Abstract
Background
Accountable care organizations (ACOs) seek to reduce growth in healthcare spending while ensuring high-quality care. We hypothesized that ACO implementation would selectively limit utilization of discretionary cardiovascular care (defined as care occurring in the absence of indications such as myocardial infarction or stroke), while maintaining high-quality care such as non-discretionary cardiovascular imaging and procedures.
Methods and Results
The intervention group was composed of fee-for-service Medicare patients (n=819,779) from 10 groups participating in a Medicare pilot ACO, the Physician Group Practice Demonstration (PGPD). Matched controls were patients (n=934,621) from non-participating groups in the same regions. We compared utilization of cardiovascular care before (2002-2004) and after (2005-2009) PGPD implementation, studying both discretionary and non-discretionary carotid and coronary imaging and procedures. Our main outcome measure was the difference in the proportion of patients treated with imaging and procedures, among patients of PGPD practices compared to patients in control practices, before and after PGPD implementation (difference-in-difference). For discretionary imaging, the difference-in-difference between PGPD practices and controls was not statistically significant for discretionary carotid imaging (0.17%; 95% CI -0.51% to 0.85%, p=0.595) or discretionary coronary imaging (-0.19%; 95% CI -0.73% to 0.35%, p=0.468). Similarly, the difference-in-difference was also minimal for discretionary carotid revascularization (0.003%; 95% CI -0.008% to 0.002%, p=0.705) and coronary revascularization (-0.02%, 95% CI -0.11% to 0.07%, p=0.06). The difference-in-difference associated with PGPD implementation was also essentially zero for non-discretionary cardiovascular imaging or procedures.
Conclusions
Implementation of a pilot ACO did not limit the utilization of discretionary or non-discretionary cardiovascular care in ten large health systems.
Keywords: health policy and outcomes research, stroke care, myocardial infarction, outcomes research, health economics
Introduction
Accountable care organizations (ACOs) are payment models which utilize a combination of financial incentives and quality measures to focus health care spending on evidence–based treatments.1-4 Unlike their predecessors of the 1990s, managed care organizations, ACOs incorporate quality measures to help ensure cost savings focus on discretionary treatments where the benefits of treatment are low or uncertain, rather than indiscriminate limits on care.5, 6 Early reports from pilot ACO implementation projects have described improvements in quality with savings reported in selected regions and populations.7, 8
Specialty care often includes costly imaging tests and invasive procedures, a prime target for reducing spending growth. The treatment of common cardiovascular conditions, such as acute myocardial infarction and stroke, offers an interesting opportunity to test how ACO implementation might affect the use of specialty care. Cardiovascular imaging and procedures vary considerably, from evidence-based, non-discretionary treatments (such as invasive cardiac catheterization in the setting of acute myocardial infarction) to discretionary treatments where the benefits are much less clear (such as imaging or treatments for asymptomatic coronary or carotid atherosclerosis).9-11 Imaging and procedures for coronary and carotid atherosclerosis contribute over 6 billion dollars annually to Medicare spending, and recent population-based reports suggest up to 60% of this spending is discretionary, occurring in the absence of definitive indications such as myocardial infarction or stroke.12-14 Further, evidence from managed care plans (which similarly shift away from volume incentives) show that specialist staffing levels are traditionally lower, fewer specialist services are used, and physicians are less likely to order discretionary tests under capitation15-17.
We hypothesized that the incentives in ACO contracts would encourage providers to selectively limit utilization of discretionary cardiovascular care, while maintaining high-quality care such as non-discretionary cardiovascular imaging and procedures. However, it remains unknown how ACOs affected specialty-related spending in the pilot programs of the ACO care model.18, 19 To test this hypothesis, we studied discretionary and non-discretionary cardiovascular care provided before and after the implementation of Medicare's Physician Group Practice Demonstration Project (PGPD), an ACO pilot project implemented in 2005 for more than 2 million Medicare beneficiaries' served by ten large health care systems.8
Methods
Data Sources
We used Medicare administrative fee-for-service claims data from 2001-2010 to create treatment and control groups following the regulations set out in the Physician Group Practice Demonstration8. We used a 20% sample of Carrier file claims from 2001-2005 and 100% of claims from 2006-2009. Medicare beneficiaries were assigned to a PGPD participant group if the plurality of their evaluation and management visits came from affiliated PGPD providers. The control groups were comprised of Medicare beneficiaries residing in the same counties as their PGPD counterparts, but receiving their care from non-PGPD providers.8 Previous studies have reported in greater detail both the structure of the PGPD and methods for replicating the intervention and control groups.8
Categories used to Study Discretionary and Non-Discretionary Imaging and Procedures
We sought to test whether ACOs would limit spending growth for health care services where the benefits to patients are least evident while retaining the ability to provide non-discretionary, evidence-based care. Evidence from other care settings suggests that high intensity areas tend to provide more discretionary and non-discretionary care20, 21. Therefore, within common cardiovascular conditions, we studied the provision of discretionary and non-discretionary care, before and after ACO implementation, comparing patients receiving care from PGPD and non-PGPD sites.
We studied two common cardiovascular conditions (coronary and carotid atherosclerosis) because these conditions offered the opportunity to examine the treatment of asymptomatic coronary and carotid disease as discretionary conditions.22, 23 These categories were created based on pre-existing, claims-based evidence of symptoms using coding algorithms from prior published reports.14, 24, 25 For coronary care, we included the following three services: stress testing, diagnostic catheterization alone or catheterization with revascularization procedures such as stenting or bypass surgery. Each service was coded as non-discretionary if delivered to patients with recent (<1 year) symptoms of coronary disease, and discretionary in all other cases.26 For example, a cardiac catheterization provided to a patient without any evidence of a broad list of diagnosis codes indicative of symptomatic cardiac disease (Appendix 1) would be categorized as “discretionary”. Similarly, we examined four carotid services: duplex imaging, computed tomography imaging, carotid endartectomy, and stenting. Each of these four services was coded as non-discretionary if delivered to patients with recent (<1 year) symptoms of stroke or transient ischemic attack (TIA), and discretionary in all other cases. Appendix 1 describes the ICD-9 and CPT codes used to define the diagnoses and imaging and procedural tests described above. The codes used to define non-discretionary care were intentionally broad, so as to be conservative in defining imaging and procedural care without these codes as discretionary. However, sensitivity analyses which used narrower coding schema to define non-discretionary care did not change our study findings.
Based on the coding above, our study design focused on four outcomes, measured over the course of a calendar year: the proportion of patients with at least one coronary imaging test, the proportion of patients with at least one carotid imaging test, the proportion of patients treated with at least one invasive coronary revascularization procedure, and the proportion of patients treated with at least one invasive carotid revascularization procedure. We studied the first occurrence of a defined imaging test or procedure per beneficiary per year. The denominators used to form these proportions were based on patient populations, with numerators and denominators calculated separately for patients assigned to a PGPD site or local non-PGPD control patients. We estimated effects on each outcome stratified by a patient's status as discretionary or non-discretionary, yielding 8 distinct outcomes in total.
Difference-in-Difference Study Design and Statistical Analysis
Using a difference-in-difference study design, we examined the utilization of imaging tests and revascularization procedures, among patients receiving care in PGPD and non-PGPD settings before and after ACO implementation. We defined the number of patients attributed to each of the ten PGPD health systems and their geographically matched controls. By comparing patients in a PGPD site to geographically matched controls over time, the difference-in-difference design, increasingly used in health services and clinical research,8, 27, 28 controls for fixed differences between PGPD participants and non-participants. The characteristics of patients were determined by looking back one year in Medicare claims from the date of the imaging test or procedure (Table 1).
Table 1.
Sample characteristics. Demographics, before and after ACO implementations status, for patients undergoing treatment for cardiovascular disease, as well as their regionally matched controls.
| All Patients Treated for Carotid and Coronary Disease | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Pre (2002-2004) | Post (2005-2009) | |||||
|
| ||||||
| Participants (n=114,130) | Controls (n=114,243) | p value | Participants (n=819,779) | Controls (n=820,378) | p value | |
|
| ||||||
| Age (Mean) | 71.9 | 72.0 | 0.3472 | 71.2 | 71.4 | <0.0001 |
| % Female | 57.8 | 58.5 | <0.0001 | 57.7 | 57.6 | 0.0006 |
| % Black | 1.9 | 2.8 | <0.0001 | 2.3 | 3.3 | <0.0001 |
| Mean HCC | 0.9 | 0.9 | <0.0001* | 1.0 | 0.9 | <0.0001 |
| % Nursing Home Residents | 2.3 | 2.6 | <0.0001 | 2.2 | 2.5 | <0.0001 |
| Mean Comrobidity Count (of the 10 below) | 0.5 | 0.5 | <0.0001* | 0.6 | 0.5 | <0.0001 |
| % Malignancy | 2.9 | 2.2 | <0.0001 | 3.0 | 2.2 | <0.0001 |
| % Chronic Obstructive Pulmonary Disease | 11.2 | 11.5 | <0.0001 | 11.4 | 11.4 | 0.0036 |
| % Coronary Artery Disease | 16.2 | 15.7 | <0.0001 | 15.3 | 15.0 | <0.0001 |
| % Congestive Heart Failure | 8.0 | 7.9 | 0.0090 | 7.3 | 7.0 | <0.0001 |
| % Peripheral Vascular Disease | 6.3 | 6.1 | <0.0001 | 6.7 | 6.6 | <0.0001 |
| % Chronic Liver Disease | 0.3 | 0.3 | 0.0524 | 0.4 | 0.3 | <0.0001 |
| % Diabetes with End Organ Damage | 2.1 | 1.9 | <0.0001 | 2.4 | 1.9 | <0.0001 |
| % Chronic Renal Failure | 2.4 | 2.3 | 0.0052 | 4.1 | 3.6 | <0.0001 |
| % Dementia | 4.2 | 4.3 | 0.1131 | 4.3 | 4.5 | <0.0001 |
| % Diabetes | 18.0 | 17.9 | 0.1118 | 20.3 | 19.4 | <0.0001 |
| % Medicaid | 12.6 | 13.3 | <0.0001 | 15.2 | 14.9 | <0.0001 |
Significant differences arise from decimal places not shown here.
We estimated the differential change in coronary and carotid procedures in two steps. For each PGPD site, we estimated patient-level models of our outcomes, using linear regression. Each model included an indicator for being attributed to a PGPD participant, an indicator for the post-PGPD period, and an indicator for the interaction of the two variables (post*PGPD). The estimate of interest is the coefficient on this interaction term, which describes the differential change in a given outcome at PGPD sites in the post-implementation period compared with controls. If negative, the estimate would indicate that rates of a given imaging or revascularization procedure declined more (or grew more slowly) in PGPD patients compared with controls, comparing the pre- and post- demonstration period. Our models adjusted for patient age category, sex, race, interactions between age, sex, and race, Medicaid status and disability status. We also adjust for the proportion with income under the federal poverty line in the beneficiary's ZIP code. To calculate an overall estimate, we averaged site-specific estimates from the 10 PGPD sites, weighting the estimates and variances by the number of beneficiaries assigned to the PGPD site. Residual errors were corrected for clustering at the individual and site-specific levels, and within years.
All analyses were performed using SAS (Cary, NC) and STATA 12 MP (College Station, TX). Dartmouth's Committee for the Protection of Human Subjects approved our research protocol and approved the waiver of need for informed consent.
Results
Patient characteristics
The intervention group was composed of fee-for-service Medicare patients (n=819,779 patients) receiving cardiovascular care from ten physician groups participating in a Medicare pilot ACO project, the Physician Group Practice Demonstration (PGPD). Controls were Medicare patients (n=934,621 patients) from the same regions who received care from non-PGPD physicians. As shown in Table 1, patients treated within PGPD health systems were similar to patients treated in control health systems for both discretionary and non-discretionary cardiovascular care, in terms of mean age, gender distribution, and comorbidities. While minor differences were statistically significant given our large sample size, but few clinically relevant differences existed between patients at PGPD sites and controls. While both carotid and coronary patients are aggregated together in Table 1, when categorized by procedure type and discretionary status these patient populations again showed only minor differences (Appendix 2).
As expected, when compared to patients undergoing non-discretionary procedures, patients undergoing discretionary imaging or procedures were younger, and a smaller proportion had serious comorbidities such as diabetes, chronic renal insufficiency, and congestive heart failure. Among all patients without symptoms of myocardial infarction or stroke, 17.0% (95 % CI =17.01-17.07%) were treated with discretionary coronary imaging, and 27.7% (95 % CI = 27.67-27.74) were treated with discretionary carotid imaging. In terms of discretionary revascularization procedures, 1.4% (95 % CI =1.39-1.41) of patients were treated with discretionary coronary revascularization, and 0.06% (95 % CI =0.05-0.06) were treated with discretionary carotid revascularization. Among patients with a history of myocardial infarction or stroke, the proportion of patients treated with imaging was higher, as expected. Overall, 42.6% (95 % CI = 42.50-42.68) of patients with a history of myocardial infarction had coronary imaging, and 68.7% (95 % CI = 68.58-68.89%) of patients with stroke had carotid imaging. Similarly, when compared with rates of use in discretionary patients, rates of revascularization were higher among patients with symptoms of myocardial infarction or stroke (13.2% (95 % CI =13.15-13.28) of patients with myocardial infarction received coronary revascularization, and 4.3% (95 % CI = 4.18-4.32%) of patients with stroke received carotid revascularization.
Trends in use of imaging and revascularization
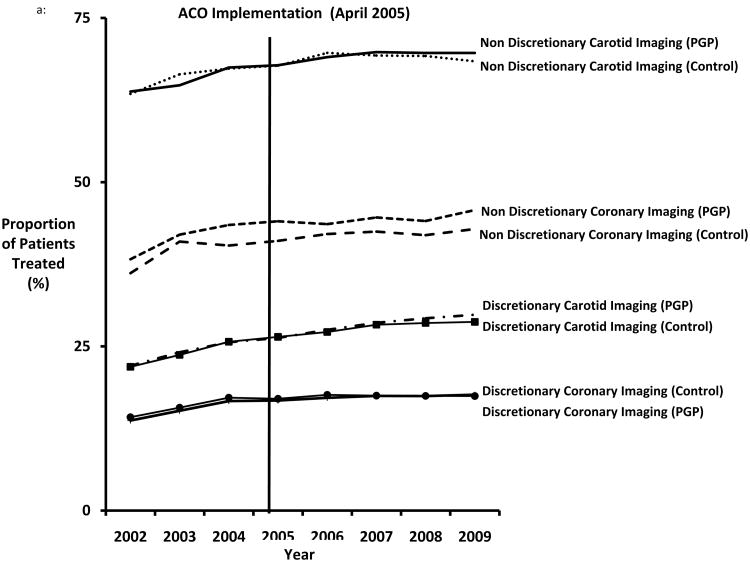
Cardiovascular imaging use increased over time in both patients treated at PGPD sites as well as patients treated in non-PGPD sites in the same region (Figure 1a). For example, among all patients with symptomatic coronary disease, rates of coronary imaging increased from 37% (95% CI = 36.78-37.60) in 2002, to 44% (95% CI = 44.12-44.49) by 2009 (p<.0001). Cardiac imaging tests were less common over time, but still increased in patients without evidence of myocardial infarction, growing from 14% (95% CI = 13.82-14.11) of asymptomatic patients in 2002 to 17% (95% CI = 17.48-17.60) by 2009 (p=0.01). Similar trends were noted in carotid imaging (Figure 1a).
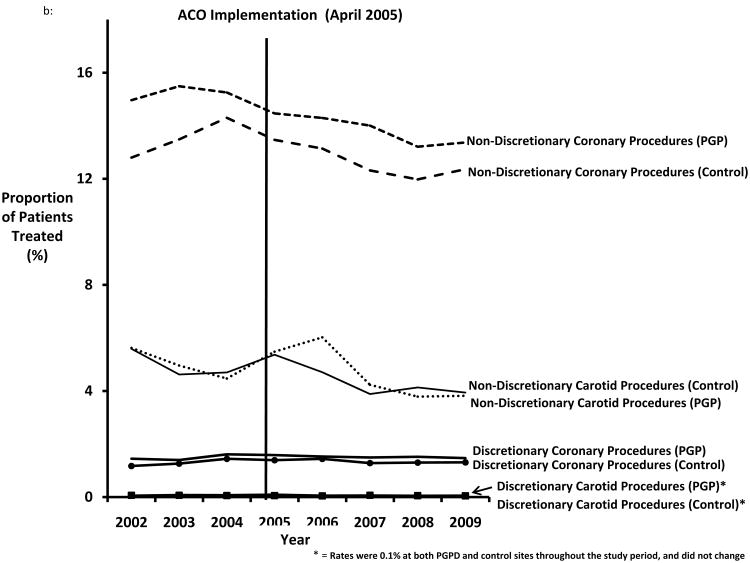
Figure 1.
A. Proportion of patients treated with discretionary and non-discretionary cardiovascular imaging, by year. B. Proportion of patients treated with discretionary and non-discretionary cardiovascular procedures, by year.
The proportion of patients with a diagnosis of myocardial infarction treated with invasive procedures declined slightly during the study period, ranging from 13.9 (95% CI = 13.6-14.2) in 2002 to 12.9% (95% CI = 12.8-13.0) in 2009 (p<.001), although the proportion treated was slightly higher in patients treated in PGPD hospitals (average difference in PGP vs. non-PGPD = 1.3%; 95% CI 1.23-1.47, p<.001 (Figure 1b). Invasive carotid revascularization for patients with symptoms of stroke also declined slightly between 2002 and 2009, from 5.6% (95% CI = 5.28-5.93) to 3.9% (95% CI 3.74-4.01) (p<.001). The rates of discretionary cardiovascular procedures remained low and constant throughout the study period. Coronary revascularization procedures were used in fewer than 2% of patients without symptoms of myocardial infarction. Similarly, throughout the study period carotid revascularization was used in less than 1% of all patients without symptoms of stroke in both PGPD and control sites.
Association between ACO implementation and imaging and procedure use, in discretionary and non-discretionary settings
Overall, we found no significant differences in the percentage of patients treated with cardiovascular imaging or procedure use between PGPD and controls before and after ACO implementation. As shown in Figures 1a and 1b, there was little evidence of a decline in the proportion of patients treated with discretionary cardiovascular imaging or procedures in PGPD health systems or their controls. The overall difference-in-difference estimate (comparing the change in use at PGPD versus control sites) was essentially zero for all imaging tests and procedures studied (Table 2). While these models showed no significant associations with PGPD implementation, we also considered, in relative terms, what the largest possible declines in care utilization could be given within 95% confidence intervals surrounding our estimates. The largest potential relative reductions within our 95% confidence intervals were a 6% relative decline in non-discretionary coronary procedures and 16% relative decline for non-discretionary carotid procedures, suggesting that any change within the confidence bounds of our estimates would imply only modest change. Given these findings, it seemed unlikely that our models have failed to identify any statistically significant, or clinically meaningful, declines in the proportion of patients treated with imaging or procedures associated with ACO implementation.
Table 2.
Models showing the risk-adjusted difference in difference (in absolute % change) between PGPD and controls.
| Type of Cardiovascular Care | Absolute Proportion of Patients Treated (mean, %) | 95% CI | Proportion of Patients Treated Before ACO Implementation (2002-2004) | Proportion of Patients Treated After ACO Implementation (2005-2009) | Percentage Point Change in Proportion of Patients Treated PGPD Vs. Controls | 95% CI | p value | ||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Discretionary Imaging | |||||||||
|
| |||||||||
| Carotid | 27.93 | 27.90 | 27.97 | 23.69 | 27.97 | 0.17 | 0.51 | 0.85 | 0.595 |
| Coronary | 17.16 | 17.13 | 17.19 | 15.41 | 17.21 | -0.19 | 0.73 | 0.35 | 0.468 |
|
| |||||||||
| Non-discretionary Imaging | |||||||||
|
| |||||||||
| Carotid | 68.75 | 68.60 | 68.90 | 65.61 | 69.25 | 0.73 | 1.15 | 2.61 | 0.427 |
| Coronary | 42.99 | 42.90 | 43.08 | 40.27 | 43.47 | -0.27 | 1.81 | 1.25 | 0.711 |
|
| |||||||||
| Discretionary procedures | |||||||||
|
| |||||||||
| Carotid | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0 | 0.01 | 0.01 | 0.705 |
| Coronary | 1.40 | 1.39 | 1.41 | 1.38 | 1.42 | -0.02 | 0.11 | 0.07 | 0.0631 |
|
| |||||||||
| Non-discretionary procedures | |||||||||
|
| |||||||||
| Carotid | 4.74 | 4.67 | 4.81 | 5.76 | 4.58 | 0.14 | 0.63 | 0.92 | 0.707 |
| Coronary | 13.21 | 13.15 | 13.28 | 14.41 | 13.00 | -0.39 | 0.73 | 0.35 | 0.319 |
Finally, while there were no significant overall declines in imaging or procedures with ACO implementation, the effect of implementation was heterogeneous across the ten PGPD sites (Appendix 3). For example, when we studied the use of discretionary carotid imaging, we found that certain sites, such as the Marshfield Clinic, were able to achieve a small but significant decline in imaging utilization (a decline of 1.6%, 95 % CI: -1.7 to -1.5%, p<0.001, Appendix 3). However, other health systems, such as the Park Nicollet Clinic, actually performed significantly more discretionary carotid imaging after ACO implementation (an increase of 2.2%, 95% CI 2.15 to 2.29%, p<0.001). On balance, reductions in any one PGPD site were offset by increasing use in other sites, for an overall association of zero across the ten PGPD sites.
Discussion
Specialty care, such as the care of patients with cardiovascular disease, is an important cost center for Medicare patients, and many argue that much of the spending on these services is discretionary in nature.13, 14 ACO models seek to limit health care spending growth. However, we found no evidence to suggest that an early ACO Medicare demonstration had any effect on the utilization of discretionary cardiovascular care. Our study suggests that better tools and implementation strategies may be necessary to limit growth in discretionary, specialty-related spending under ACO care contracts.
A previous study of the PGPD demonstrated small differences in overall spending before and after ACO implementation, with the exception of spending related to patients dually eligible for Medicare and Medicaid.8 In this analysis, we hypothesized that although overall spending may have declined only slightly as a result of ACO implementation, spending within cardiovascular care might represent a potential “bright spot” in cost reduction. In addition to being expensive, cardiovascular care has discrete, concrete treatment algorithms for imaging tests and procedures for both symptomatic and asymptomatic cardiovascular disease processes.26, 29 We hypothesized that cardiovascular care could potentially represent an ideal specialty setting to attempt to limit discretionary procedures, especially those at the margin of plausible benefit. Marshfield Clinic and The University of Michigan Faculty Group Practice significantly reduced spending overall in the Demonstration; while there is some indication of reductions in discretionary use in these practices (e.g. Marshfield's carotid and coronary imaging) the results overall are mixed (Appendix 2)8. Despite the availability of these guidelines and the financial incentives inherent in the ACO payment model, the use of discretionary cardiovascular imaging and procedures did not differ between the sites that participated in the PGPD and comparisons.
Why did the use of discretionary cardiovascular care not decline in ACO settings? Several potential explanations exist. First, the heterogeneity seen across health systems in utilization changes suggests that variation exists in terms of how each health system approached the goal of achieving savings with the ACO. Studying and learning from those centers that were able to deliver less discretionary care may prove beneficial towards understanding how to effectively achieve savings. Second, our results suggest initial ACO efforts were targeted at primary care treatment patterns, rather than specialists such as cardiovascular physicians and services.30 Participating groups largely chose to focus their efforts on increasing patient engagement, expanding care management, improving care transitions, and expanding the role of non-physician providers, rather than focusing on specialty care31. Third, while the health systems engaged in the PGPD were incentivized to limit spending by the downstream promise of shared savings, it remains unknown if the incentives of the ACO's cardiovascular physicians were similarly aligned. If these physicians typically remained in productivity-based remuneration arrangements, they would have little incentive to limit the use of cardiovascular care.
Finally, either PGPD groups or individual physicians may have been unwilling to forego guaranteed imaging or procedure-related payment for the possibility of future savings bonuses. For example, the Centers for Medicare and Medicaid Services estimated hospital payment for percutaneous coronary artery stent placement in 2012 at about $16,50032. A crude calculation would suggest that for an individual ACO site, six fewer coronary revascularizations would save approximately $100,000, and of this total, $78,400 (savings are calculated over a 2% savings threshold) would be returned to the ACO. However, this “shared savings” would only be realized if (1) the 2% savings threshold was achieved, and (2) the organization had recovered the costs of initial ACO implementation - which averaged $1.7 million across the ten PGPD participants33. Further, because savings are measured in aggregate at the level of the health system, any “shared savings” generated by any one particular specialty (such as cardiovascular care) would result in a downstream reward only if similar efforts were mirrored by other types of specialists in the ACO. In sum, whether or not the savings are “worth it” depends on the perceived probability of receiving savings, the facilities margin for the procedure, and how revenue is shared. Perhaps these potential downstream savings provided too little of an incentive for physicians and hospitals to limit utilization of cardiovascular imaging and procedure based-care – typically an up-front profit center for both physicians and hospitals alike. This concern may have been especially salient for the PGPD, a pilot with an uncertain future at its start in 2005. Further, imagine a hypothetical cardiovascular program that generates a profit margin of one million dollars per year for a hospital system. This cardiovascular program would have to willingly perform 77 fewer cardiac catheterizations – and forfeit more than 1.26 million dollars in revenue –to generate a million dollars in shared savings for their institution. Therefore, incentives that will be adequate to change practice patterns for specialists are unlikely to be trivial in magnitude if fee-for-service payment options remain available.
Nonetheless, policymakers, payers, and health care organizations should consider several potential solutions to encourage limiting use of discretionary specialty care in ACO settings. First, administrative complexities in ACO structures across institutions, which contribute toward greater heterogeneity and higher administrative costs, should be simplified to allow transparency and ease identification of strategies that effectively reduce spending on discretionary cardiovascular care. Second, while many of the initial attempts at limiting cost growth in ACOs focused on primary care settings, policymakers need to “go where the money is”, and prioritize attempts to limit spending growth not only in primary care settings, but in the delivery of specialty care as well. Third, health care provider organizations will need to align incentives throughout their organization – from administrative targets to individual physician goals – to ensure that the entire system is working toward the same goal of providing value based care to its patients. Finally, the landscape of ACOs currently operating in the Medicare program is quite diverse – some are large integrated delivery systems, while others are made up of solely primary care practices34. Effective control of referrals for discretionary care in ACO settings will likely require a uniform approach, although this task will be difficult to design and implement.35, 36
Is success plausible? We believe that a pathway exists to achieve savings, even in specialty care, in ACO settings. The Physician Group Practice Demonstration differed from the current Medicare Shared Savings Program in important ways. First, attribution was allowed through any physician, not focused around primary care. Second, the savings rate was much higher for practices in the Demonstration. Finally, and perhaps most importantly, as mentioned above the Demonstration was temporary in nature and the future of payment reform was unknown. The Medicare Shared Savings Program is a permanent program and the move away from fee-for-service payment is likely to become broader with time. Going forward, qualitative and quantitative methods will be needed to identify structural, process, and outcome-oriented approaches associated with reduced utilization of discretionary procedures. If successful strategies are identified and shared with a broader network of providers, it will accelerate the ability of ACOs to limit the use of discretionary cardiovascular care. Due to the small sample size and similarity across participating groups, it is not possible to analyze the characteristics of groups in the PGPD associated with success. Evaluation of the PGP showed some evidence that cost savings and the number of physicians in each network were correlated8.
Our study has limitations. First, while we studied two common cardiovascular conditions, they may not be representative of other types of cardiovascular care associated with high use of discretionary imaging and procedures, such as the treatment of congestive heart failure or lower extremity peripheral arterial disease. However, the similarity of findings across two disease processes carotid and coronary disease, both discretionary and non-discretionary – suggests that our findings are likely generalizable. Second, well-described weaknesses exist when applying clinical descriptions to administrative-based analyses.37-39 To account for this, the exposures and outcomes selected for our analysis were intentionally common, broadly occurring, and easily identifiable, making a large type II error resultant from poor risk stratification unlikely. Third, while this study of cardiovascular care in the PGPD –which only included a small number of health systems - has a null finding, other ACO implementation projects have shown small savings.7 Future studies in a much greater number of health care systems will be necessary to clearly understand what current ACO programs can and cannot accomplish in limiting the health care spending growth, especially for spending related to specialty care.
In conclusion, implementation of pilot ACO payment models did not limit the utilization of discretionary or non-discretionary cardiovascular imaging or procedures in ten large health systems. Future work is needed to see if ACOs can achieve cost reductions for cardiovascular specialty care within appropriately targeted and incentivized care settings.
Supplementary Material
Acknowledgments
Funding Sources: Dr. Goodney was supported by a K08 from NHLBI (1K08HL05676-01), and Dr. Colla and Dr. Meara were supported by an R21 from NIA R21 (AG044251) as well as support from Dr. Jon Skinner's NIA P01AG01978.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Lewis VA, Colla CH, Carluzzo KL, Kler SE, Fisher ES. Accountable care organizations in the united states: Market and demographic factors associated with formation. Health Serv Res. 2013;48:1840–1858. doi: 10.1111/1475-6773.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher ES, McClellan MB, Safran DG. Building the path to accountable care. N Engl J Med. 2011;365:2445–2447. doi: 10.1056/NEJMp1112442. [DOI] [PubMed] [Google Scholar]
- 3.McClellan M, McKethan AN, Lewis JL, Roski J, Fisher ES. A national strategy to put accountable care into practice. Health Affairs. 2010;29:982–990. doi: 10.1377/hlthaff.2010.0194. [DOI] [PubMed] [Google Scholar]
- 4.Fisher ES, Staiger DO, Bynum JP, Gottlieb DJ. Creating accountable care organizations: The extended hospital medical staff. Health Affairs. 2007;26:w44–57. doi: 10.1377/hlthaff.26.1.w44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis VA, Larson BK, McClurg AB, Boswell RG, Fisher ES. The promise and peril of accountable care for vulnerable populations: A framework for overcoming obstacles. Health Affairs. 2012;31:1777–1785. doi: 10.1377/hlthaff.2012.0490. [DOI] [PubMed] [Google Scholar]
- 6.McWilliams JM, Landon BE, Chernew ME. Changes in health care spending and quality for medicare beneficiaries associated with a commercial aco contract. JAMA. 2013;310:829–836. doi: 10.1001/jama.2013.276302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song Z, Landon BE. Controlling health care spending--the massachusetts experiment. N Engl J Med. 2012;366:1560–1561. doi: 10.1056/NEJMp1201261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colla CH, Wennberg DE, Meara E, Skinner JS, Gottlieb D, Lewis VA, Snyder CM, Fisher ES. Spending differences associated with the medicare physician group practice demonstration. JAMA. 2012;308:1015–1023. doi: 10.1001/2012.jama.10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss L, Golzarian J, Gornik HL, Jaff MR, Moneta GL, Olin JW, Stanley JC, White CJ, White JV, Zierler RE American College of Cardiology Foundation Task F, American Heart Association Task F. Management of patients with peripheral artery disease (compilation of 2005 and 2011 accf/aha guideline recommendations): A report of the american college of cardiology foundation/american heart association task force on practice guidelines. J Am Coll Cardiol. 2013;61:1555–1570. doi: 10.1016/j.jacc.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss LK, Golzarian J, Gornik HL, Halperin JL, Jaff MR, Moneta GL, Olin JW, Stanley JC, White CJ, White JV, Zierler RE Society for Cardiovascular A, Interventions, Society of Interventional R, Society for Vascular M, Society for Vascular S. 2011 accf/aha focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): A report of the american college of cardiology foundation/american heart association task force on practice guidelines. J Am Coll Cardiol. 2011;58:2020–2045. doi: 10.1016/j.jacc.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snow V, Barry P, Fihn SD, Gibbons RJ, Owens DK, Williams SV, Weiss KB, Mottur-Pilson C Acp, Panel ACCCSA. Evaluation of primary care patients with chronic stable angina: Guidelines from the american college of physicians. Ann Intern Med. 2004;141:57–64. doi: 10.7326/0003-4819-141-1-200407060-00015. [DOI] [PubMed] [Google Scholar]
- 12.Shaw LJ, Marwick TH, Zoghbi WA, Hundley WG, Kramer CM, Achenbach S, Dilsizian V, Kern MJ, Chandrashekhar Y, Narula J. Why all the focus on cardiac imaging? JACC Cardiovasc Imaging. 2010;3:789–794. doi: 10.1016/j.jcmg.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Hannan EL, Samadashvili Z, Cozzens K, Walford G, Holmes DR, Jr, Jacobs AK, Stamato NJ, Venditti FJ, Sharma S, King SB., 3rd Appropriateness of diagnostic catheterization for suspected coronary artery disease in new york state. Circ Cardiovasc Interv. 2014;7:19–27. doi: 10.1161/CIRCINTERVENTIONS.113.000741. [DOI] [PubMed] [Google Scholar]
- 14.Nallamothu BK, Gurm HS, Ting HH, Goodney PP, Rogers MA, Curtis JP, Dimick JB, Bates ER, Krumholz HM, Birkmeyer JD. Operator experience and carotid stenting outcomes in medicare beneficiaries. JAMA. 2011;306:1338–1343. doi: 10.1001/jama.2011.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiner JP. Forecasting the effects of health reform on us physician workforce requirement. Evidence from hmo staffing patterns. JAMA. 1994;272:222–230. [PubMed] [Google Scholar]
- 16.Martin DP, Diehr P, Price KF, Richardson WC. Effect of a gatekeeper plan on health services use and charges: A randomized trial. Am J Public Health. 1989;79:1628–1632. doi: 10.2105/ajph.79.12.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clancy CM, Hillner BE. Physicians as gatekeepers. The impact of financial incentives. Arch Intern Med. 1989;149:917–920. doi: 10.1001/archinte.149.4.917. [DOI] [PubMed] [Google Scholar]
- 18.Tallia AF, Howard J. An academic health center sees both challenges and enabling forces as it creates an accountable care organization. Health affairs. 2012;31:2388–2394. doi: 10.1377/hlthaff.2012.0155. [DOI] [PubMed] [Google Scholar]
- 19.Goodney PP, Fisher ES, Cambria RP. Roles for specialty societies and vascular surgeons in accountable care organizations. J Vasc Surg. 2012;55:875–882. doi: 10.1016/j.jvs.2011.10.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandra A, Staiger DO. Productivity spillovers in healthcare: Evidence from the treatment of heart attacks. J Polit Econ. 2007;115:103–140. doi: 10.1086/512249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landrum MB, Meara ER, Chandra A, Guadagnoli E, Keating NL. Is spending more always wasteful? The appropriateness of care and outcomes among colorectal cancer patients. Health Affairs. 2008;27:159–168. doi: 10.1377/hlthaff.27.1.159. [DOI] [PubMed] [Google Scholar]
- 22.Ng KH, Oczkowski W. Acp journal club. Review: Treatment options for asymptomatic carotid artery stenosis were compared. Ann Intern Med. 2013;159:JC6. doi: 10.7326/0003-4819-159-4-201308200-02006. [DOI] [PubMed] [Google Scholar]
- 23.Matos P. screening for coronary artery disease (cad) in asymptomatic diabetics. How to and in whom to screen? Rev Port Cardiol. 2013;32(Suppl 1):9–14. doi: 10.1016/S0870-2551(13)70042-8. [DOI] [PubMed] [Google Scholar]
- 24.Krumholz HM, Radford MJ, Ellerbeck EF, Hennen J, Meehan TP, Petrillo M, Wang Y, Kresowik TF, Jencks SF. Aspirin in the treatment of acute myocardial infarction in elderly medicare beneficiaries. Patterns of use and outcomes. Circulation. 1995;92:2841–2847. doi: 10.1161/01.cir.92.10.2841. [DOI] [PubMed] [Google Scholar]
- 25.Goodney PP, Travis LL, Malenka D, Bronner KK, Lucas FL, Cronenwett JL, Goodman DC, Fisher ES. Regional variation in carotid artery stenting and endarterectomy in the medicare population. Circ Cardiovasc Qual Outcomes. 2010;3:15–24. doi: 10.1161/CIRCOUTCOMES.109.864736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scanlon PJ, Faxon DP, Audet AM, Carabello B, Dehmer GJ, Eagle KA, Legako RD, Leon DF, Murray JA, Nissen SE, Pepine CJ, Watson RM, Ritchie JL, Gibbons RJ, Cheitlin MD, Gardner TJ, Garson A, Jr, Russell RO, Jr, Ryan TJ, Smith SC., Jr Acc/aha guidelines for coronary angiography: Executive summary and recommendations. A report of the american college of cardiology/american heart association task force on practice guidelines (committee on coronary angiography) developed in collaboration with the society for cardiac angiography and interventions. Circulation. 1999;99:2345–2357. doi: 10.1161/01.cir.99.17.2345. [DOI] [PubMed] [Google Scholar]
- 27.McWilliams JM, Meara E, Zaslavsky AM, Ayanian JZ. Use of health services by previously uninsured medicare beneficiaries. N Engl J Med. 2007;357:143–153. doi: 10.1056/NEJMsa067712. [DOI] [PubMed] [Google Scholar]
- 28.Dimick JB, Nicholas LH, Ryan AM, Thumma JR, Birkmeyer JD. Bariatric surgery complications before vs after implementation of a national policy restricting coverage to centers of excellence. JAMA. 2013;309:792–799. doi: 10.1001/jama.2013.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, Cates CU, Creager MA, Fowler SB, Friday G, Hertzberg VS, McIff EB, Moore WS, Panagos PD, Riles TS, Rosenwasser RH, Taylor AJ, Jacobs AK, Smith SC, Jr, Anderson JL, Adams CD, Albert N, Buller CE, Creager MA, Ettinger SM, Guyton RA, Halperin JL, Hochman JS, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Ohman EM, Page RL, Riegel B, Stevenson WG, Tarkington LG, Yancy CW. 2011 asa/accf/aha/aann/aans/acr/asnr/cns/saip/scai/sir/snis/svm/svs guideline on the management of patients with extracranial carotid and vertebral artery disease: Executive summary: A report of the american college of cardiology foundation/american heart association task force on practice guidelines, and the american stroke association, american association of neuroscience nurses, american association of neurological surgeons, american college of radiology, american society of neuroradiology, congress of neurological surgeons, society of atherosclerosis imaging and prevention, society for cardiovascular angiography and interventions, society of interventional radiology, society of neurointerventional surgery, society for vascular medicine, and society for vascular surgery. Developed in collaboration with the american academy of neurology and society of cardiovascular computed tomography. Catheter Cardiovasc Interv. 2013;81:E76–123. doi: 10.1002/ccd.22983. [DOI] [PubMed] [Google Scholar]
- 30.Hacker K, Walker DK. Achieving population health in accountable care organizations. Am J Public Health. 2013;103:1163–1167. doi: 10.2105/AJPH.2013.301254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trisolini M, Agarwal R. The medicare physician group practice demonstration: Lessons learned on improving quality and efficiency in health care. A report from the commonwealth fund. 2008 [Google Scholar]
- 32.Physician Fee Schedule from the Centers for Medicare and Medicaid Services. 2014 http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/index.html?redirect=/physicianfeesched.
- 33.Health policy brief: Next steps for acos. Health Affairs. 2012 http://healthaffairs.org/healthpolicybriefs/brief_pdfs/healthpolicybrief_61.pdf.
- 34.Colla CH, Lewis VA, Sharp SM, Fisher ES. First national survey of acos shows physicians are playing strong leadership and ownership roles. Health Affairs. 2014;33:964–71. doi: 10.1377/hlthaff.2013.1463. [DOI] [PubMed] [Google Scholar]
- 35.Vierra M. Death panels. Ann Intern Med. 2012;156:394–395. doi: 10.7326/0003-4819-156-5-201203060-00016. [DOI] [PubMed] [Google Scholar]
- 36.Bishop JP, Brothers KB, Perry JE, Ahmad A. Finite knowledge/finite power: “Death panels” and the limits of medicine. Am J Bioeth. 2010;10:W7–9. doi: 10.1080/15265160903493070. [DOI] [PubMed] [Google Scholar]
- 37.Finlayson E, Birkmeyer JD. Research based on administrative data. Surgery. 2009;145:610–616. doi: 10.1016/j.surg.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Riley GF. Administrative and claims records as sources of health care cost data. Med Care. 2009;47:S51–55. doi: 10.1097/MLR.0b013e31819c95aa. [DOI] [PubMed] [Google Scholar]
- 39.Iezzoni LI. The risks of risk adjustment. JAMA. 1997;278:1600–1607. doi: 10.1001/jama.278.19.1600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.