Abstract
Aims:
To compare the consistency and accuracy in ocular biometric measurements and intraocular lens (IOL) power calculations using the new optical low-coherence reflectometry and partial coherence interferometry.
Subjects and Methods:
The clinical data of 122 eyes of 72 cataract patients were analyzed retrospectively. All patients were measured with a new optical low-coherence reflectometry system, using the LENSTAR LS 900 (Haag Streit AG)/ALLEGRO BioGraph biometer (Wavelight., AG), and partial coherence interferometry (IOLMaster V.5.4 [Carl Zeiss., Meditec, AG]) before phacoemulsification and IOL implantation. Repeated measurements, as recommended by the manufacturers, were performed by the same examiner with both devices. Using the parameters of axial length (AL), corneal refractive power (K1 and K2), and anterior chamber depth (ACD), power calculations for AcrySof SA60AT IOL were compared between the two devices using five formulas. The target was emmetropia. Statistical analysis was performed using Statistical Package for the Social Sciences software (SPSS 13.0) with t-test as well as linear regression. A P value < 0.05 was considered to be statistically significant.
Results:
The mean age of 72 cataract patients was 64.6 years ± 13.4 [standard deviation]. Of the biometry parameters, K1, K2 and [K1 + K2]/2 values were significantly different between the two devices (mean difference, K1: −0.05 ± 0.21 D; K2: −0.12 ± 0.20 D; [K1 + K2]/2: −0.08 ± 0.14 D. P <0.05). There was no statistically significant difference in AL and ACD between the two devices. The correlations of AL, K1, K2, and ACD between the two devices were high. The mean differences in IOL power calculations using the five formulas were not statistically significant between the two devices.
Conclusions:
New optical low-coherence reflectometry provides measurements that correlate well to those of partial coherence interferometry, thus it is a precise device that can be used for the pre-operative examination of cataract patients.
Keywords: Cataract, intraocular lens, optical low-coherence reflectometry, partial coherence interferometry
Precise and predictable intraocular lens (IOL) power calculation is essential for high post-operative patient satisfaction. With premium IOLs being used more frequently, such as multi-focal and accommodating IOLs, and also toric IOLs, the accuracy of pre-operative calculations is becoming increasingly critical. Meeting target refraction is important for ophthalmologists and patients alike. The first step to achieve this goal is to ensure that the parameters needed for accurate IOL calculation are measured as precisely as possible. Ocular biometric parameters, including: Axial length (AL), corneal refractive power (K1 and K2), and anterior chamber depth (ACD), must be precisely measured to determine the correct IOL power to achieve the target refraction.[1]
Manual keratometry and ultrasound AL measurements have been the gold standard for a long time. However, the introduction of automated keratometry and partial coherence interferometry (PCI) biometry measurements have been an important step in modern cataract surgery. The IOLMaster was the first device that combined PCI technology for AL measurements with automated keratometry, ACD and corneal white-to-white distance measurements in one machine.[2,3] So it is possible to evaluate all required parameters with a single device. There is no need for topical anesthesia, the evaluation is quick, and the training required for examiner competence is minimal. Patients feel more comfortable during measurement as it is a non-contact technique. Furthermore, the IOLMaster biometer shows good interexaminer repeatability regardless of the examiner's medical training and provides excellent results in both long and short eyes.[4,5,6] The IOLMaster can also be used in patients with asymmetrically shaped globes, silicone oil-filled eyes and is particularly useful in anxious or nervous patients.[7] The IOLMaster is also just as precise as an immersion ultrasound for AL assessment.[3,8] Bhatt et al.,[9] compared the refractive outcomes after cataract surgery between IOLMaster optical biometry and ultrasound biometry and found that the former gave better post-operative results.
The new ALLEGRO BioGraph (Wavelight., AG)/LENSTAR LS 900 (Haag Streit., AG) biometer is based on optical low-coherence reflectometry (OLCR), and permits synchronous measurement of AL, ACD, keratometry, and white-to-white distance; and also corneal, lens, and retinal thickness parameters, and distance from the endothelium to the anterior surface of the lens, which are very important for phakic IOL implantation and pupillometry studies. It also permits IOL power to be calculated using different formulas.[10,11] All parameters may be determined in a single step with a single alignment.
The purpose of this study was to evaluate the accuracy of biometry and precision of IOL power calculations in cataract patients with the LENSTAR device, and to compare the results with those obtained from the IOLMaster.
Subjects and Methods
In this retrospective study, seventy-two cataract patients (122 eyes) who received phacoemulsification and IOL implantation were analyzed.
Measurement technique
During the pre-operative examination, biometry was first performed using an IOLMaster V5.4 followed by assessment with the LENSTAR device. Patients were excluded if measurement of any parameter was not possible to obtain with either device. Five AL and ACD measurements, and three keratometry measurements, were taken using the IOLMaster, followed by five consecutive measurements with the LENSTAR. The same examiner, who was trained according to the manufacturer's recommendations, performed all the biometry testing.
The AcrySof SA60AT IOL (Alcon, Inc.) was used for the purpose of IOL power calculations and the target in each patient was emmetropia. The IOL powers were calculated with both biometers using five formulae: SRK II, SRK/T, Hoffer Q, Holladay, and Haigis.
Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences software (SPSS 13.0) with t-test as well as linear regression. A P < 0.05 was considered to be statistically significant.
Results
We measured 160 cataract eyes. 28 eyes were excluded from analysis due to measurements failures for IOLMaster, and 38 eyes were excluded for LENSTAR. 122 eyes were successfully measured by both IOLMaster and LENSTAR.
The mean age of the patients was 64.6 years ± 13.4 (standard deviation). The mean values and standard deviations for AL, K1, K2, and ACD with both biometers, as well as the difference between the devices, are shown in Table 1. The two biometers provided generally similar and reliable results. The differences of K1, K2 and [K1 + K2]/2 between the two devices were statistically significant (mean difference, K1= −0.05 ± 0.21 D; K2= −0.12 ± 0.20 D; [K1 + K2]/2= −0.08 ± 0.14 D. P <0.05; t-test). The difference of AL between the two devices was (0.02 ± 0.10 mm) and (−0.97 to 0.33 mm). The difference of ACD between the two devices was (−0.02 ± 0.17 mm) and (−0.68 to 0.58 mm). The differences of AL and ACD between the two devices were not found to be statistically significant (P < 0.05; t-test).
Table 1.
Comparison of parameter measurements
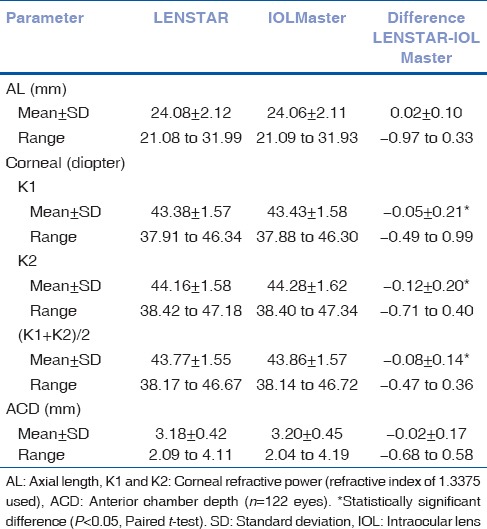
Table 2 shows the SA60AT IOL power calculation results using the five formulas. The differences of IOL power calculation between the two devices were 0.00 ± 0.17 (SRK/formula), 0.01 ± 0.22 (SRK/T formula), 0.03 ± 0.25 (Hoffer Q formula), 0.03 ± 0.22 (Holladay formula) and 0.04 ± 0.25 (Haigis formula), respectively. There were no statistically significant differences between the two devices (P > 0.05; t-test).
Table 2.
Comparisons of IOL SA60AT powers (D)
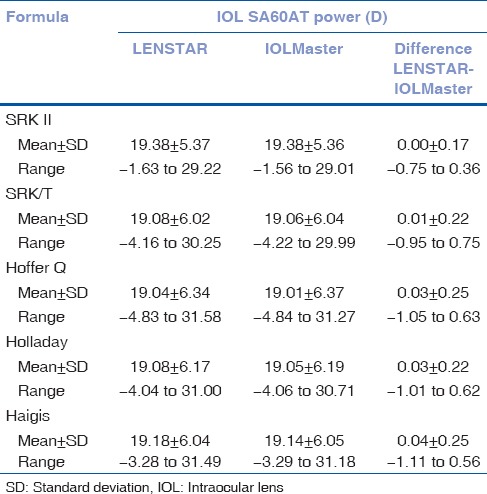
Fig. 1 demonstrates the distribution of the differences in SA60AT IOL power for each formula. Overall, a mean of 45.6% ±5.8% of eyes were within a ± 0.10 D difference; 71.2% ±8.2% were within ± 0.20 D; 85.9% ±5.4% were within ± 0.30 D; 93.6% ±2.3% were within ± 0.40 D, 96.7% ±1.7% were within ± 0.50 D; 99.5% ±0.4% were within ± 1.00 D, and all were within ±1.50 D. The difference in SA60AT IOL power calculations, between the two devices, was lowest with the SRK II formula, and was highest with the Haigis formula.
Figure 1.
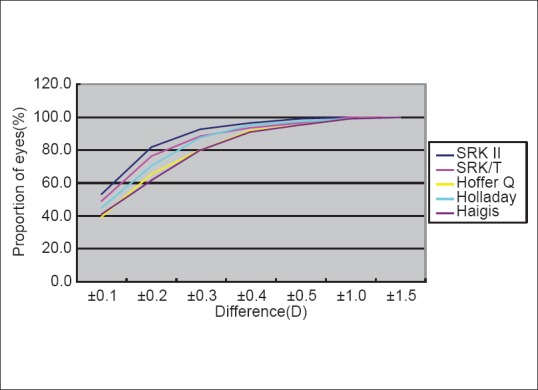
Distribution of IOL SA60AT power differences: LENSTAR–IOLMaster
Fig. 2 shows the correlations of the AL, K1, K2, and ACD measurements between the LENSTAR and IOLMaster. Linear regression showed an excellent correlation. The Pearson r for AL, K1, K2, and ACD were 0.999, 0.991, 0.992, and 0.927, respectively.
Figure 2.
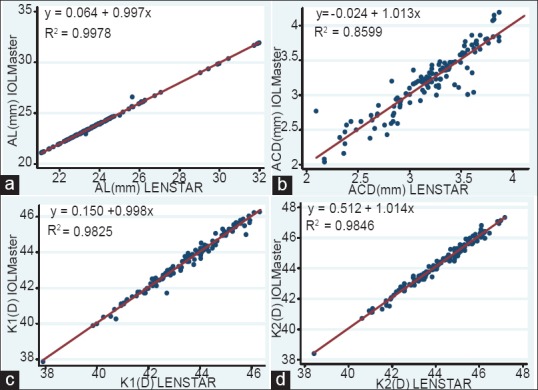
Correlation between LENSTAR and IOLMaster measurements, AL = axial length; ACD = anterior chamber depth; K1 and K2 = corneal refractive power (refractive index of 1.3375 used;). n = 122 eyes
Fig. 3 shows the Bland-Altman plots for the SA60AT IOL power calculations. There was good agreement between the LENSTAR and IOLMaster in terms of power calculation.
Figure 3.
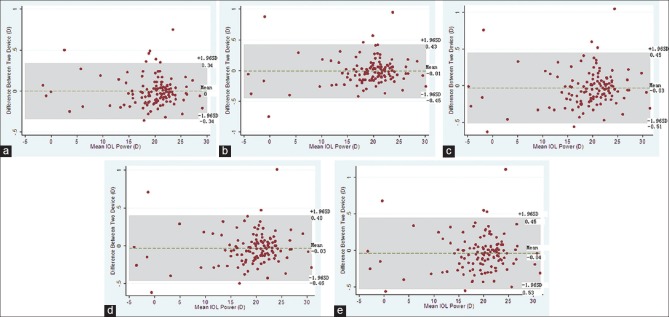
Bland-Altman plots by intraocular lens (IOL) power calculation formula A: SRK/formula, (a) SRK II formula, (b) SRK/T formula,(c) Hoffer Q formula, (d) Holladay formula, (e) Haigis formula
Discussion
Accurate measurements of AL, K, and ACD are essential in modern cataract surgery. Both IOLMaster and LENSTAR are based on non-contact laser interferometry to assess AL. The IOLMaster uses PCI in a dual-beam configuration to measure AL, and it is powered with a multi-mode laser diode,[2,3] whereas the LENSTAR uses OLCR powered with an super luminescent diode (SLD).[10,11] With the SLD, reflective structures within the cornea, anterior chamber, lens, and retina are measured. Hence, the LENSTAR can simultaneous measure AL, ACD, keratometry, and white-to-white distance; and also corneal, lens, and retinal thickness parameters, and distance from the endothelium to the anterior surface of the lens.
Altogether 160 cataract eyes were measured. Of which 28 eyes were excluded from analysis due to measurements failures for IOLMaster, and 38 eyes were excluded for LENSTAR. 122 eyes were successfully measured by both IOLMaster and LENSTAR. Dense cataracts, poor fixation, and unable to co-operate with examination were causes of measurement failures. Due to the different technologies used, the IOLMaster requires 3 different positions and releases procedures, whereas the LENSTAR acquires all parameters in a single position with one release procedure. Hence, measurements with the LENSTAR need better co-operation of the patients than IOLMaster.
In a comparison of the two devices in 200 phakic healthy eyes without cataract, Holzer et al.,[10] found a good correlation in AL, keratometry, and ACD measurements. A study by Buckhurst et al.,[11] evaluated 112 cataract patients using both devices. The corneal curvature measurements were similar. AL and ACD values were significantly higher with the OLCR device, than with the PCI device, but the differences were not clinically significant. In a subgroup of 32 patients, the OLCR measurements were highly repeatable. Rabsilber et al.,[12] evaluated IOL power calculations on 100 cataract eyes using parameters obtained from the LENSTAR and IOLMaster. Of all the biometry parameters, the only statistically significant differences between the two devices were in terms of corneal radii and ACD. The mean difference in IOL power calculations using four formulas were not statistically significant between the two devices. Rohrer K et al.,[13] evaluated LENSTAR in 144 eyes of 80 persons and compared the measurements with those obtained from the IOLMaster. Measurements with LENSTAR and IOLMaster for AL, ACD, corneal radius, and axis of the flattest radius in cataractous, pseudophakic, aphakic, silicon oil-filled, and normal eyes correlated well. These previous studies support our findings of reliable measurements using the LENSTAR biometry device. In our study, of the biometry parameters, there was no statistically significant difference in AL and ACD between the two devices for cataract patients who received phacoemulsification and IOL implantation. The correlations of AL, K1, K2, and ACD between the two devices were high. K1, K2 and [K1 + K2]/2 values were significantly different between the two devices, but the differences were not clinically significant. The mean differences in IOL power calculations using the five formulas were not statistically significant between the two devices.
The difference between the two devices in ACD measurement might be related to the difference in the measuring technique of the two devices. While the IOLMaster uses lateral slit illumination to determine the distance between the corneal epithelium and the anterior surface of the crystalline lens, the LENSTAR uses OLCR technology to detect the corneal thickness from epithelium to endothelium and the distance from the endothelium to the anterior surface of the crystalline lens which represents the anatomic ACD. Furthermore, the keratometry is required before ACD measurement with IOLMaster.
As for corneal refractive power measurement, both IOLMaster and LENSTAR evaluate corneal radius in the flat and steep meridian. The LENSTAR takes readings in two circles, 16 points in each circle, for a total of 32 readings. The inner circle has a diameter of 1.65 mm, and the outer circle has a diameter of 2.3 mm. However, the IOLMaster takes readings from 6 points in one circle which has a diameter of 2.3 mm. In our study, the differences of K1, K2 and [K1 + K2]/2 between the two devices were statistically significant; however, in practice, there was no clinical significance.
A limitation of both devices is that they are unable to accurately measure severely compromised eyes, such as those with retinal detachment, severe opacities along the visual axis, and in case of poor patient cooperation.[7]
In summary, the new optical low-coherence reflectometry LENSTAR device provides precise biometry and IOL power calculation in cataract patients, and has good correlation with measurements obtained using the partial coherence interferometry IOLMaster. Therefore, the LENSTAR is a useful new tool for accurate IOL power calculation in cataract patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Olsen T. Calculation of intraocular lens power: A review. Acta Ophthalmol Scand. 2007;85:472–85. doi: 10.1111/j.1600-0420.2007.00879.x. [DOI] [PubMed] [Google Scholar]
- 2.Drexler W, Findl O, Menapace R, Rainer G, Vass C, Hitzenberger CK, et al. Partial coherence interferometry: A novel approach to biometry in cataract surgery. Am J Ophthalmol. 1998;126:524–34. doi: 10.1016/s0002-9394(98)00113-5. [DOI] [PubMed] [Google Scholar]
- 3.Haigis W, Lege B, Miller N, Schneider B. Comparison of immersion ultrasound biometry and partial coherence interferometry for intraocular lens calculation according to Haigis. Graefes Arch Clin Exp Ophthalmol. 2000;238:765–73. doi: 10.1007/s004170000188. [DOI] [PubMed] [Google Scholar]
- 4.Kielhorn I, Rajan MS, Tesha PM, Subryan VR, Bell JA. Clinical assessment of the Zeiss IOLMaster. J Cataract Refract Surg. 2003;29:518–22. doi: 10.1016/s0886-3350(02)01819-9. [DOI] [PubMed] [Google Scholar]
- 5.Wang JK, Hu CY, Chang SW. Intraocular lens power calculation using the IOLMaster and various formulas in eyes with long axial length. J Cataract Refract Surg. 2008;34:262–7. doi: 10.1016/j.jcrs.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 6.MacLaren RE, Natkunarajah M, Riaz Y, Bourne RR, Restori M, Allan BD. Biometry and formula accuracy with intraocular lenses used for cataract surgery in extreme hyperopia. Am J Ophthalmol. 2007;143:920–31. doi: 10.1016/j.ajo.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 7.Lege BA, Haigis W. Laser interference biometry versus ultrasound biometry in certain clinical conditions. Graefes Arch Clin Exp Ophthalmol. 2004;242:8–12. doi: 10.1007/s00417-003-0672-2. [DOI] [PubMed] [Google Scholar]
- 8.Narváez J, Cherwek DH, Stulting RD, Waldron R, Zimmerman GJ, Wessels IF, et al. Comparing immersion ultrasound with partial coherence interferometry for intraocular lens power calculation. Ophthalmic Surg Lasers Imaging. 2008;39:30–4. doi: 10.3928/15428877-20080101-08. [DOI] [PubMed] [Google Scholar]
- 9.Bhatt AB, Schefler AC, Feuer WJ, Yoo SH, Murray TG. Comparison of predictions made by the intraocular lens master and ultrasound biometry. Arch Ophthalmol. 2008;126:929–33. doi: 10.1001/archopht.126.7.929. [DOI] [PubMed] [Google Scholar]
- 10.Holzer MP, Mamusa M, Auffarth GU. Accuracy of a new partial coherence interferometry analyzer for biometric measurements. Br J Ophthalmol. 2009;93:807–10. doi: 10.1136/bjo.2008.152736. [DOI] [PubMed] [Google Scholar]
- 11.Buckhurst PJ, Wolffsohn JS, Shah S, Naroo SA, Davies LN, Berrow EJ. A new optical low coherence reflectometry device for ocular biometry in cataract patients. Br J Ophthalmol. 2009;93:949–53. doi: 10.1136/bjo.2008.156554. [DOI] [PubMed] [Google Scholar]
- 12.Rabsilber TM, Jepsen C, Auffarth GU, Holzer MP. Intraocular lens power calculation: Clinical comparison of 2 optical biometry devices. J Cataract Refract Surg. 2010;36:230–4. doi: 10.1016/j.jcrs.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Rohrer K, Frueh BE, Wälti R, Clemetson IA, Tappeiner C, Goldblum D. Comparison and evaluation of ocular biometry using a new noncontact optical low-coherence reflectometer. Ophthalmology. 2009;116:2087–92. doi: 10.1016/j.ophtha.2009.04.019. [DOI] [PubMed] [Google Scholar]


