Abstract
Portal cavernoma develops as a bunch of hepatopetal collaterals in response to portomesenteric venous obstruction and induces morphological changes in the biliary ducts, referred to as portal cavernoma cholangiopathy. This article briefly reviews the available literature on the vascular supply of the biliary tract in the light of biliary changes induced by portal cavernoma. Literature pertaining to venous drainage of the biliary tract is scanty whereas more attention was focused on the arterial supply probably because of its significant surgical implications in liver transplantation and development of ischemic changes and strictures in the bile duct due to vasculobiliary injuries. Since the general pattern of arterial supply and venous drainage of the bile ducts is quite similar, the arterial supply of the biliary tract is also reviewed. Fine branches from the posterior superior pancreaticoduodenal, retroportal, gastroduodenal, hepatic and cystic arteries form two plexuses to supply the bile ducts. The paracholedochal plexus, as right and left marginal arteries, run along the margins of the bile duct and the reticular epicholedochal plexus lie on the surface. The retropancreatic, hilar and intrahepatic parts of biliary tract has copious supply, but the supraduodenal bile duct has the poorest vascularization and hence susceptible to ischemic changes. Two venous plexuses drain the biliary tract. A fine reticular epicholedochal venous plexus on the wall of the bile duct drains into the paracholedochal venous plexus (also called as marginal veins or parabiliary venous system) which in turn is connected to the posterior superior pancreaticoduodenal vein, gastrocolic trunk, right gastric vein, superior mesenteric vein inferiorly and intrahepatic portal vein branches superiorly. These pericholedochal venous plexuses constitute the porto-portal collaterals and dilate in portomesenteric venous obstruction forming the portal cavernoma.
Keywords: epicholedochal plexus, paracholedochal plexus, parabiliary venous system, porto-portal collaterals
Abbreviations: LHD, left hepatic duct; RASD, right anterior sectoral duct; RPSD, right posterior sectoral duct; RHD, right hepatic duct; CHD, common hepatic duct; CBD, common bile duct; GDA, gastroduodenal artery; PSPDA, posterior superior pancreaticoduodenal artery; PSPDV, posterior superior pancreaticoduodenal vein; CHA, common hepatic artery; RHA, right hepatic artery; LHA, left hepatic artery; CD, cystic duct; CBD, common bile duct; PD, pancreatic duct; SMV, superior mesenteric vein; SV, splenic vein; SRCV, superior right colic vein; RGV, right gastric vein; IHBD, intrahepatic bile ductules; PBP, peribiliary plexus; HABr, hepatic arteriolar branches; PVBr, portal vein branches; CA, communicating arcade; FJV, first jejunal vein; GCT, gastrocolic trunk; AIPDV, anterior inferior pancreaticoduodenal vein; ASPDV, anterior superior pancreaticoduodenal vein
Portal cavernoma develops as a bunch of dilated and tortuous hepatopetal collateral venous channels in response to portomesenteric venous obstruction and induces morphological changes in the biliary ducts, referred to as portal biliopathy or portal cavernoma cholagiopathy. Available literature suggest that the venous plexuses draining the bile duct and veins around the head of pancreas act as preferential collateral venous pathways and dilate to form the portal cavernoma. It is believed that anatomically distinct collateral venous channels are available in cases of portal venous obstruction involving portal vein alone or superior mesenteric and splenic veins also. This article is an attempt to review the existing literature on the venous drainage of biliary tract to understand the mechanisms of development of portal cavernoma. Arterial supply of the biliary tract is more extensively studied than the venous drainage and since the general pattern of arterial supply and venous drainage is similar, this article includes the review of arterial supply also.
Anatomy of Intrahepatic Biliary Tract
Biliary tract is composed of intrahepatic and extrahepatic components. Intrahepatic biliary drainage system parallels the portal venous and hepatic arterial supply and based on their branching pattern the liver is divided into physiological right and left lobes and segments. The left lobe is divided into medial and lateral sections or sectors by the umbilical fissure. The left lateral section is divided into superior (segment II) and inferior (segment III) segments. Union of ducts of segment II and III behind the umbilical part of left portal vein form the left hepatic duct (LHD) which then receives the duct from segment IV. Average length of the LHD is 1.7 cm and diameter is 3.0 mm (±1.08). Right lobe is divided into anterior and posterior sections or sectors, each of which is further divided into superior and inferior segments. The right anterior sectoral duct (RASD) drains segments V and VIII and the right posterior sectoral duct (RPSD) drains segments VI and VII. The RPSD passes horizontally and generally curves round the RASD to join its medial side to form the right hepatic duct (RHD). Average length of RHD is 0.9 cm and diameter is 2.6 mm (±1.2). Both right and left hepatic ducts drain the caudate lobe (segment I). This pattern of formation of RHD is observed in 57% and LHD in 67% population [Figure 1].
Figure 1.
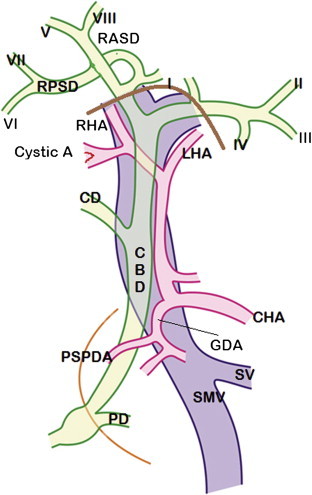
Normal anatomy of biliary tract: Extrahepatic and intrahepatic segmental bile ducts along with branches of hepatic artery and portal vein are shown. I – VIII are segmental ducts. CHA = Common hepatic artery; RHA = Right hepatic artery; LHA = Left hepatic artery; GDA = Gastroduodenal artery; PSPDA = Posterior superior pancreaticoduodenal artery; CD = Cystic duct; CBD = Common bile duct; PD = Pancreatic duct; RASD = Right anterior sectoral duct; RPSD = Right posterior sectoral duct; SMV = Superior mesenteric vein; SV = Splenic vein. Thick brown line – outline of porta hepatis. Thin orange line – outline of second part of duodenum.
Anatomy of Extrahepatic Biliary Tract
The right and left hepatic ducts unite in the hilar plate, close to the right end of porta, in front of right branch of portal vein, to form the common hepatic duct (CHD). Its lower end is defined by its junction with cystic duct, on its right margin in an acute angle, to form the common bile duct (CBD). Its length varies from 1.0 cm to 7.5 cm and average diameter is 4.0 mm. Cystic duct, 3–4 cm long with a mean diameter of 4.0 mm, runs posteroinferiorly and to the left to join the right border of CHD to form the CBD.
Common Bile duct (CBD), 6.0–8.0 cm long, is generally divided into supraduodenal, retroduodenal, retropancreatic and intraduodenal segments. The supraduodenal CBD lies in the right border of lesser omentum (hepato-duodenal ligament) anterior to portal vein and to the left of hepatic artery proper. Its mean external diameter is 9 mm (range 5–13 mm) and mean internal diameter is 8 mm (range 4–12.5 mm).1 The retroduodenal part passes behind the superior part of duodenum to the right of gastroduodenal artery (GDA) and in front of portal vein. Posterior superior pancreaticoduodenal artery (PSPDA), branch of GDA, crosses the CBD anteriorly. Retropancreatic part (more appropriately intrapancreatic) runs downwards and to the right behind the head of the pancreas to reach the medial border of second part of duodenum. In this course CBD may sometimes groove the head of pancreas or course intrapancreatically. In 83% cases a part of pancreatic tissue covers both surfaces of the duct and only in 17% cases the CBD is truly retropancreatic.2 Near the middle of medial border of descending duodenum, the CBD and the main pancreatic duct (of Wirsung) pierce the duodenal wall and unite to form the hepatopancreatic ampulla (of Vater) which opens on the major duodenal papilla 8 cm distal to pylorus. The formation of this common channel occurs in 85% cases and in the rest 15% cases, the two ducts either open separately or form a V junction before opening. A sheath of circular muscle fibers, the sphincter of Oddi, surrounds the ampulla and terminal parts of CBD and main pancreatic duct. The mean internal diameter of CBD near the ampulla is only 4.0 mm.1
Arterial Supply of Biliary Tract
Arterial supply of the biliary tract is more extensively investigated than the venous drainage, because of its important surgical implications in liver transplantation and the development of ischemic changes and strictures after vasculobiliary injuries.3
In contrast to hepatic parenchyma which has a dual blood supply by portal vein and hepatic artery, the intrahepatic and extrahepatic bile ducts are conventionally believed to be totally dependent on the hepatic arterial supply for oxygenation. Evidence to the contrary was recently provided by Slieker et al4 who demonstrated a 40% contribution of portal vein to the microvascular blood flow through the CBD in a surgically simulated condition by transecting the CBD mimicking the situation in liver transplantation. Whether such a contribution occurs under normal physiological conditions remain unanswered and the evidence provided is circumstantial and inconclusive. The authors do agree that the hepatic artery is an important contributor to blood flow through the CBD and suggest that disturbances of portal venous blood flow after liver transplantation should be taken into consideration for any intervention to prevent biliary ischemia. It is stated that about 50% of hepatic arterial blood is meant for the supply of biliary tract.5 The vascularization of extrahepatic biliary system can be considered under supraduodenal CBD including CHD, retropancreatic CBD, hilar ducts and intrahepatic bile ducts. It is generally believed that the supraduodenal bile duct has the poorest vascularization and hence more vulnerable to ischemic changes even though no end arteries were demonstrated.6–8 On the contrary, Shapiro and Robillard9 suggested that the development of biliary strictures is due to presence of end arteries to the CBD. The retropancreatic, hilar and intrahepatic portions of the bile ducts have an excellent and copious blood supply.6 CT angiographic studies have demonstrated that the blood supply to the entire biliary tract including the ampulla of Vater is derived from the branches of the celiac trunk.10
The supraduodenal CBD including CHD is primarily supplied by 6–8 small arteries of about 0.30 mm in diameter having a longitudinal ascending or descending course and anastomosing with each other. The most prominent axial vessels run along the lateral borders and named as 3 o'clock and 9 o'clock arteries,7,11 right and left arch arteries,12 right and left marginal arteries8 [Figure 2]. Ascending branches arise from the posterior superior pancreaticoduodenal artery (PSPDA – frequently mentioned as retroduodenal artery in earlier literature), supraduodenal artery, gastroduodenal artery (GDA) and retroportal artery, while descending branches come from right hepatic artery (RHA), cystic artery. Two thirds of arterial input came from ascending vessels and only one third from descending vessels. At the level of inferior border of the cystic duct there is a zone of overlap between ascending and descending vessels. The 3 o'clock (left) and 9 o'clock (right) marginal arteries (the paracholedochal arteries) give small branches which form a fine network of epicholedochal plexus on the surface of the CBD.
Figure 2.
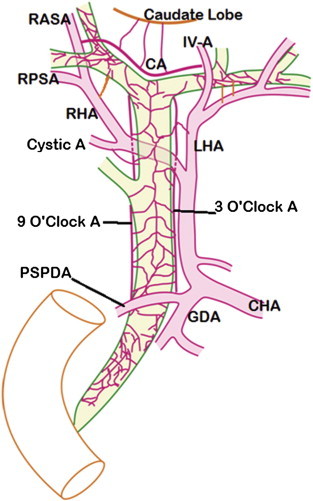
Normal arterial pattern of extrahepatic biliary tract. Supraduodenal CBD is primarily supplied by 3 o'clock (left) and 9 o'clock (right) marginal arteries contributed from posterior superior pancreaticoduodenal artery (PSPDA) from below and right hepatic (RHA), Left hepatic (LHA) and Cystic arteries from above. These marginal arteries are comparable to Paracholedochal Plexus (of Petren). Marginal arteries give small branches which form the Epicholedochal plexus. Above, the marginal arteries join the hilar plexus which supply the hilar ducts. Communicating Arcade (CA) connects RHA and LHA and is present cranial to the confluence of right and left hepatic ducts. The CA arises from right anterior sectoral artery (RASA) on the right and segment IV artery (IV A) on the left. RPSA – Right posterior sectoral artery.
The retroportal artery arising either from celiac trunk (42% cases) or from superior mesenteric artery (58% cases) is an important source of arterial supply to supraduodenal and retroduodenal parts of CBD.7,8 This artery ascends on the posterior surface of portal vein and head of pancreas and may join the PSPDA (designated Type I) or ascend on the posterior surface of supraduodenal CBD to join RHA (designated Type II). The retroportal artery is of larger caliber than the right and left marginal arteries measuring 0.92 mm in diameter (range 0.46–2.3 mm) and is present in more than 90% cases7,8 but Rath et al12 reported its presence only in 1 case out of 60 cases studied. A marginal artery on the posterior surface of CBD, probably similar to the retroportal artery, was also reported in few cases.11
Rath et al12 described three patterns of arterial vascularization named as axial pattern with right and left arches, ladder pattern with horizontally directed branches and mixed pattern [Figure 3]. The left marginal artery (3 o'clock artery) is present in 95% cases8 and arises inferiorly from PSPDA or GDA and joins the RHA superiorly. The right marginal artery (9 o'clock artery) is present in 82.5% cases8 and arises from PSPDA and generally joins the cystic artery distally. In cases where the marginal arteries are not formed, the CBD is supplied only by the epicholedochal plexus or a single epicholedochal artery [Figure 4].13
Figure 3.
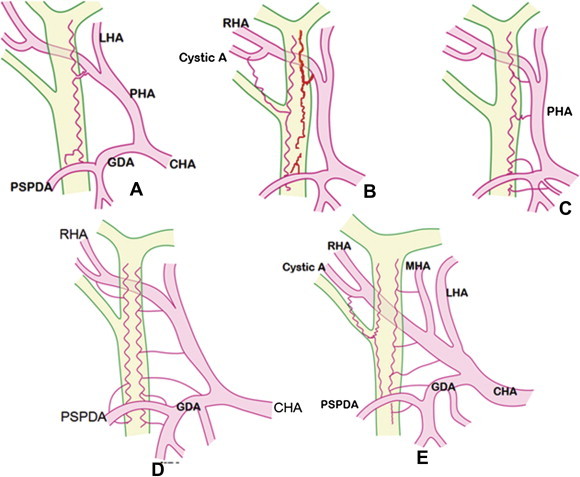
Different types of vascularizaton of supraduodenal biliary tract. [A] Axial type with single arch along the left border of bile duct. [B] Axial type with two arches along the right and left borders of the bile duct The arches are formed by posterior superior pancreaticoduodenal artery (PSPDA) from below and by right hepatic artery (RHA) and cystic artery from above. [C] Single Ladder type along the left border. Small horizontally directed branches arising from RHA, proper hepatic artery (PHA), gastroduodenal artery (GDA), and supraduodenal artery supply the bile duct. [D] Ladder type, Two ladders along the right and left borders of the bile duct. [E] Mixed type, Ladder type along the left border and axial type with single arch along the right border. CHA = Common hepatic artery; LHA = Left hepatic artery; RHA = Right hepatic artery; MHA = Middle hepatic artery; GDA = Gastroduodenal artery; PSPDA = Posterior superior pancreaticoduodenal artery.
Figure 4.
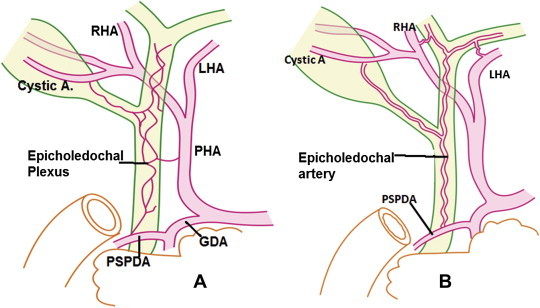
Variable pattern of arterial supply to bile duct: [A] Marginal arteries are not formed. Bile ducts are supplied only by the epicholedochal plexus of Saint. The plexus is formed by branches from PSPDA, PHA and Cystic arteries. [B] Marginal arteries are not formed. A single epicholedochal artery supplies extrahepatic bile ducts. The epicholedochal artery is formed by contributions from cystic A., PSPDA, RHA and LHA.
The retropancreatic segment of CBD is supplied by branches from PSPDA and retroportal arteries. The PSPDA is the dominant artery supplying this segment.14 The blood supply of the cystic and hilar hepatic ducts come from cystic artery and right and left hepatic arteries forming a hilar plexus which can provide collateral connections between the right and left livers.11,15 An extrahepatically located communicating arcade connecting left and right hepatic arteries not only plays an important role in the blood supply of caudate lobe and hilar bile ducts but also in the interlobar arterial collateral system16,17 [Figure 5]. The communicating arcade has been named as caudate arcade15 or transverse hilar marginal artery.18 On the left side the communicating arcade originates from segment IV artery (54%) or left hepatic artery (45%) and on the right side from right anterior sectoral artery (46%) or right hepatic artery (27%) or both (27%).16,17 Vellar11 has also reported that segment IV artery was the most important source of blood supply to left hepatic duct.
Figure 5.
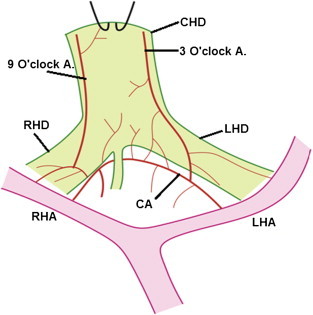
Communicating arcade: The communicating arcade (CA) in the hilar plate supplies the hilar ducts and connect the right hepatic artery (RHA) with left hepatic artery (LHA). The 3 o'clock and 9 o'clock marginal arteries join the communicating arcade. Common hepatic duct (CHD) is turned up to show the communicating arcade. RHD = Right hepatic duct; LHD = Left hepatic duct.
The intrahepatic biliary ducts are closely accompanied by intrahepatic arteries which form a rich microvascular network surrounding the biliary ducts named as the peribiliary plexus.19 This plexus drains into venules joining the intrahepatic portal system so as to reach the hepatic sinusoids [Figure 6]. Some experimental studies including animal studies have supported the presence of arterioportal communications in the peribiliary plexus.20 Injuries to the arteries supplying CBD might induce biliary ischemia. Ischemic changes are least common in intrahepatic biliary ducts because of existence of arterial and arterioportal collateral channels.5 The peribiliary plexus around intrahepatic bile ducts is continuous with the plexus around the extrahepatic bile ducts and acts as an important communication between hepatic and gastroduodenal arteries and represents a collateral source of arterial supply to the liver in the event of occlusion of the hepatic artery.15
Figure 6.
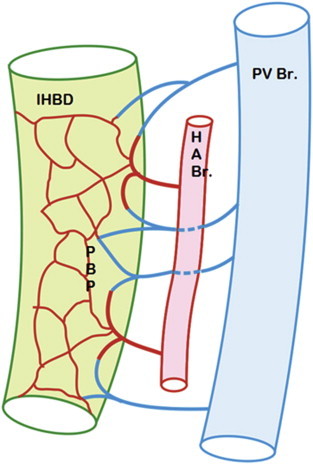
Blood supply of intrahepatic bile ducts: Intrahepatic bile ductules (IHBD) are surrounded by a delicate peribiliary plexus (PBP) formed by hepatic arteriolar branches (HABr). Hepatic arteriolar branches not only form the peribiliary capillary plexus but also form arterioportal communications being continuous with the venules draining into adjacent portal vein branches (PVBr).
The general arrangement of arterial and venous plexuses of CBD is similar.21 The paracholedochal vessels give branches to form the epicholedochal plexus on the surface of the CBD. Perforator vessels pierce the wall and connect the para- and epicholedochal plexuses with intramural and subepithelial plexuses in the wall of the CBD [Figure 7].
Figure 7.
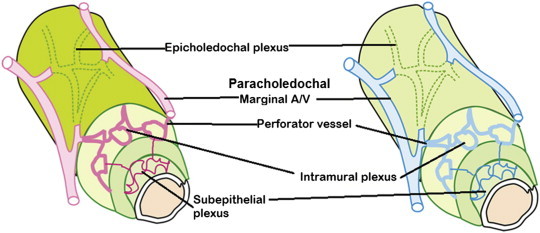
Similarity in the vascular pattern of bile duct: In the wall of the bile duct, the arrangement of arterial and venous plexuses are similar. Extrinsic plexus represented by the epicholedochal plexus on the surface of the CBD is formed by branches from the paracholedochal marginal arteries or veins. Intrinsic plexuses are intramural and subepithelial plexuses connected by perforator vessels to the epicholedochal plexus.
Venous Drainage of Biliary Tract
Relatively few reports are available in the literature pertaining to the normal venous drainage of biliary tract. Brief historical review of the earlier reports on the venous drainage of the bile duct was given by Couinaud22 and Vellar.23 The veins draining the CBD are arranged in the form of two plexuses. The epicholedochal venous plexus, described by Saint,24 is a fine reticular plexus on the surface of the bile ducts where as the paracholedochal venous plexus of Petren25 lies outside the bile ducts and courses parallel to the ducts. The veins of the epicholedochal plexus are not larger than 1 mm.26 The paracholedochal venous plexus generally forms two distinct marginal veins known as 3 o'clock and 9 o'clock marginal veins [Figure 8].23 In few cases an additional 6 o'clock marginal vein was also observed on the posterior surface of CBD [Figure 9]. Inferiorly the marginal veins and the venous plexus communicate with gastric veins, posterior superior pancreaticoduodenal vein (PSPDV), and gastrocolic trunk (GCT). Superiorly the marginal veins enter into the hepatic substance or join the hilar venous plexus which eventually drain into adjacent branches of portal vein. Hilar venous plexus communicates with the veins of caudate lobe or segment IV and cystic veins. An important observation was that the cystic vein always joined the 9 o'clock marginal vein and never joined the right branch of portal vein and that the 3 o'clock marginal vein always connected with the right gastric vein.23 In postmortem specimens Vellar23 has demonstrated retrograde filling of the marginal veins and the venous plexus on the CBD after transecting the CBD and all communicating veins at the upper border of duodenum and suggested that this bidirectional pathway ensures good results seen in end-to-end anastomosis in liver transplantation.
Figure 8.
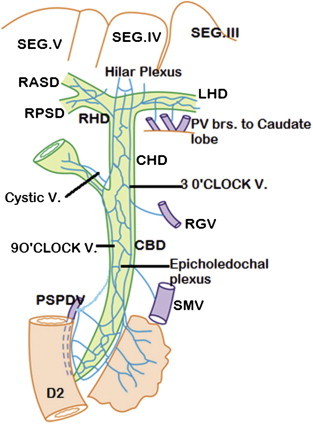
Venous drainage of extrahepatic bile ducts (Anterior view): The epicholedochal venous plexus (of Saint) drain into two marginal veins – 3 o'clock and 9 o'clock marginal veins (paracholedochal venous plexus of Petren) which drain into right gastric vein (RGV), posterior superior pancreaticoduodenal vein (PSPDV) and superior mesenteric vein (SMV) and connect to hilar plexus. The cystic vein always drains into 9 o'clock marginal vein. The hilar plexus and the venous plexus on the right and left hepatic ducts drain into portal venous branches to caudate lobe, quadrate lobe and segments adjacent to porta hepatis (segments III, IV, V).
Figure 9.
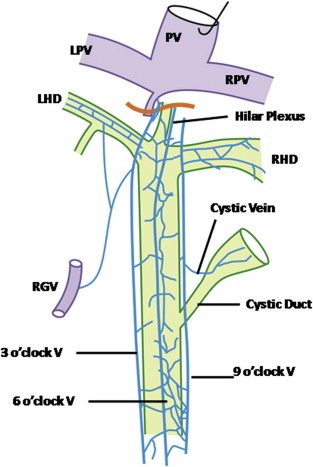
Venous drainage of extrahepatic bile ducts (Posterior view). The 6 o'clock marginal vein on the posterior surface of the bile duct is present only in few cases. Cystic vein joins 9 o'clock marginal vein. PV = Portal vein; RPV = Right portal vein; LPV = Left portal vein; RHD = Right hepatic duct; LHD = Left hepatic duct; RGV = Right gastric vein.
A parabiliary venous system, apparently having an embryological origin independent of the portal vein was described as an accessory portal venous system.22 This system runs along the CBD and HA in the arteriobiliary portion of the portal pedicle anterior to the portal vein and is composed of at least one big vessel along the CBD or hepatic artery or both. This system is comparable to the marginal veins described by Vellar23 and begins from PSPDV and pyloric veins and divides to form a venous network in the hilar plate. This hilar venous network sends branches to the veins of segments adjacent to the hilum like caudate and quadrate lobes. This system is a collateral channel for the portal vein extending from stomach, duodenum and pancreas to the liver and is a potential anastomosis between right and left livers. Couinaud22 observed that the cystic vein joined this parabiliary venous system only in 41% cases. It is suggested that the “cavernoma” is nothing more than a massive enlargement of this plexiform parabiliary venous system resulting from thrombosis of the portal vein [Figure 10].
Figure 10.
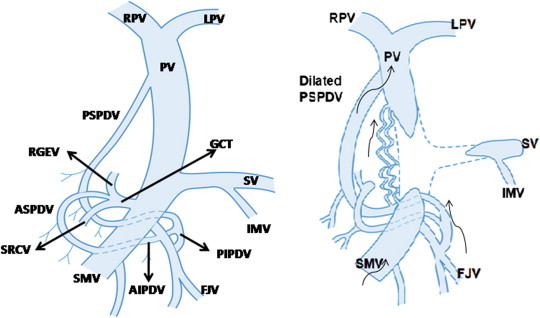
Formation of portal cavernoma: Normally the posterior superior pancreaticoduodenal vein (PSPDV) drains into portal vein (PV) close to porta hepatis and connects with posterior inferior pancreaticoduodenal vein (PIPDV) which drains into first jejunal vein (FJV), a tributary of superior mesenteric vein (SMV). The gastrocolic trunk (GCT), another tributary of SMV, is formed by union of right gastroepiploic vein (RGEV), anterior superior pancreaticoduodenal vein (ASPDV) and superior right colic vein (SRCV). In portal vein thrombosis involving the splenomesenteric confluence, the PSPDV and the pericholedochal venous plexus dilate and acts as a porto-portal collateral channels and develop into a cavernoma. Arrows indicate the direction of venous blood flow.
Discussion
Clinical studies on portal cavernoma cholangiopathy have given additional information about the venous drainage of the biliary tract.27,28 It has been well established that multiple hepatopetal collateral veins called portal cavernoma form in response to extrahepatic portal vein obstruction to provide alternate route around the obstructed segment of the main portal vein and involve the paracholedochal and epicholedochal venous plexuses and cholecystic veins.29 Mori et al30 suggested that the PSPDV is an important hepatopetal collateral. The paracholedochal veins will dilate first in portal hypertension causing external compression of ductal wall and protrusion of varicose paracholedochal veins into the thin and pliable wall of the CBD. The dilatation of epicholedochal venous collaterals may make the normally smooth intraluminal surface of CBD irregular. Perforator veins piercing through the muscular wall connect the paracholedochal and epicholedochal veins outside the CBD wall with those inside the wall present in a subepithelial position. Enlargement of the subepithelial plexus results in the formation of subepithelial varices in the CBD wall which is a source of troublesome bleeding.31 Denys et al32 in their color Doppler study showed an unusual cavernoma composed of a network of tiny vessels in the wall of CBD and suggested that veins that accompany the biliary tree (choledochal and cystic veins) serve as the most frequent porto-portal bypass routes in the situation of portal vein thrombosis. De Gaetano et al33 evaluated patients with cavernous transformation of portal vein by color Doppler sonography and observed formation of the cavernoma with in 6–20 days of acute thrombosis. Intrahepatic extension of the cavernoma was seen in 76% patients. Both portosystemic (mainly through left gastric vein) and porto-portal (periportal and pericholecystic venous channels) collaterals were observed. Flow in the venous channels of the portal cavernoma at the porta hepatis was hepatopetal in all patients.33
Some important morphological features observed in extrahepatic portal biliopathy (PB) include the presence of splenomesenteric and portal venous thrombosis (18 out of 19 cases with PB versus 1 out of 41 cases with no-PB), more acute angulation of CBD at the top of pancreas (110° in PB versus 128° in no-PB) and gastrocolic trunk as the large collateral vein (18 out of 19 cases with PB).34 It is suggested that a different collateral pathway is preferentially employed in the setting of portal biliopathy. When the portal vein thrombosis extend into superior mesenteric and splenic veins, the hepatopetal collateral venous flow is preferentially routed around pancreatic head involving the PSPDV, GCT and venous plexuses of bile duct rather than via the gastric veins and gastroesophageal variceal circuit.34 Two venous arcades, an anterior and a posterior, drain the head of pancreas and duodenum. The posterior arcade consists of posterior superior and posterior inferior pancreaticoduodenal veins (PSPDV and PIPDV) and the anterior superior and anterior inferior pancreaticoduodenal veins (ASPDV and AIPDV) constitute the anterior arcade. The AIPDV and PIPDV generally join the first jejunal vein (FJV) either independently or forming a common trunk. FJV joins the SMV at the level of the uncinnate process of pancreas. The ASPDV along with right gastroepiploic and right colic veins form the gastrocolic trunk which is a major tributary of the SMV. The PSPDV receives the paracholedochal marginal veins and accompanies its artery behind the head of pancreas up to its upper border. Then it passes vertically upwards with the CBD in the hepato-duodenal ligament and drains into posterolateral aspect of main portal vein close to porta hepatis [Figure 10]. Small communicating veins passing around the CBD at the upper border of pancreas connect the PSPDV with ASPDV. Dilatations of these veins in portomesenteric venous thrombosis compress and displace the CBD resulting in more acute angulation of the CBD at the upper border of pancreas.
It is also noted that dilatation of large paracholedochal veins result in compression and distortion of the extrahepatic bile duct producing a varicoid appearance while the fibrotic appearance results from enlargement of smaller intramural epicholedochal veins. The importance of this distinction is that varicoid portal biliopathy, caused by mechanical compression is reversible with decompression of the collateral veins, while the fibrotic type, caused by ischemic injury, is not. One possible explanation is that the variceal enlargement of epicholedochal plexus compromises the arterial supply of the ductal wall producing ischemic changes and fibrosis, though clear evidence for such a mechanism is wanting.
Conclusions
Available evidence suggests that the epicholedochal and paracholedochal venous plexuses draining the biliary tract act as important porto-portal collateral channels. In conditions of portal vein obstruction these collaterals enlarge to form portal cavernoma surrounding the bile duct and bring about morphological changes observed in portal cavernoma cholangiopathy. Most of the studies, employing different radiological procedures, have attempted to focus on the biliary changes induced by portal cavernoma. It would be relevant to study the development of different types of collateral channels in different scenarios of thrombosis of portal vein alone and thrombus extending into superior mesenteric and splenic veins and reasons for formation of portosystemic collaterals in some and porto-portal collaterals in others.
Conflicts of interest
All authors have none to declare.
Acknowledgments
The authors acknowledge the help rendered by Mr. Pran Prakash in preparing the figures of the article and Dr.R.K. Dhiman and Dr.Sandeep Goyal in the collection of some references.
References
- 1.Vakili K., Pomfret E.A. Biliary anatomy and embryology. Surg Clin N Am. 2008;88:1159–1174. doi: 10.1016/j.suc.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Skandalakis J.E. vol. II. PMP; Athens: 2004. pp. 1095–1150. (Surgical Anatomy – The Embryologic and Anatomic Basis of Modern Surgery). [Google Scholar]
- 3.Jablonska B. The arterial blood supply of the extrahepatic biliary tract – surgical aspects. Polish J Surg. 2007;80(6):336–342. [Google Scholar]
- 4.Slieker J.C., Farid W.R.R., van Eijck C.H.J. Significant contribution of the portal vein to blood flow through the common bile duct. Ann Surg. 2012;255(3):523–527. doi: 10.1097/SLA.0b013e31824714d0. [DOI] [PubMed] [Google Scholar]
- 5.Deltenre P., Valla D.C. Ischemic cholangiopathy. Semin Liv Dis. 2008;28(3):235–246. doi: 10.1055/s-0028-1085092. [DOI] [PubMed] [Google Scholar]
- 6.Northover J.M., Terblanche J. Bile duct blood supply. Its importance in human liver transplantation. Transplantation. 1978;26:67–69. [PubMed] [Google Scholar]
- 7.Northover J.M., Terblanche J. A new look at the arterial supply of the bile duct in man and its surgical implications. Br J Surg. 1979;66:379–384. doi: 10.1002/bjs.1800660603. [DOI] [PubMed] [Google Scholar]
- 8.Chen W.J., Ying D.J., Liu Z.J., He Z.P. Analysis of the arterial supply of the extrahepatic bile ducts and its clinical significance. Clin Anat. 1999;12(4):245–249. doi: 10.1002/(SICI)1098-2353(1999)12:4<245::AID-CA2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro A.L., Robillard G.L. The arterial blood supply of the common and hepatic bile ducts with reference to the problem of common duct injury and repair based on a series of twenty-three dissections. Surgery. 1948;23:1. [PubMed] [Google Scholar]
- 10.Furukawa H., Iwata R., Moriyama N., Kosuge T. Blood supply to the pancreatic head, bile duct and duodenum: evaluation by computed tomography during arteriography. Arch Surg. 1999;134(10):1086–1090. doi: 10.1001/archsurg.134.10.1086. [DOI] [PubMed] [Google Scholar]
- 11.Vellar I.D. The blood supply of the biliary ductal system and its relevance to vasculobiliary injuries following cholecystectomy. Aust NZ J Surg. 1999;69(11):816–820. doi: 10.1046/j.1440-1622.1999.01702.x. [DOI] [PubMed] [Google Scholar]
- 12.Rath A.M., Zhang J., Bourdelat D., Chevrel J.P. Arterial vascularization of the extrahepatic biliary tract. Surg Radiol Anat. 1993;15:105–111. doi: 10.1007/BF01628308. [DOI] [PubMed] [Google Scholar]
- 13.Parke W.W., Mitchels N.A., Ghosh G.M. Blood supply of the common bile duct. Surg Obstet Gynecol. 1963;117:47. [PubMed] [Google Scholar]
- 14.Wang X.L., Fang C.H., Quan X.Y. Sub millimeter CT observations of the blood supplying arterioles of the extrahepatic bile duct. Zhongguo Zuzhi Gongcheng Yanjiu. 2012;16(18):3305–3309. [Google Scholar]
- 15.Stapleton G.N., Hickman R., Terblanche J. Blood supply of the right and left hepatic ducts. Br J Surg. 1998;85(2):202–207. doi: 10.1046/j.1365-2168.1998.00511.x. [DOI] [PubMed] [Google Scholar]
- 16.Tohma T., Cho A., Okazumi S. Communicating arcade between the right and left hepatic arteries: evaluation with CT and angiography during temporary balloon occlusion of the right or left hepatic artery. Radiology. 2005;237:361–365. doi: 10.1148/radiol.2371040919. [DOI] [PubMed] [Google Scholar]
- 17.Gunji H., Cho A., Tohma T. The blood supply of the hilar bile duct and its relationship to the communicating arcade located between the right and left hepatic arteries. Am J Surg. 2006;192(3):276–280. doi: 10.1016/j.amjsurg.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 18.Strasberg S.M., Helton W.S. An analytical review of vasculobiliary injury in laparoscopic and open cholecystectomy. HPB. 2011;13:1–14. doi: 10.1111/j.1477-2574.2010.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terada T., Ishida F., Nakanuma Y. Vascular plexus around intrahepatic bile ducts in normal livers and portal hypertension. J Hepatol. 1989;8(2):139–149. doi: 10.1016/0168-8278(89)90001-9. [DOI] [PubMed] [Google Scholar]
- 20.Cho K.J., Lunderquist A. The peribiliary vascular plexus: the microvascular architecture of the bile duct in the rabbit and in clinical cases. Radiology. 1983;147(2):357–364. doi: 10.1148/radiology.147.2.6836115. [DOI] [PubMed] [Google Scholar]
- 21.Northover J.M., Terblanche J. Applied surgical anatomy of the biliary tree. In: Blumgart L.H., editor. vol. 5. Churchill Livingstone; Edinburgh: 1982. (The Biliary Tract). Ch.1. [Google Scholar]
- 22.Couinaud C. The parabiliary venous system. Surg Radiol Anat. 1988;10(4):311–316. doi: 10.1007/BF02107904. [DOI] [PubMed] [Google Scholar]
- 23.Vellar I.D. Preliminary study of the anatomy of venous drainage of the intrahepatic and extrahepatic bile ducts and its relevance to the practice of hepatobiliary surgery. Aust NZ J Surg. 2001;71:418–422. doi: 10.1046/j.1440-1622.2001.02150.x. [DOI] [PubMed] [Google Scholar]
- 24.Saint J.H. The epicholedochal venous plexus and its importance as a means of identifying the common duct during operation on the extrahepatic biliary tract. Br J Surg. 1961;48:489–498. doi: 10.1002/bjs.18004821104. [DOI] [PubMed] [Google Scholar]
- 25.Petren T. The veins of the extrahepatic biliary system and their pathologic-anatomic significance. Verh Anat Ges. 1932;41:139–143. [Google Scholar]
- 26.Kim S., Chew F.S. Choledochal varices. Case report. AJR. 1988;150:578–580. doi: 10.2214/ajr.150.3.578. [DOI] [PubMed] [Google Scholar]
- 27.Sharma M., Pathak A. Intracholedochal varices in portal hypertensive biliopathy. Eur J Radiol Extra. 2009;72:e119–e123. [Google Scholar]
- 28.Dhiman R.K., Puri P., Chawla Y. Biliary changes in extrahepatic portal venous obstruction: compression by collaterals or ischemic. Gastrointest Endosc. 1999;50:646–652. doi: 10.1016/s0016-5107(99)80013-3. [DOI] [PubMed] [Google Scholar]
- 29.Shin S.M., Kim S., Lee J.W. Biliary abnormalities associated with portal biliopathy: evaluation on MR cholangiography. AJR. 2007;188:W341–W347. doi: 10.2214/AJR.05.1649. [DOI] [PubMed] [Google Scholar]
- 30.Mori H., Miyake H., Aikawa H. Dilated posterior superior pancreaticoduodenal vein: recognition with CT and clinical significance in patients with pancreaticobiliary carcinomas. Radiology. 1991;181:793–800. doi: 10.1148/radiology.181.3.1947099. [DOI] [PubMed] [Google Scholar]
- 31.Sharma M., Pathak A. Perforators of common bile duct wall with portal hypertensive biliopathy (with videos) Gastrointest Endosc. 2009;70(5):1041–1043. doi: 10.1016/j.gie.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 32.Denys A., Helenon O., Lafortune M. Thickening of the wall of the bile duct due to intramural collaterals in three patients with portal vein thrombosis. AJR. 1998;171:455–456. doi: 10.2214/ajr.171.2.9694474. [DOI] [PubMed] [Google Scholar]
- 33.De Gaetano A.M., Lafortune M., Patriquin H., De Franco A., Aubin B., Paradis K. Cavernous transformation of the portal vein. Patterns of intrahepatic and splanchnic collateral circulation detected with Doppler sonography. AJR. 1995;165:1151–1155. doi: 10.2214/ajr.165.5.7572494. [DOI] [PubMed] [Google Scholar]
- 34.Walser E.M., Runyan B.R., Heckman M.G. Extrahepatic portal biliopathy: proposed etiology on the basis of anatomic and clinical features. Radiology. 2011;258:146–153. doi: 10.1148/radiol.10090923. [DOI] [PubMed] [Google Scholar]


