Abstract
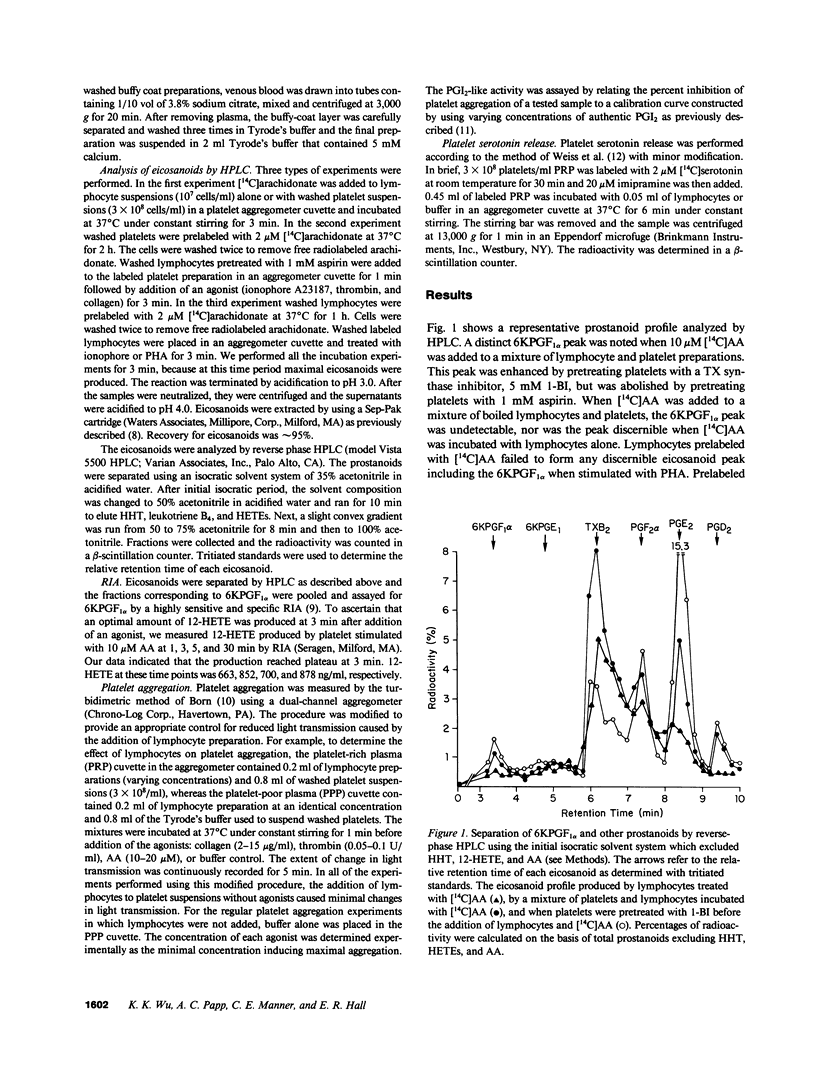
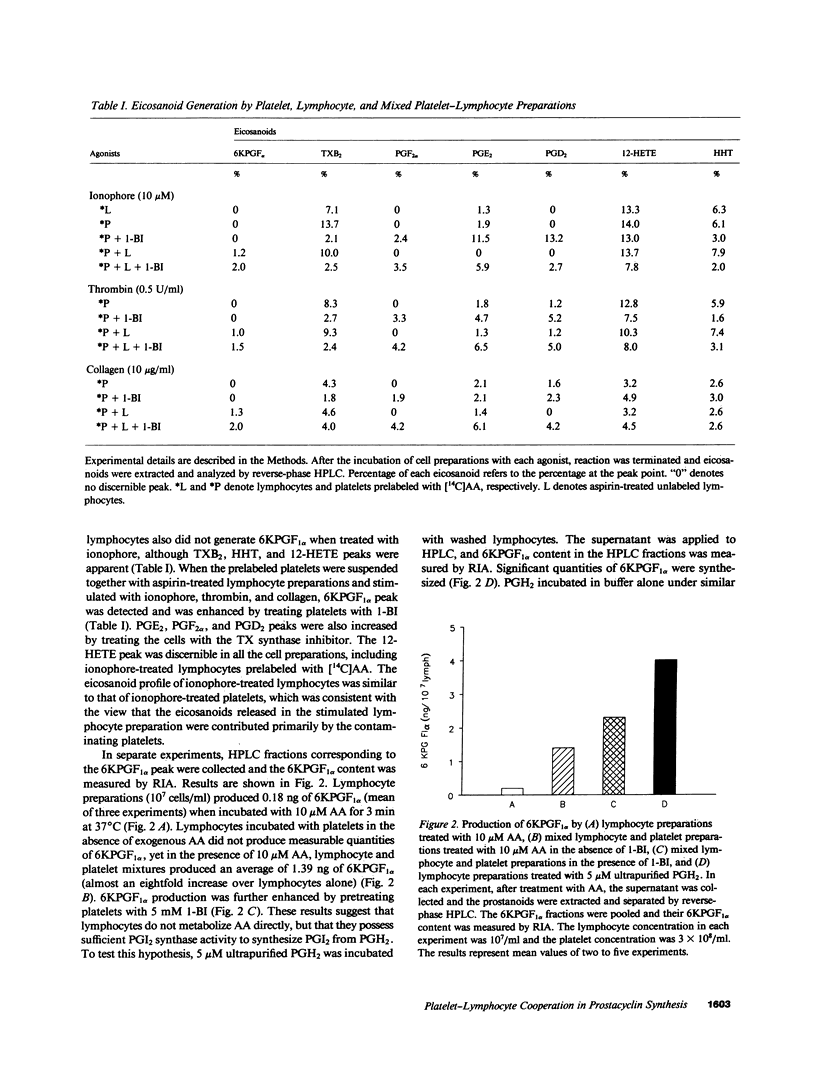
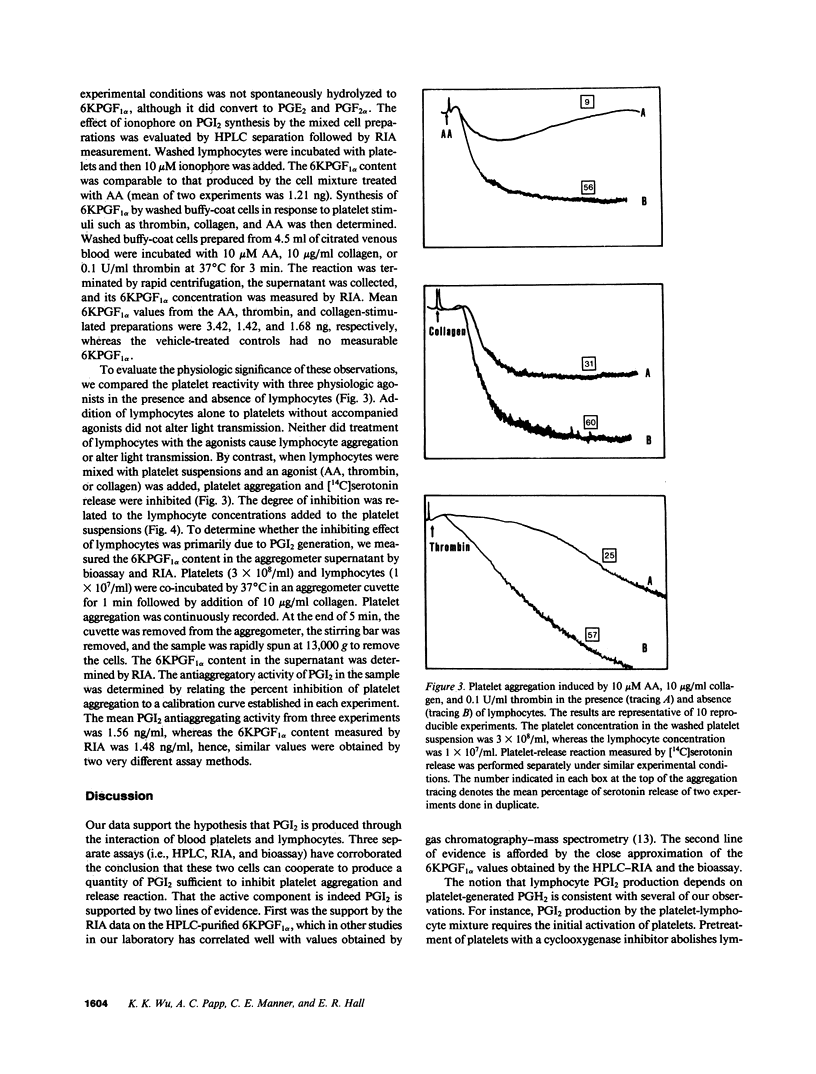
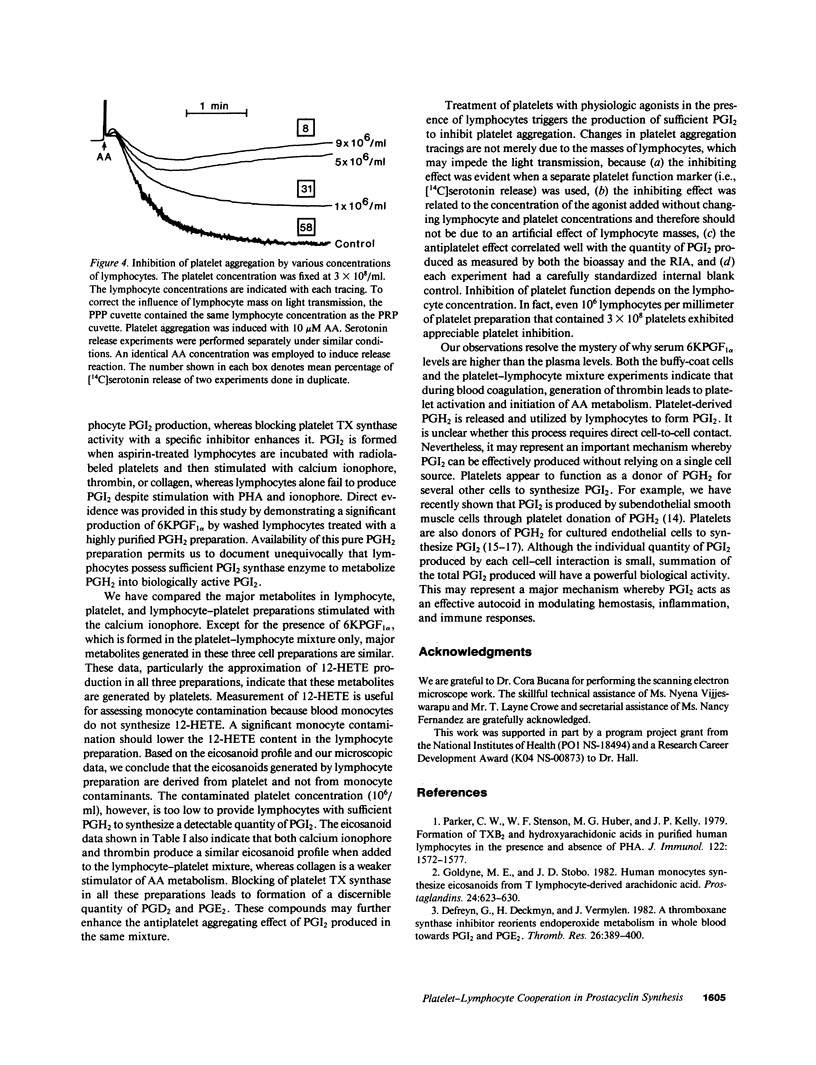
To test the hypothesis that prostacyclin (PGI2) is formed via a biochemical interaction between platelets and lymphocytes, we measured eicosanoids by high performance liquid chromatography (HPLC) and radioimmunoassay (RIA). A distinct 6-keto-prostaglandin F1 alpha (6KPGF1 alpha) peak was noted when [14C]arachidonic acid ([14C]AA) was added to the mixed cell preparations which was increased by pretreating platelets with 1-benzylimidazole (1-BI). Lymphocytes prelabeled with [14C]AA failed to form 6KPGF1 alpha when stimulated with phytohemagglutinin (PHA) or ionophore A23187. When the prelabeled platelets were suspended together with aspirin-treated lymphocytes and stimulated with ionophore, thrombin, or collagen, a 6KPGF1 alpha peak was detected and enhanced by 1-BI. These results were supported by quantifying the 6KPGF1 alpha content in the HPLC-purified fraction by RIA. Adding prostaglandin H2 (PGH2) directly to lymphocytes led to 6KPGF1 alpha production. Platelet aggregation and release were inhibited by lymphocytes in a dose-related manner. We conclude that lymphocytes possess PGI2 synthase activity which is capable of converting platelet-derived PGH2 into PGI2. PGI2 formed is sufficient to inhibit platelet function.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORN G. V. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962 Jun 9;194:927–929. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- Bøyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol. 1976 Jun;Suppl 5:9–15. [PubMed] [Google Scholar]
- Chen Y. C., McLeod B., Hall E. R., Wu K. K. Accelerated prostacyclin degradation in the thrombotic thrombocytopenic purpura. Lancet. 1981 Aug 8;2(8241):267–269. doi: 10.1016/s0140-6736(81)90522-5. [DOI] [PubMed] [Google Scholar]
- Defreyn G., Deckmyn H., Vermylen J. A thromboxane synthetase inhibitor reorients endoperoxide metabolism in whole blood towards prostacyclin and prostaglandin E2. Thromb Res. 1982 Jun 15;26(6):389–400. doi: 10.1016/0049-3848(82)90311-5. [DOI] [PubMed] [Google Scholar]
- Goldyne M. E., Stobo J. D. Human monocytes synthesize eicosanoids from T lymphocyte-derived arachidonic acid. Prostaglandins. 1982 Nov;24(5):623–630. doi: 10.1016/0090-6980(82)90032-6. [DOI] [PubMed] [Google Scholar]
- Hall E. R., Papp A. C., Seifert W. E., Jr, Wu K. K. Stimulation of endothelial cell prostacyclin formation by interleukin-2. Lymphokine Res. 1986 Spring;5(2):87–96. [PubMed] [Google Scholar]
- Hall E. R., Tuan W. M., Venton D. L. Production of platelet thromboxane A2 inactivates purified human platelet thromboxane synthase. Biochem J. 1986 Feb 1;233(3):637–641. doi: 10.1042/bj2330637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus A. J., Weksler B. B., Jaffe E. A., Broekman M. J. Synthesis of prostacyclin from platelet-derived endoperoxides by cultured human endothelial cells. J Clin Invest. 1980 Nov;66(5):979–986. doi: 10.1172/JCI109967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman P., Wyche A., Raz A. Platelet and blood vessel arachidonate metabolism and interactions. J Clin Invest. 1979 Feb;63(2):345–349. doi: 10.1172/JCI109309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp A. C., Crowe L., Pettigrew L. C., Wu K. K. Production of eicosanoids by deendothelialized rabbit aorta: interaction between platelets and vascular wall in the synthesis of prostacyclin. Thromb Res. 1986 May 15;42(4):549–556. doi: 10.1016/0049-3848(86)90218-5. [DOI] [PubMed] [Google Scholar]
- Parker C. W., Stenson W. F., Huber M. G., Kelly J. P. Formation of thromboxane B2 and hydroxyarachidonic acids in purified human lymphocytes in the presence and absence of PHA. J Immunol. 1979 Apr;122(4):1572–1577. [PubMed] [Google Scholar]
- Pedersen A. K., Watson M. L., FitzGerald G. A. Inhibition of thromboxane biosynthesis in serum: limitations of the measurement of immunoreactive 6-keto-PGF1 alpha. Thromb Res. 1984 Jan 1;33(1):99–103. doi: 10.1016/0049-3848(84)90159-2. [DOI] [PubMed] [Google Scholar]
- Schafer A. I., Crawford D. D., Gimbrone M. A., Jr Unidirectional transfer of prostaglandin endoperoxides between platelets and endothelial cells. J Clin Invest. 1984 Apr;73(4):1105–1112. doi: 10.1172/JCI111296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh P. N. Albumin density gradient separation and washing of platelets and the study of platelet coagulant activities. Br J Haematol. 1972 Feb;22(2):205–217. doi: 10.1111/j.1365-2141.1972.tb08801.x. [DOI] [PubMed] [Google Scholar]
- Weiss H. J., Tschopp T. B., Rogers J., Brand H. Studies of platelet 5-hydroxytryptamine (serotonin) in storage pool disease and albinism. J Clin Invest. 1974 Aug;54(2):421–433. doi: 10.1172/JCI107778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K. K., Hall E. R., Rossi E. C., Papp A. C. Serum prostacyclin binding defects in thrombotic thrombocytopenic purpura. J Clin Invest. 1985 Jan;75(1):168–174. doi: 10.1172/JCI111670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulak I. M., Puttemans M. L., Schilling A. B., Hall E. R., Venton D. L. A fast, nondestructive purification scheme for prostaglandin H2 using a nonaqueous, bonded-phase high-performance liquid chromatography system. Anal Biochem. 1986 Apr;154(1):152–161. doi: 10.1016/0003-2697(86)90509-9. [DOI] [PubMed] [Google Scholar]



