high prevalence of end-stage kidney diseases demands new treatment strategies. Decellularization approach may provide a viable option grow organs using a regenerative medicine approach. Goat kidney was decellularized by perfusion decellularization using detergents to produce an cellular construct for kidney scaffold. After pre-treatment with anticoagulant, the decellularized scaffold was analyzed for its intact three-dimensional natural architecture and vasculature. Perfusion of decellularized kidney preserved the structure and composition of renal extra-cellular matrix and vascular structures within the scaffold. No evidence of residual cellular components was found. This approach provides a model for understanding of whole organ regeneration.
Keywords: Extra-cellular matrix, goat kidney, organ engineering, perfusion decellularization
Introduction
The increasing prevalence of patients with end-stage kidney diseases and limited organ donors suggests need for innovative treatment approaches. Some renal assist devices have reached the stage of preclinical evaluation and hold promise to improve the quality of life of patients suffering from end-stage renal failure.[1,2,3] However, these strategies provide only a part of the functional kidney and fail to provide life-long protection and alternative to the natural kidney.
Whole organ decellularization represents a novel approach to create bio-artificial natural three-dimensional (3D) architecture. A number of methods have been used to remove the cells and its components from different organ leaving its extra-cellular matrix (ECM) and organ scaffold.[4,5,6] There is a need to find a more appropriate decellularization agent to improve the quality of the scaffold. Several studies have demonstrated the use of a variety of chemical, biological and physical substance for whole organ decellularization.
Whole organ decellularization eliminates potentially immunogenic cells, while maintaining the scaffold and vascular structures for efficient supply of oxygen and nutrients.[7] It provides a natural 3D platform for cell transplantation, migration, engraftment, and survival. It also acts as natural model for drug testing. The ECM provides important factors for cellular proliferation and differentiation. Several bioactive molecules are released after transplantation which makes the tissue architecture close to natural.[6]
Getting a suitable source for organ donors remains a challenge. Previous studies have demonstrated the potential of decellularization on rat, pig and monkey. These sources suffer with various serious hurdles of zoonosis and compatibility with humans. Hence, there is a need to find alternative xenogenic sources to fill the gap between the shortage of available organ donors. Goat is closer to humans and the organ size and complexity is similar. Hence, the present study was undertaken to develop a 3D goat kidney scaffold by standardizing appropriate protocol for perfusion decellularization to provide a model for organ engineering.
Materials and Methods
Experimental design
Goat kidney was decellularized by perfusion decellularization using a detergent to produce acellular construct. After pre-treatment with anticoagulant, the decellularized kidney scaffold was analyzed for its intact 3D natural architecture and vasculature. The study was approved by Institutional Ethics Committee of Deccan College of Medical Sciences, Hyderabad, India.
Heparin pre-treatment
Goat (age: <1 year, sex: M) kidney was dissected from the abdomen along with renal artery, vein and ureter. Renal artery was cannulated with a prefilled cannula and was perfused using 50 U/mL heparin and antibiotic-containing physiological saline (pH 7.4) for 30 min to prevent blood clotting.
Perfusion decellularization
After heparinization, cannula of the renal artery was connected with ionic detergent, sodium do-decyl sulfate (0.1% SDS) to solubilize and disrupt cell membranes, thereby lysing cells; and perfused for 30 min at 100 mm Hg pressure and 37°C temperature. Further the kidney was subjected to perfusion decellularization using gradient of SDS (0.5% SDS for 48 h and 1.0% SDS for 24 h) and 0.1% Triton-X-100 for 30 min at constant physiological fluid pressure. 5 mM solution of calcium chloride and magnesium sulfate was also used for perfusion to activate endogenous nucleases and digest bulky nuclear material. Finally 0.0025% deoxyribonuclease 1 was used for enzymatic digestion of deoxyribonucleic acid (DNA).
Chemical sterilization of decellularized kidney
After perfusion decellularization the cannula of renal artery was connected with deionized water to rinse the preparation for 15 min and sterilized by perfusing with × 1 phosphate buffer saline (PBS) containing 10,000 U/mL penicillin G, 10 mg/mL streptomycin and 25 μg/mL amphotericin B at 1 mL/min constant perfusion for 48 h.
Histological evaluation
Decellularized kidney was fixed in paraffin (5% formalin buffered × 1 PBS) for 24 h at room temperature. Sections to be frozen were fixed in 4% paraformaldehyde at 4°C. Sections were prepared by cutting into 5-μm thin sections and subjected to hematoxylin and eosin staining using standard protocols.
Deoxyribonucleic acid estimation
Total ribonucleic acid was extracted from the decellularized kidney scaffold using guanidinium thiocyanate method. cDNA was synthesized using oligo dT primers. Qualitative polymerase chain reaction (PCR) was performed using primers specific for Pax-2, Ksp-cadherin and glyceraldehyde-3-phosphate dehydrogenase. Cells from other goat kidney were used as a positive control to check the efficiency of the primers and PCR conditions.
Nuclei were stained using 4', 6-diamidino-2-phenylindole (DAPI) to check for any residual DNA.
Decellularized kidney angiogram
Whole decellularized kidney was kept under the live imaging system. Contrast was infused through renal artery and the vasculature of the decellularized kidney was imaged.
Results
Decellularized goat kidney
We decellularized goat kidney using renal artery perfusion with SDS, Triton-X-100, calcium chloride and magnesium sulfate at a constant pressure of 100 Hg [Figure 1]. Histology of the decellularized kidney showed preservation of ECM, which is integral in filtration, secretion and reabsorption. The kidney capsule was found intact retaining its complete vasculature [Figure 2].
Figure 1.

In house set-up for perfusion decellularization of whole kidney
Figure 2.

Decellularized goat kidney (a) after 24 h (b) after 48 h (c) after 72 h (d) after 96 h
Validation of heparin pretreatment
Pretreatment of goat kidney using heparin through the renal artery didn't show any blockage or rupture in the kidney vasculature showing a crucial step before starting the decellularization process. Blood clot was not observed in any of the part of kidney scaffold generated after decellularization.
Determination of deoxyribonucleic acid and other cellular components
Sodium do-decyl sulfate, deionized water, Triton-X-100, calcium chloride and magnesium sulfate reduced the DNA content within the decellularized kidney scaffold. PBS washing removed all the SDS present in the scaffold used during decellularization process. PCR analysis and DAPI staining of whole decellularized goat kidney scaffold didn't show the presence of any residual DNA or cell nuclear component within the organ scaffold [Figure 3].
Figure 3.
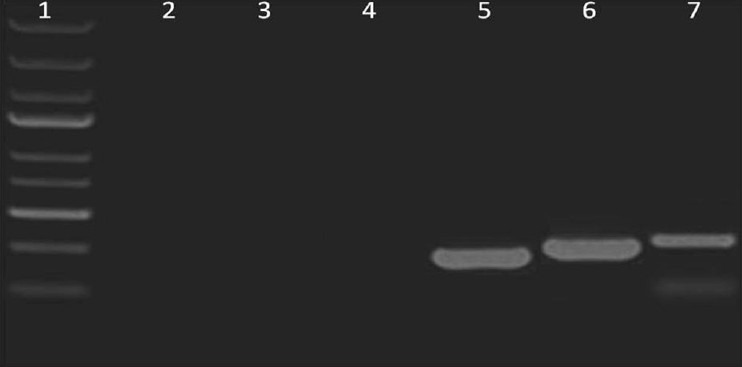
Expression analysis of kidney cell specific markers using polymerase chain reaction: Lane 1: 1Kb deoxyribonucleic acid ladder, Lane 2: Pax-2 (165 bp) not expressed in decellularized kidney, Lane 3: Ksp-Cadherin (188 bp) not expressed in decellularized kidney, Lane 4: glyceraldehyde-3-phosphate dehydrogenase (205 bp) was not expressed, Lane 5-7: Positive expression was observed for GAPDH, Pax-2 and Ksp- Cadherin in freshly harvested kidney tissue (positive control)
Determination of kidney vasculature
Angiogram showed intact vascular structure within the organ scaffold [Figure 4]. No sign of spillage of contrast showed integrity of the vascular structures within the scaffold.
Figure 4.
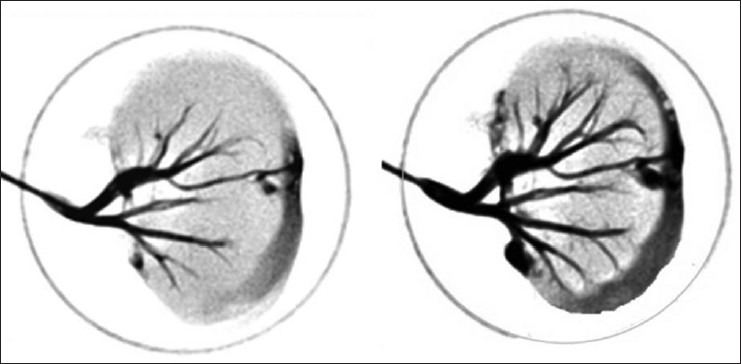
Kidney angiogram showing intact vascular tree of whole decellularized goat kidney
Discussion
Organization of kidney cells is very complex where each type of cell has a specific function. Unlike liver and other organs where cells are distributed throughout the organ and perform the same function, in kidney each cell is committed to perform a specific function depending upon their location. And loss of any cell type leads to functional impairment. Standardization of decellularization protocols for higher mammals that are closer to human can be taken for further applications. ECM composition within a single organ system varies from animal to animal. Various studies have demonstrated the decellularization approach on small mammals such as rat where the organ size is very small and architecture is much far as compared to human.[5,6,8] Hence, there is a need to standardize the protocols to ideal xenogenic sources of organs.
Goat can be an ideal source for the development of organ scaffold and further to engineer the bioartificial organ support because of low risk of zoonosis. The organ architectures and size of the organ is almost similar to human.[9,10] However, among the higher animals, the pig and monkey are considered suitable xenogenic source because of the similarity in size and functions of the organs. Transplantation of pig organ/cells for human therapy suffers with several limitations such as improper functioning of xenografts and the major risk of transmission of transmission of endogenous retroviruses to the recipient. Hence, decellularized goat kidneys may provide a viable option to bridge the gap between the donor and the recipients waiting for organ transplantation with further advancements.
Conclusion
In summary, we showed goat kidney as a source for the development of natural 3D organ scaffold and the need of standardization of decellularization protocols required for producing whole goat kidney scaffold. This approach provides a model for better understanding of whole organ regeneration and shows promise for a suitable organ donor for the recipients waiting for organ transplantation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Rogers SA, Hammerman MR. Prolongation of life in anephric rats following de novo renal organogenesis. Organogenesis. 2004;1:22–5. doi: 10.4161/org.1.1.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gura V, Macy AS, Beizai M, Ezon C, Golper TA. Technical breakthroughs in the wearable artificial kidney. Clin J Am Soc Nephrol. 2009;4:1441–8. doi: 10.2215/CJN.02790409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fissell WH, Roy S. The implantable artificial kidney. Semin Dial. 2009;22:665–70. doi: 10.1111/j.1525-139X.2009.00662.x. [DOI] [PubMed] [Google Scholar]
- 4.Ross EA, Williams MJ, Hamazaki T, Terada N, Clapp WL, Adin C, et al. Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J Am Soc Nephrol. 2009;20:2338–47. doi: 10.1681/ASN.2008111196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross EA, Abrahamson DR, St John P, Clapp WL, Williams MJ, Terada N, et al. Mouse stem cells seeded into decellularized rat kidney scaffolds endothelialize and remodel basement membranes. Organogenesis. 2012;8:49–55. doi: 10.4161/org.20209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song JJ, Guyette JP, Gilpin SE, Gonzalez G, Vacanti JP, Ott HC. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med. 2013;19:646–51. doi: 10.1038/nm.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baptista PM, Orlando G, Mirmalek-Sani SH, Siddiqui M, Atala A, Soker S. Whole organ decellularization-A tool for bioscaffold fabrication and organ bioengineering. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:6526–9. doi: 10.1109/IEMBS.2009.5333145. [DOI] [PubMed] [Google Scholar]
- 8.Nakayama KH, Lee CC, Batchelder CA, Tarantal AF. Tissue specificity of decellularized rhesus monkey kidney and lung scaffolds. PLoS One. 2013;8:e64134. doi: 10.1371/journal.pone.0064134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Habibullah CM, Vijayalakshmi V, Naseem B, Habeeb MH, Shashi S, Rao M. Hepatofunctional study of UV-B (302 nm) irradiated goat hepatocytes. Am J Gastroenterol. 2000;95:2511–2. [Google Scholar]
- 10.Khan AA, Capoor AK, Parveen N, Naseem S, Venkatesan V, 5th, Habibullah CM. In vitro studies on a bioreactor module containing encapsulated goat hepatocytes for the development of bioartificial liver. Indian J Gastroenterol. 2002;21:55–8. [PubMed] [Google Scholar]


