Abstract
Objective:
Analysis of red blood cell acetyl cholinesterase (AChE) in a familial Alzheimer's diseases (AD) Parkinson's disease dementia (PDD) and their first generation.
Setting:
General hospital, Mahad district, Raigad.
Patients and Methods:
Clinically diagnosed patients of AD and PDD and their asymptomatic relatives. Their blood was collected in EDTA tube and transferred to laboratory at Mumbai.
Result:
Median red blood cell (RBC) cholinesterase levels amongst PDD, their first generation asymptomatic relatives, familial AD, asymptomatic relatives of AD, healthy controls, farmers exposed to pesticides (positive control) and other neurological condition without dementia (hypertension with TIA 1, sub-dural hematoma 2, hypothyroid 1, non-familial unilateral parkinsonism without dementia 3, writers cramps 2, hyponitremia 1 and cerebral palsy with non-fluent aphasia 1). Median values of RBC AChE were 19086.78 U/L, 15666.05 U/L, 9013.11 U/L, 7806.19 U/L, 14334.57 U/L, 9785.05 U/L and 13162.60 U/L, respectively. As compared to controls, RBC AChE levels were statistically significant among PDD (P = 0.004) and significantly lowered among familial AD patients (P = 0.010), relatives of patients (P = 0.010).
Interpretations:
Below the normal RBC AChE level is a potential biomarker in asymptomatic relatives of familial AD patients. RBC AChE is raised than normal level in patients suffering from PDD, where AChE inhibitors are helpful. However, RBC AChE level below the normal where AChE inhibitor may not be effective.
Keywords: Acetyl choline, acetyl cholinesterase inhibitors, Alzheimer's diseases, donepzil, mementine, Parkinson's diseases dementia
Introduction
Alzheimer's disease (AD) and Parkinson's disease dementia (PDD) are the most common dementia. Incidence of dementia is directly related to advanced age. Its prevalence in India is 1-3%. Studies have shown that pathophysiology changes occurred decade before the appearance of first symptoms in AD.[1] Twenty-five percent of all the people aged below 55 have family history of dementia.[2,3,4] About 40-70% patients of PDD that manifest 2 years after the clinical diagnosis of Parkinsonism. More than 10 autosomal dominant and recessive gene loci have been linked with Parkinson's disease. The 10-15% will have an affected first or second degree relative.[5] The severity of cognitive symptoms correlates with the extent of cholinergic deficiency, though there is lesser damage to neocortical neurons, which results in better outcome with cholinergic repletion.[6] Neurotransmitters responsible for memory is acetyl choline. Acetyl choline esterase (AChE) is a key enzyme in the cholinergic nervous system, it inactivates the acetyl choline. AChE has an important role in neuronal development including regular cell growth. The AChE activity in plasma is correlated with brain beta amyloid load.[7] Raised plasma AChE seen in early AD and is a pre-symptomatic biomarker.[8,9,10,11,12,13] In an asymptomatic person with positive family history of AD, early diagnosis with biomarkers is important for preventing morbidity due to late detection, advanced care planning and reproductive counseling.[4] In a situation with restricted resources like that in rural India, we evaluated role of RBC AChE level as a simple cheap and easily available biomarker for early prediction of dementia in an asymptomatic population.
Patients and Methods
This study is conducted at general hospital in Mahad, from June 2013 to February 2014. Written consent was obtained from blood relatives of patients. We evaluated a total of 44 subjects. AD 5, their first-generation relatives 6, PDD 5, their first generation relatives 5, farmers exposed to pesticide (occupational hazards) 6, other 11 (hypertension with transient ischemic attack 1, sub-dural hematoma 2, hypothyroid 1, non-familial unilateral parkinsonism without dementia 3, writer's cramps 2, hyponitremia 1 and cerebral palsy with non-fluent aphasia1) and healthy control 6 [Table 1].
Table 1.
Age and sexwise distribution of 44 cases

History, clinical examination and diagnosis
We tried our best to evaluate these AD and PDD cases by the mini-mental state examination and Wechsler memory score. The cognitive function was measured with score ranging from 0 (severe impairment) to 30 (no impairment). Participants recall details as they can from a short story containing 25 bits of information after it was read aloud by examiner and again after 30 minutes delay with score ranging from 0 (no recall) to 25 (complete recall). The present studied cases of AD and PDD are almost in terminal phase of disease and were impossible to classify according to these tests. However, asymptomatic cleared all the above tests except the case of hypothyroidism, sub-dural hematoma and hyponitremia who had transient memory deficit; all of these were totally recovered with treatment.[14] Diagnosis of AD was made by criteria including progressive neurological disease of brain leading to the irreversible loss of intellectual abilities, including memory and reasoning, which become severe enough to impede social or occupational functioning,[3] and PDD was defined as dementia that develop at least 2 years after the diagnosis of Parkinson's disease.[15]
These patients of AD and PDD are neglected in family as being extra burden because of maximum money, time is already spend for specialist consultations, biochemical and radiological investigations. Care takers are much sufferer. Relatives felt that there is no use of any therapy and investigations hence the patient's records are destroyed or burned. Relatives denied moreover, it was very difficult or rather impossible to bring these severely demented cases to hospital [Figures 1–3]. We authors visited regularly to their residents of respective patients, examined in details, took detail family history of similar illness including maternal and paternal side. In case of positive family history, details of pattern of illness, age of onset of illness and provided treatment if any. Whatever investigations were available were noted and recorded in standard pro forma. PDD patients of onset of tremors and progressive development of dementia was recorded, including delusions, hallucinations, anxiety and mood symptoms were noted in these cases.
Figure 1.
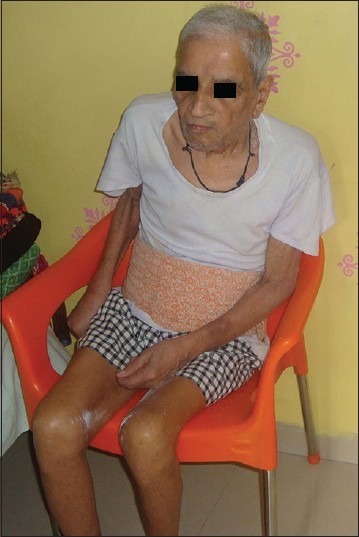
WB/84 years old male is suffering of parkinsonism since last 11 years and since last 7 years progressive loss of memory and cognitive function. At present total dementia, flexed deformity, rigidity with contractures, mute and incontinence
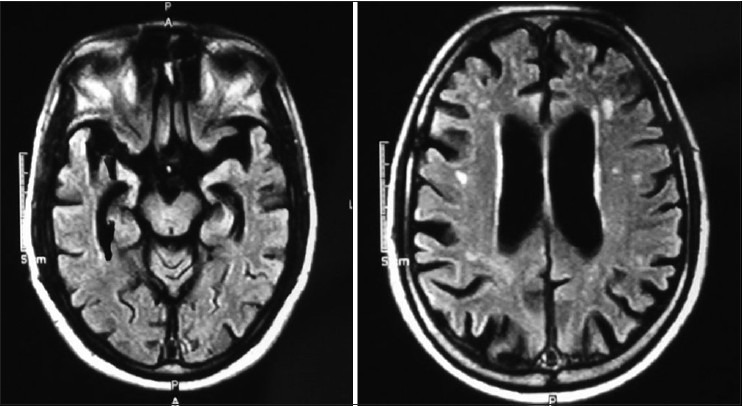
Figure 3.
MRI of VMS/81 years male progressive loss of memory, mute, incontinence since last 5 year. His mother had similar illness. MRI shows Basal ganglia infarct generalized cortical atrophy and degeneration of hippocampus (RBC AChE is 7880.22 U/L (normal 11.188–16698 u/L)
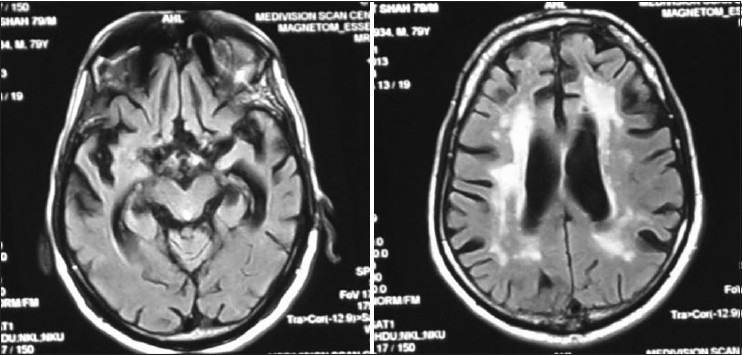
Figure 2.
WB - MRI 10/10/2001 showed diffuse atrophy of brain and basal nuclei, widened ventricles and degeneration of hippocampus (RBC AChE is 20544.68 (normal (11.188– 16698)
RBC AChE detection
Exclusion
Patients with history of smoking, tobacco chewing, application of tobacco paste, exposure to manganese, depression, consumption of non-steroid anti-inflammatory, benzodiazepine and AChE inhibitors or memantine drugs or exposure to pesticides except farmers were excluded. Routine laboratory evaluation included complete blood count, fasting blood sugar, renal and liver chemistries, thyroid stimulating hormone, vitamin B12 and folate levels. Total red blood cell (RBC) AChE level was done in all the study subjects. Neuroimaging with MRI was not possible in all subjects due to financial and logistic problems. Whenever available with old record, we studied the available CT or MRI plates. In 10 patients who were totally demented, written consent was obtained from their blood relatives and control and asymptomatic person gave their own consent. Being incurable disease data is kept secret and anonymous.
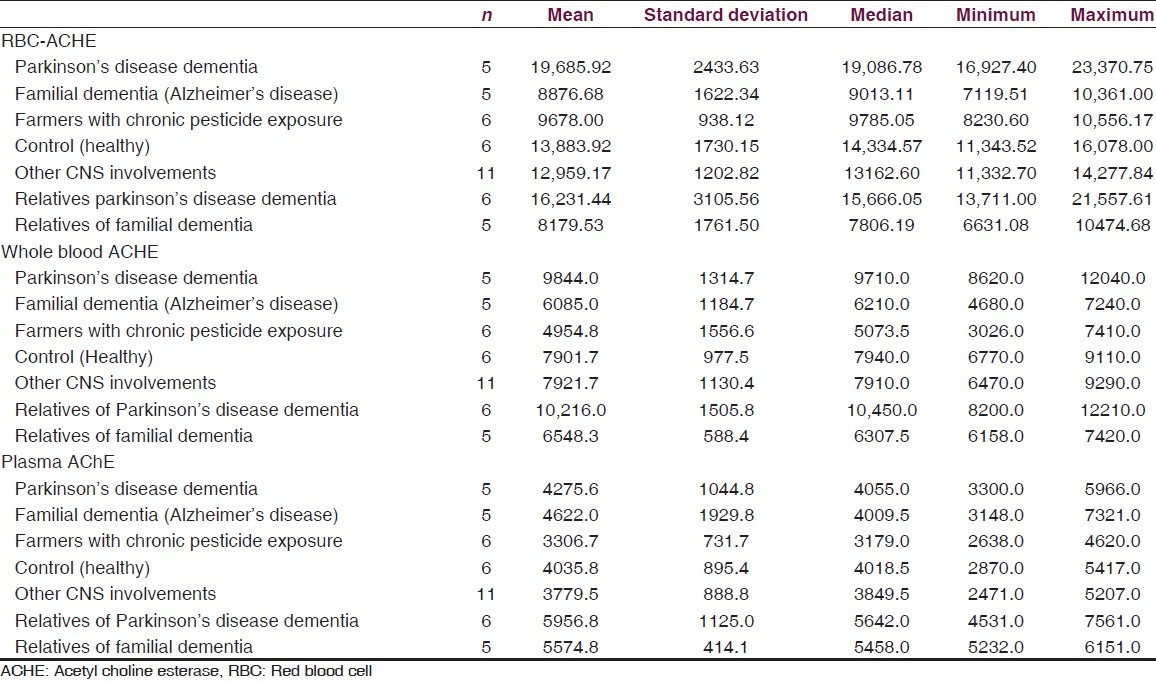
Blood sample was collected in EDTA bulb and was transferred to laboratory in cold chain by courier. RBC AChE level was analyzed by measure of the enzyme activity in U/L unit [Tables 1 and 2].
Table 2.
Standard deviation of mean, median, maximum and minimum average red blood cell, Whole blood and plasma acetyl choline esterase level in U/L in total 44 cases
Results and Statistical Analysis
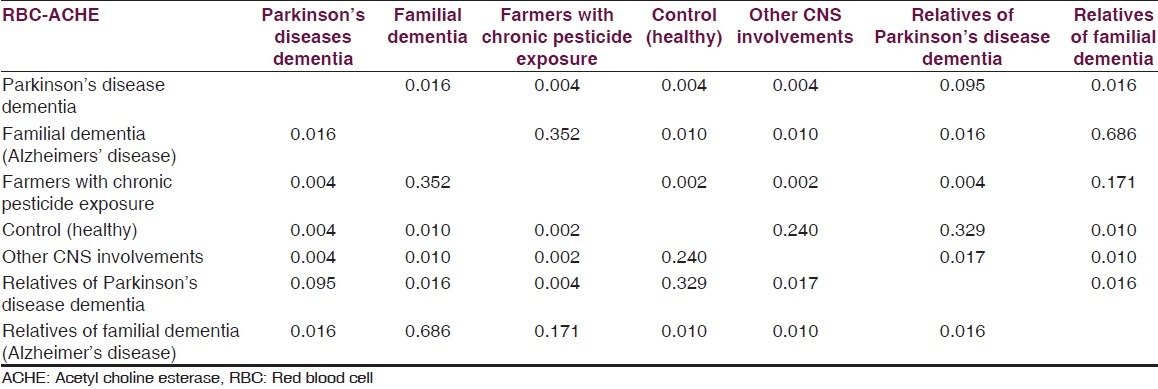
Data is presented as, mean, median, minimum and maximum for numerical variables. Mann-Whitney test was used for comparison of AChE values between the two groups. For this study, we have considered a P value less than 0.05 as statistically significant. All analyses were performed using IBM-SPSS version 21. Mean RBC AChE level was significantly lower (Mann-Whitney test for each comparison) in the familial AD group when compared to controls and patients with PDD. Levels of RBC AChE in asymptomatic first degree relatives of AD patients were significantly lower than controls. Mean RBC AChE level was significantly higher (Mann-Whitney test for each comparison) in PDD as compared to controls, patients with familial AD dementia and relatives of familial dementia and farmers with pesticide exposure. Mean RBC AChE level was statistically significantly lower (Mann-Whitney test for each comparison) in farmers with chronic pesticide exposure as compared to controls, patients with AD and familial PDD [Tables 2–4].
Table 4.
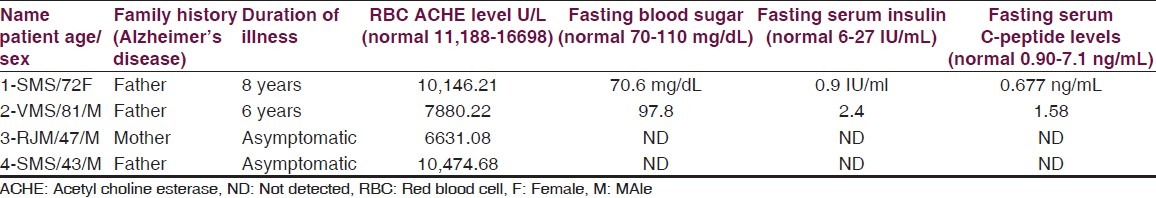
RBC AChE levels and fasting insulin and C-peptide levels in of two patients and RBC ACHE levels their first generation relatives
Discussion
In the present study we have recorded that the level of plasma and RBC AChE familial dementia of AD is significantly below the normal value (P = 0.010), being similar in relatives of familial dementia (P = 0.010). However, RBC AChE significantly rose in PDD (P = 0.004) compared to control, AD. However, both levels are raised in PDD patients and their first generation relatives as compared to AD patients and their relatives. This is the first report of its kind showed an altered level of plasma and RBC AChE level in AD and PDD patients and their first generation relatives.
Though RBC AChE gets significantly reduced in AD brain but the butyrycholinestarase is increased.
Thus, RBC AChE might be a diagnostic biomarker for early diagnosis of dementia and is worth investigating.[11,12,13]
We evaluated role of RBC AChE level as a simple cheap and easily available biomarker for early prediction of dementia in asymptomatic population. RBC AChE level correlates with the cholinergic cortical AChE level. Because of no improvement and deterioration in patient with AD and PDD, these cases are totally neglected from family members. Moreover, lethargic and silence approach of scientists and neurologists of India toward these non-treatable diseases result a miserable outcome. The patient with PDD have greater cholinergic deficit than those with AD. The extent of deficit correlates with severity of cognitive symptoms and inhibition of pro-inflammatory markers [Tables 2 and 3].[15,16] Dysfunction and loss of basal forebrain cholinergic neuron and reduction in acetyl choline level also contribute to cognitive impairment in AD. Presynaptic alpha-7 nicotinic acetyl choline receptors have a vital role in cognitive processing and their levels increases in early AD before reducing later. Activation of nicotinic acetyl choline receptors or muscarinic type-1 receptors limit TAU phosphorylation.[17]
Table 3.
Results (P value) for Mann-Whitney test RBC AChE in 44 cases
AChE plays important role in AB fibrinogenesis. Plasma and RBC AChE level is consistently reduced in AD brain. In the present report we observed plasma and RBC AChE levels are significantly reduced in severe AD patients and also in their first degree relatives [Tables 2 and 3]. Patient with PDD have a greater cholinergic deficit than AD. The severity of deficiency so relates with severity of cognitive symptoms and responds to AChE inhibitor.[6]
Farmers are chronically exposed to pesticides and persistent inhibition of AChE result in raised level of acetyl choline that may mask the symptoms of early dementia or delays the dementia.
At rural setting these dementia cases are reported in terminal phase, when RBC AChE level, a simple biomarker test, may help a treating physician regarding selection of appropriate therapy. At rural India aging population of age more than 70 years are increasing in numbers. In such situation we found a simple promise of RBC AChE as non-invasive, simple and easily available biomarker in an asymptomatic person with strong family history of dementia.[13] Acetyl cholinesterase inhibitors (donepzil, rivastigmine and galantamine) and the N-methyl-D-aspirate receptor antagonist memantine are the only treatment for AD that have been approved by the Food and Drug administration.[18] Randomized, placebo-controlled clinical trials of cholinesterase inhibitors have included patients with mainly mild to moderate AD and have shown significant but clinically marginal benefits with respect to cognition, daily function and behavior a selective muscarinic agonist has been shown to improve vocal outbursts and psychological symptoms.[19,20]
Over excitations of NMDA receptors results in neuronal excitotoxicity, which has been complicated in the loss of neurons associated with ischemic strokes, AD, Parkinson's and Huntington disease and amyotrophic lateral sclerosis; mementine, an uncompetitive NMDA-receptor antagonist, could be therapeutically important in moderate to severe AD.[21,22]
Recently therapeutic effects of PPARy agonists thiozolidinediones in AD attributed to their action on insulin and insulin-resistant peripheral hyperinsulinemia seem to promote neuropathology of AD.[22,23] These agents are known to increase peripheral insulin sensitivity and reduce the circulating insulin, which competes with amyloidal beta for degradation by the insulin-degrading enzymes (23). In present two patients (patients 1 and 2) of familial AD fasting serum insulin and serum C-peptide levels are below the normal value [Table 4] but their fasting blood sugar is normal. Excessive eating in these two patients was reduced after regular administration of pioglitazone 7.5 mg without hypoglycaemia [Table 4].
Acetyl salicylic acid may generate messengers that are anti-pro-inflammatory signals and may shed new insight into how the brain modulates its response to Alzheimer's injury.[24] To date, treatment of AD relies on the symptomatic effects of cholinesterase inhibitors and NMDA receptor antagonists.
Limitations of study
We have performed analysis of RBC acetylcholine esterase levels in various groups made on clinical examination. We could not perform further investigation like validated tests (PET or CSF Amyloid plaque or phosphorylated-Tau) and genetic analysis for AD diagnosis. Also, numbers of participants in a study are less considering patient and resource availability in our area. Further work is in progress.
Acknowledgements
We are grateful to professor Dr. Tukaram Jamale Nephologist KEM Mumbai for reviewing the manuscript.
Dr. Abhijit Pakhare Professor of preventive and social medicine AIIMS Bhopal for statically analysis of data.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–28. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aprahamian I, Stella F, Forlenza OV. New treatment strategies for Alzheimer's disease: Is there a hope? Indian J Med Res. 2013;138:449–60. [PMC free article] [PubMed] [Google Scholar]
- 3.Mayeux R. Clinical practice. Early Alzheimer's disease. N Engl J Med. 2010;362:2194–201. doi: 10.1056/NEJMcp0910236. [DOI] [PubMed] [Google Scholar]
- 4.Loy CT, Schofield PR, Turner AM, Kwok JB. Genetics of dementia. Lancet. 2014;383:828–40. doi: 10.1016/S0140-6736(13)60630-3. [DOI] [PubMed] [Google Scholar]
- 5.Nutt JG, Wooten GF. Clinical practice. Diagnosis and initiual management of Parkinson's disease. N Engl J Med. 2005;353:1021–7. doi: 10.1056/NEJMcp043908. [DOI] [PubMed] [Google Scholar]
- 6.Bohnen NI, Kaufer DI, Ivanco LS, Lopresti B, Koeppe RA, Davis JG, et al. Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: An in vivo positron emission tomographic study. Arch Neurol. 2003;60:1745–8. doi: 10.1001/archneur.60.12.1745. [DOI] [PubMed] [Google Scholar]
- 7.Grenfield S. Discovering and targeting the basic mechanism of neurodegeneration: The role of peptides from the C-terminus of acetylcholinesterase: Non-hydrolytic effects of ache: The actions of peptides derived from the C-terminal and their relevance to neurodegenertaion. Chemico-pathological interactions. Chem Biol Interact. 2013;203:543–6. doi: 10.1016/j.cbi.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 8.García-Ayllón M, Riba-Llena I, Serra-Basante C, Alom J, Boopathy R, Sáez-Valero J. Altered levels of acetylcholinesterase in Alzheimer plasma. PLoS One. 2010;5:e8701. doi: 10.1371/journal.pone.0008701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García-Ayllón MS, Small DH, Avila J, Sáez-Valero J. Revisiting the role of acetylchoinesterese in Alzheimer's disease: Cross-talk with P-tau and β-amyloid. Mol Neurosci. 2011;4:22. doi: 10.3389/fnmol.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet. 1976;2:1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- 11.Chipperfield B, Newman PM, Moyes IC. Decreased erythrocyte cholinesterase activity in dementia. Lancet. 1981;2:199. doi: 10.1016/s0140-6736(81)90377-9. [DOI] [PubMed] [Google Scholar]
- 12.Perry RH, Wilson ID, Bober MJ, Atack J, Blessed G, Tomlinson BE, et al. Plasma and erythrocyte acetylcholinesterase in senile dementia of Alzheimer type. Lancet. 1982;319:174–5. doi: 10.1016/s0140-6736(82)90429-9. [DOI] [PubMed] [Google Scholar]
- 13.Bird TD. Genetic factors in Alzheimer's disease. N Engl J Med. 2005;352:862–4. doi: 10.1056/NEJMp058027. [DOI] [PubMed] [Google Scholar]
- 14.Bateman R, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Dominantly Inherited Alzheimer Network. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Press DZ. Parkinson's disease dementia--a first step? N Engl J Med. 2004;351:2547–9. doi: 10.1056/NEJMe048305. [DOI] [PubMed] [Google Scholar]
- 16.van Gool WA, van de Beek D, Eikelenboom P. Systemic infection and delirium: When cytokines and acetylcholine collide. Lancet. 2010;375:773–5. doi: 10.1016/S0140-6736(09)61158-2. [DOI] [PubMed] [Google Scholar]
- 17.Ridha BH, Barnes J, Bartlett JW, Godbolt A, Pepple T, Rossor MN, et al. Trackling atrophy progression in familial Alzheimer's disease: A serial MRI study. Lancet Neurol. 2006;5:828–34. doi: 10.1016/S1474-4422(06)70550-6. [DOI] [PubMed] [Google Scholar]
- 18.Reisberg B, Doody R, Stöffler A, Schmitt F, Ferris S, Möbius HJ. Memantine Study Group. Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med. 2003;348:1333–41. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 19.Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I. Memantine Study Group. Memantine treatment in a patients with moderate to severe Alzheimer disease already receiving Donepezil: A randomized controlled trial. JAMA. 2004;291:317–24. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- 20.Rösler M, Anand R, Cicin-Sain A, Gauthier S, Agid Y, Dal-Bianco P, et al. Efficacy and safety of rivastigmine in patients with Alzheimer's disease: International randomised controlled trial. BMJ. 1999;318:633–8. doi: 10.1136/bmj.318.7184.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The European Federation of Neurological Societies Annual Meeting. Stokholm, Sweden: [Last accessed on 2014 May 31-Jun 1]. Available from: http://www.efns.org . [Google Scholar]
- 22.Craft S. The role of metabolic disorders in Alzeheimer disease and vascular dementia: Two roads converged. Arch Neurol. 2009;66:300–5. doi: 10.1001/archneurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landreth G, Jiang Q, Mandrekar S, Heneka M. PPARgamma agonists as therapeutics for the treatment of Alzheimer's disease. Neurotherapeutics. 2008;5:481–9. doi: 10.1016/j.nurt.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pomponi M, Pomponi MF. Alzheimer's disease prevention and acetyl salicylic acid: A believable story. Indian J Med Res. 2014;139:1–3. [PMC free article] [PubMed] [Google Scholar]


