Abstract
Portal cavernoma cholangiopathy (PCC) refers to a constellation of secondary changes in the biliary tree in patients with chronic portal vein (PV) thrombosis and portal cavernoma formation. These findings of PCC are seen in the extra-hepatic bile duct(s), with or without involvement of the 1st or 2nd degree intra-hepatic bile ducts.
Of all patients with chronic PV thrombosis, cholangiographic features of PCC are found in 80%–100%. The biliary changes are symptomatic in a smaller proportion of 5%–38% patients. Choledocholithiasis and hepatolithiasis occur in 5%–20%, independent of the occurrence of cholelithiasis. We review the published literature on cholangiographic description of PCC. We also propose standardized nomenclature for the cholangiographic findings, namely: extrinsic impressions/indentations, shallow impressions, irregular ductal contour, stricture (s), upstream dilatation, filling defects, bile duct angulation, and ectasia.
Keywords: portal cavernoma cholangiopathy, portal vein, cholelithiasis, extra-hepatic portal venous obstruction
Abbreviations: PCC, portal cavernoma cholangiopathy; PV, portal vein; MRC, magnetic resonance cholangiography; EHPVO, extra-hepatic portal venous obstruction; MRA, magnetic resonance angiography
Cholangiographic changes in the extra- and intra-hepatic biliary ducts are seen in a large proportion of patients with chronic portal vein obstruction and exuberant collateral channels in the hepatic-duodenal ligament - the so-called ‘portal cavernoma.’ Previously similar biliary changes were attributed to cirrhosis and non-cirrhotic portal fibrosis with collateral circulation due to portal hypertension. However, most authorities now agree that the term ‘portal cavernoma cholangiopathy (PCC; previously called ‘portal biliopathy’), should be restricted to patients with portal cavernoma and secondary changes in the biliary tree. This review describes the cholangiographic findings in PCC, and proposes uniform descriptive terminology.
Pathogenesis of Portal Cavernoma Cholangiopathy– Anatomical Factors
The cholangiographic changes seen in PCC can be due to:
-
1.
Compression of the bile duct by dilated collateral venous channels, in and around the wall of the duct.
-
2.
Neo-vascularization, with compression of the bile duct by newly formed vessels.
-
3.
Bile duct ischemia with resultant mural fibrosis, due to extension of thrombosis to the smaller draining veins, is suspected but unproven.
-
4.
Increased connective tissue deposition, forming a solid tumor-like meshwork of fine vessels embedded in a large amount of fibrous tissue.
Compression of the bile duct in PCC is due to bridging porto-portal collaterals running in the hepato-duodenal ligament.1 Mechanical obstruction of the pliable bile duct by impinging extrinsic venous collateral channels is possible, as the portal pressure is greater than the pressure inside the common bile duct.2 Biliary obstruction caused by dilated intramural veins, and protruding into the bile duct is also possible. Enhancement of the bile duct wall after contrast injection supports the presence of intramural vascular channels. These collateral channels bypass venous blood across the obstructed segment of the portal vein and/or superior mesenteric vein. The para-biliary venous plexus of Petren runs parallel to the bile duct in the hepato-duodenal ligament, and is supplied by the gastric and pancreaticoduodenal veins, which connect to the portal vein branches around the hepatic hilum. Dilatation of the para-biliary venous plexus gives rise to extrinsic compression and indentations on the bile duct wall. Smaller veins measuring ≤1 mm normally form a mesh on the surface of the bile duct, and constitute the peri-biliary venous plexus of Saint.3,4 When dilated, these small veins lead to fine irregularity of the bile duct wall on cholangiography.5,6 Perforator channels connect the extra-mural vascular channels to intramural bile duct varices.7
Need for Structured Definition of Cholangiographic Findings
Different operators have used different descriptive terms to describe the cholangiographic findings of PCC. There is a need for uniform terminology of cholangiographic findings to compare results between centers, and also compare interval findings in the same patient. We therefore propose structured reporting of cholangiographic findings in PCC, using identical Magnetic resonance cholangiography (MRC) and ERC terminology.
Proposed Cholangiographic Definition of Portal Cavernoma Cholangiopathy
PCC can be defined as a spectrum of changes in the extra-hepatic bile duct(s), with or without involvement of 1st or 2nd degree intra-hepatic bile ducts, with resulting symptomatic or asymptomatic bile duct morphological changes, with or without stasis, and sequel of stasis including stones and cholangitis, in patients with chronic portal vein obstruction. This definition excludes cirrhosis with portal vein thrombosis as an etiology of PCC. The pattern of biliary changes should be consistent with known pattern of morphologic changes in PCC. These cholangiographic changes of PCC are described later.
Frequency of Cholangiographic Findings
Of all patients with extra-hepatic portal venous obstruction (EHPVO), changes suggestive of PCC are found in 80%–100% on endoscopic retrograde cholangiography (ERC). The biliary changes are symptomatic in a smaller proportion of 5%–38% patients. Choledocholithiasis and hepatolithiasis occur in 5%–20% of all patients with PCC, independent of the occurrence of cholelithiasis.8 The frequency of choledocholithiasis is significantly more in symptomatic patients and patients with deranged liver tests, as compared with asymptomatic patients. Different series that have included symptomatic patients with PCC have reported that 22%–77% patients have stones or debris in the common bile duct.9–11
Clinical studies of Cholangiographic Changes in Portal Cavernoma Cholangiopathy
There are 9 published papers and several case-reports and abstracts, describing the cholangiographic changes in PCC (Table 1). These studies are summarized below in chronological order.
Table 1.
Studies Describing Cholangiographic Changes in Portal Cavernoma Cholangiopathy.
| Study | n | Overall cholangiographic abnormalities | Extra-hepatic duct changes | Intra-hepatic duct changes | Left intra-hepatic ducts | Right intra-hepatic ducts |
|---|---|---|---|---|---|---|
| Dilawari J. Gut 1992 | 20 | 100% | 90% | 100% | 100% | 56% |
| Bayraktar Y. Am J Gastroenterol 1992 | 16 | 100% | 100% | NA | – | – |
| Khuroo MS. Hepatology 1993 | 21 | 80.9% | 66.6% | 38.1% | – | – |
| Bayraktar Y. Am J Gastroenterol 1995 | 35 | 94.3% | 94.3% | NA | – | – |
| Perlemuter G. J Hepatol 1996 | 7 | 100% | 100% | 100% | – | – |
| Dhiman RK. GIE 1999 | 5 | 100% | 100% | 100% | – | – |
| Malkan GH. GIE 1999 | 20 | 85% | 60% | NA | 55% | 40% |
| Nagi B. Acta Radiologica 2000 | 43 | 93% | 100% | 57% | – | – |
| Sezgin O. GIE 2003 | 10 | 100% | 100% | 100% | – | – |
Dilawari et al12 described the cholangiographic findings in 20 patients with EHPVO diagnosed by spleno-portovenography and splenic pulp pressure measurements. Fifteen patients with abdominal pain but no biliary disease served as the control group. Only one of 20 patients had a mildly deranged liver tests, but on ERC all had biliary changes suggestive of sclerosing cholangitis. Abnormalities in the common bile duct, left intra-hepatic ducts, and right intra-hepatic ducts were seen in 90%, 100%, and 56% patients respectively. The changes in the right-sided ducts were less severe. It is possible that the right-sided ducts, which are more difficult to opacify in the supine position, were defined in less detail. It is also possible that the left sided ducts are more severely affected due to more extensive collateral circulation along the left duct, where the umbilical vein joins the left branch of the portal vein. The changes in the intra-hepatic ducts included focal narrowing, dilatations, irregular walls, and clustering of the intra-hepatic branches. No patients had associated gallstones in this study. All patients had esophageal varices.
Bayraktar et al13 prospectively studied 17 patients with cavernous transformation of portal vein, diagnosed by portography. All patients had mildly increased alkaline phosphatase and serum bilirubin levels. There were narrowing, irregularity, undulation, and nodular extrinsic defects along the extra-hepatic biliary tract in all 16 patients who underwent ERC. Similar ERC findings were not found in six patients with portal hypertension due to liver cirrhosis.
Khuroo et al14 prospectively studied 21 consecutive patients with EHPVO for evidence of biliary tract disease. Three of the patients were symptomatic with extra-hepatic cholestasis (n = 2) and recurrent cholangitis (n = 1). A significant number of patients in this series had biochemical evidence of biliary abnormalities. Elevated bilirubin levels were found in 14 (66.6%), elevated alkaline phosphatase levels in 17 (80.9%) and elevated serum ALT levels in 8 (38.0%) patients. ERC revealed abnormal findings in 17 (80.9%) patients. The changes involved the common bile duct (66.6%) more often than they did the intra-hepatic bile ducts (38.1%). Cholangiographic abnormalities included strictures (52.4%), caliber irregularity (23.8%), segmental upstream dilatation (42.8%), ectasias (9.5%), collateral veins causing extra-luminal bile duct impressions (14.3%), displacement of ducts (9.5%), angulation of ducts (4.7%), and pruning of intra-hepatic ducts (9.5%).
Bayraktar et al15 described 44 patients with cavernous transformation of portal vein, diagnosed by either splenoportography, or arterial portography with digital subtraction angiography. To evaluate the cause of increased bilirubin and alkaline phosphatase levels, 35 of the 44 cases were evaluated by ERC (n = 34) or percutaneous trans-hepatic cholangiography (n = 1). Irregular, undulating narrowing and nodular extrinsic defects along the length of the common bile duct were present in 33 (94.3%) patients. The authors termed these findings as “pseudo-cholangiocarcinoma sign” The changes in the intra-hepatic bile ducts were not described in this study. No such findings were observed in 10 cirrhotic patients with portal hypertension but without portal cavernoma, who also underwent ERC. Esophageal varices were present in all.
Perlemuter et al16 studied 8 patients with portal cavernoma, and biochemical abnormalities and/or symptoms of biliary obstruction. ERC was done in 7 patients, and all had cholangiographic changes. The common bile duct was twisted and dilated upstream from an extra-luminal stricture. These strictures were described as having an ‘hourglass appearance’ in this study. Multiple strictures and short dilated segments produced a characteristic beaded appearance. Extra-luminal ‘thumb-like smooth indentations’ were observed. The intra-hepatic bile ducts were also dilated in all the cases.
Dhiman et al17 described 5 patients with EHPVO who underwent porto-systemic shunt surgery. All patients had biliary abnormalities on pre-shunt ERC. Repeat ERC was performed 4–8 weeks after the surgery. The post-shunt ERC showed partial reversal of biliary abnormalities in 3 patients, complete reversal in 1 patient, and no reversal in 1 patient. Smooth strictures opened after shunt surgery, and proximal dilatation disappeared in most patients. The indentations and caliber irregularities disappeared after shunt surgery, whereas angulations and ectasias of biliary ducts persisted. The authors postulate that these persistent changes could have been due to ischemic damage or fibrous scarring within the porta-hepatis.
Malkan et al18 found abnormalities in the extra-hepatic or intra-hepatic biliary tree by cholangiography in 17 of 20 (85%) patients with EHPVO. Eight patients with portal venous obstruction had elevated alkaline phosphatase levels; two had elevated bilirubin levels. Abnormalities (mainly strictures, caliber irregularity, and dilatation) were seen in the common bile duct (n = 5), common hepatic duct (n = 7), and in the right (n = 8) and left (n = 11) hepatic ducts. The changes noted in the extra-hepatic bile ducts were predominantly strictures and caliber irregularity, whereas the intra-hepatic ducts showed dilatation in the majority, suggesting that the intra-hepatic changes are secondary to the extra-hepatic obstruction.
Nagi et al19 studied 43 consecutive patients with EHPVO, and found cholangiographic abnormalities in 40 (93%) patients on ERC. Eight patients had obstructive jaundice with abnormal biochemical parameters. Extra-hepatic bile ducts were involved in all 40 (100%), whereas intra-hepatic bile ducts showed changes in 23 (57%). The commonest cholangiographic abnormality noted in the extra-hepatic ductal system was contour irregularity with indentations, observed in 24 patients. Other abnormalities noted were displacement and angulation of the common bile duct, strictures of the common bile duct and multiple filling defects. Intra-hepatic bile ducts showed dilatation with areas of narrowing and filling defects. Unlike the other studies, there was no predilection for left hepatic duct involvement.
Sezgin et al20 reported 10 patients with portal cavernoma with biliary symptoms. Three patients had jaundice, 5 had cholangitis, and one each had pruritus and abdominal pain. On ERC, all the patients had biliary involvement, in the form of strictures. The strictures involved the common bile duct in 9 (90%), common hepatic duct in two (20%), and junction of the common hepatic duct and left intra-hepatic duct in one (10%) patient. The strictures were relatively long with a smooth border in 6 patients, and short localized segments in 4 patients. The intra-hepatic bile ducts were dilated in all patients. Ductal irregularity, stenosis, and ectasia were present in 3 patients.
Proposed Nomenclature for Cholangiographic Findings
With the present state of knowledge, any nomenclature for the cholangiographic changes would be arbitrary. The proposed nomenclature given below is not based on rigorous data, but will help in the standardized reporting of cholangiographic findings. These recommendations would need to be modified and refined based on data accrued from future ERC and MRC studies of patients with PCC. The proposed cholangiographic definitions, with their descriptions are given below:
-
1.
Extrinsic impressions/indentations: Smooth thumb-like impressions on the bile duct, with a nodular contour. The indentation is more than one-quarter of the width of the opacified duct (Figure 1). Impressions may be multiple.
-
2.
Shallow impressions/indentation(s): Smooth non-contiguous impressions on the bile duct, <one-quarter of ductal diameter (Figure 2).
-
3.
Irregular ductal contour: Fine-wavy, irregular contour of the bile duct walls due to contiguous shallow indentations <one-quarter of the ductal diameter.
-
4.
Stricture: Variable length narrowing of the ductal lumen, in reference to well opacified downstream duct segment (Figure 3). Strictures may be associated with upstream dilatation. Strictured bile duct segments should offer some resistance to passage of an adequately inflated extraction balloon across it. Strictures may be divided into ‘mild to moderate’ or ‘severe’ depending on whether the narrowed segment is > or <2/3rd of the diameter of adjacent normal segment.14
-
5.
Upstream dilatation: Proximal dilatation can be similarly classified as ‘mild to moderate’ or ‘severe’, depending on whether the dilated segment is between 1.5–2× or > 2× diameter of the adjacent normal duct, respectively.14
-
6.
Filling defects: Round, oval, or elongated defects in the cholangiographic image, with contrast on three or all sides. Filling defects can represent stones, prolapsing intra-luminal varices, or clots (Figure 4).
-
7.
Bile duct angulation: Bile duct angulation is usually due to ductal kinking by collateral circulation draining between anterior-superior pancreaticoduodenal and posterior-superior pancreaticoduodenal veins, along the superior aspect of pancreatic head.
Figure 1.
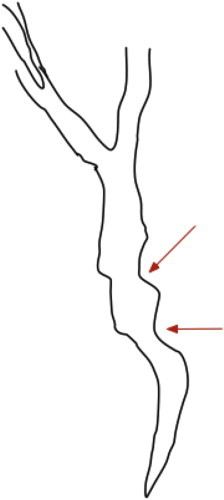
Extrinsic impression on the bile duct (arrows).
Figure 2.
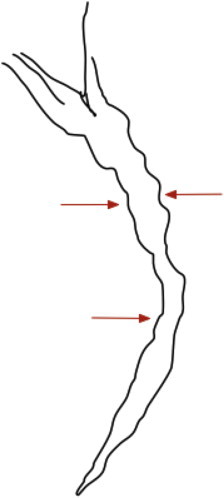
Shallow indentations or impressions (arrows).
Figure 3.
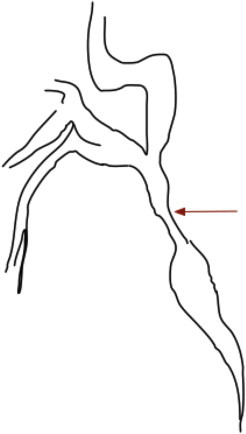
Stricture and upstream dilatation.
Figure 4.
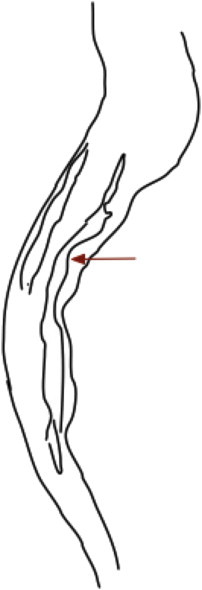
Filling defects in the bile duct (arrows).
On intra-operative cholangiogram bile duct angulation has been considered a risk factor for recurrence of choledocholithiasis after surgery, without any intrinsic bile duct disease. Keizman et al described that more acute angulation of the bile duct (≤145°) was an independent risk factor for symptomatic bile duct stone recurrence in a cohort of 232 patients.21 Similarly, Warren et al reported from a study of 126 operative cholangiograms, that the mean angulation of duct was 135.7° in patients with only cholecystolithiasis, and 103.4° in patients with concurrent choledocholithiasis.22 The cholangiographic method of measuring bile duct angulation is shown in Figure 5. It is proposed that an angle of ≤145° be considered as significant.
-
8.
Ectasia: Merriam-Webster dictionary (http://www.merriam-webster.com/medical/) defines ectasia as ‘the expansion of a hollow or tubular organ.’ In the context of portal biliopathy, ectasia may be defined as dilated segment of biliary tree without any evident downstream obstruction.
Figure 5.
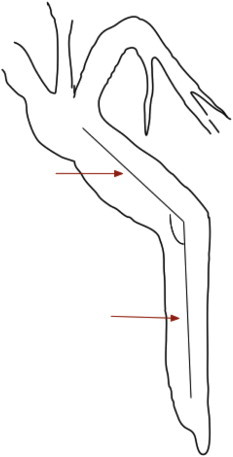
Bile duct angulation. Lines are drawn along the center of the bile duct. Bile duct angulation is measured at the intersection of these imaginary lines along the long axis of the bile duct. Lesser values indicate greater angulation.
Classification of Cholangiographic Abnormalities
Chandra et al23 proposed a classification system for PCC based on the location of cholangiographic abnormalities on direct cholangiography:
Type I: Involvement of extra-hepatic ducts alone.
Type II: Involvement of intra-hepatic bile ducts only.
Type III a: Extra-hepatic bile duct and unilateral intra-hepatic bile duct involvement.
Type III b: Extra-hepatic bile duct, and bilateral intra-hepatic bile duct involvement.
Type I or type III biliary changes are most frequent.24 This classification has subsequently also been adopted by some MRC studies.25 Changes in the intra-hepatic ducts alone may be seen in cirrhosis, but the common bile duct is usually normal. Conversely in chronic portal vein thrombosis, liver parenchymal changes of regenerating nodules or fibrosis do not occur and changes in the intra-hepatic ducts probably do not occur in isolation.
More recently, Llop et al proposed a cholangiographic classification based on MRC and magnetic resonance angiography (MRA), which may be more clinically relevant.26 They classified the changes in the biliary tree into different degrees of severity:
Grade 0: No abnormality.
Grade I: Irregularities or angulations of the biliary tree.
Grade II: indentations or strictures without dilation.
Grade III: Strictures with dilation. Dilatation was defined as extra-hepatic duct, and/or intra-hepatic ductal diameter of > 7 mm and 4 mm, respectively.
These authors described 67 patients with EHPVO (22 with acute episode). Overall, 52 patients had PCC (6 grade I, 12 grade II and 34 grade III). Symptoms developed in 14 (21%) patients, all of whom had grade III PCC. Presence of grade III changes had a 41% positive predictive value for symptom development, and a negative predictive value of 100%. Thus, the absence of grade III PCC at MRA/MRC obviates the possibility of developing symptomatic disease.
Need for Complete Ductal Opacification
A complete cholangiographic description needs complete ductal opacification. There is an understandable hesitation to completely opacify the biliary tree because of perceived risk of cholangitis. In the presence of cholangitis, complete ductal filling under pressure would entail a risk of cholangio-venous reflux and bacteremia. However inadequate ductal filling can lead to over-estimation of ductal strictures and changes in the intra-hepatic ducts. None of the published reports on ERC in PCC acknowledge incomplete ductal filling.
With the patient prone or in left lateral decubitus position, there is preferential opacification of the left intra-hepatic ducts due to gravity as they are dependent. Hence changes in the right-sided ducts may be under-appreciated, unless care is taken. Placing the patient in a right decubitus position or supine position helps to opacify the right-sided ducts. The posterior segmental branches may be best seen with the patient supine. In some patients, it is necessary to tilt the head of the table down to opacify the right intra-hepatic ducts. The best position to identify the confluence of right and left hepatic ducts is right anterior oblique.
Overfilling of the ducts with dense contrast should also be avoided. Overlying contrast can obscure stones. Varices are seen as faint linear filling defects, and can be easily obscured in a duct distended by contrast. Hence the ducts should be evaluated carefully both during the early filling phase of cholangiography, as well as after complete ductal distention.
Challenges for Disease Classification/Unanswered Questions
Any cholangiographic description should be as detailed as possible, without use of ambiguous terminology. This is important because many unanswered questions remain regarding PCC. For example, it is unclear how many changes on cholangiography are needed to diagnose PCC, and what should be the severity of these changes. Can minor changes be disregarded? In the absence of adequate understanding of the natural history of this condition, there can be no firm recommendations when to consider biliary involvement as ‘significant,’ and this interpretation has remained operator subjective.
Many patients with choledocholithiasis develop cholangitis. It then becomes difficult to decide whether the fine contour irregularities are secondary to the cholangitis, or are manifestations of the primary disease. Similarly strictures can develop due to ductal inflammation, secondary to the stones and cholangitis. It remains unsettled whether these superadded complications can alter the cholangiographic changes of PCC.
After porto-systemic shunts, some changes of PCC may resolve. However it remains uncertain what constitutes disease resolution, and what should be considered as therapeutic success.
Indications for ERC in Suspected Portal Cavernoma Cholangiopathy
In the present era, with the availability of MRC imaging, the indications for ERC with diagnostic intent are becoming less common in patients with suspected PCC. Although ERC still remains the gold standard to define the changes of PCC, diagnostic ERC now has a limited role with the improvement in MRC. MRC can give a non-invasive ‘snapshot’ image of the biliary tree in PCC. MRC paired with MRA can gives additional information about vascular collaterals and their relation with the bile ducts, which is not possible with ERC. MRA/MRC can also be used to follow up these patients. MRA/MRC should be performed 6–12 months after acute non-recanalised EHPVO, and at diagnosis of chronic EHPVO. Patients with grade III PCC, especially those with high levels of alkaline phosphatase and gamma glutamyl transpeptidase, may be at risk of developing symptomatic disease.26
However, second and third order intra-hepatic bile ducts can be demonstrated with greater detail with ERC, due to its greater spatial and contrast resolution.
The possible indications of ERC in patients with suspected portal biliopathy are:
-
1.
Cholangitis.
-
2.
Bile duct stones.
-
3.
Bile duct stricture (either symptomatic or persisting after portal decompression).
-
4.
With diagnostic intent, when there is diagnostic ambiguity.
Diagnostic ERC has a limited role in the present era. The operator must be prepared to undertake the necessary therapeutic maneuvers after the diagnostic confirmation, and drain the opacified ductal segments after confirming the diagnosis.
Differential Diagnosis of the Cholangiographic Findings in Portal Cavernoma Cholangiopathy
Multiple disease conditions can mimic the cholangiographic changes of PCC on cholangiography. MRC may be superior over ERC to clarify some of the differentials, because of its ability to demonstrate the relationship of the bile duct and the cavernoma in the same examination. The list of differential diagnosis should include:
-
1.
Primary sclerosing cholangitis
-
2.
Recurrent pyogenic cholangitis
-
3.
HIV-cholangiopathy
-
4.
Autoimmune/IgG4-related cholangiopathy
-
5.
Cholangiocarcinoma
-
6.
Extrinsic compression of bile duct by peri-choledochal lymphadenopathy
-
7.
Langerhan cell histiocytosis.
-
8.
Miscellaneous, rare conditions.
Conflicts of interest
The author has none to declare.
References
- 1.Shin S.M., Kim S., Lee J.W. Biliary abnormalities associated with portal biliopathy: evaluation on MR cholangiography. AJR Am J Roentgenol. 2007;188:W341–W347. doi: 10.2214/AJR.05.1649. [DOI] [PubMed] [Google Scholar]
- 2.Guelrud M., Mendoza S., Rossiter G., Villegas M.L. Sphincter of Oddi manometry in healthy volunteers. Dig Dis Sci. 1990;35:38–46. doi: 10.1007/BF01537220. [DOI] [PubMed] [Google Scholar]
- 3.Petren T. The veins of the extrahepatic biliary system and their pathologic-anatomic significance. Vert Anat Ges. 1932;41:139–143. [Google Scholar]
- 4.Saint J.H. The epicholedochal venous plexus and its importance as a means of identifying the common duct during operation on extra-hepatic biliary tract. Br J Surg. 1961;48:489–498. doi: 10.1002/bjs.18004821104. [DOI] [PubMed] [Google Scholar]
- 5.Don S.J., Trian J.S., Cohen B.A. Common bile duct varices: cholangiographic demonstration of a hazardous portosystemic communication. Am J Gastroenterol. 1983;78:42–43. [PubMed] [Google Scholar]
- 6.Sharma M., Pathak A. Perforators of common bile duct wall in portal hypertensive biliopathy. Gastrointest Endosc. 2009;70:1041–1043. doi: 10.1016/j.gie.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 7.Sharma M., Babu C.S., Dhiman R.K., Chawla Y. Induced hypotension in the management of acute hemobilia during therapeutic ERCP in a patient with portal biliopathy. Gastrointest Endosc. 2010;72:1317–1319. doi: 10.1016/j.gie.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Dhiman R.K., Behera A., Chawla Y.K., Dilawari J.B., Suri S. Portal hypertensive biliopathy. Gut. 2007;56:1001–1008. doi: 10.1136/gut.2006.103606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oo Y.H., Olliff S., Haydon G., Thorburn D. Symptomatic portal biliopathy: a single centre experience from the UK. Eur J Gastroenterol Hepatol. 2009;21:206–213. doi: 10.1097/MEG.0b013e3283060ee8. [DOI] [PubMed] [Google Scholar]
- 10.Dumortier J., Vaillant E., Boillot O. Diagnosis and treatment of biliary obstruction caused by portal cavernoma. Endoscopy. 2003;35:446–450. doi: 10.1055/s-2003-38779. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhary A., Dhar P., Sarin S.K. Bile duct obstruction due to portal biliopathy in extrahepatic portal hypertension: surgical management. Br J Surg. 1998;85:326–329. doi: 10.1046/j.1365-2168.1998.00591.x. [DOI] [PubMed] [Google Scholar]
- 12.Dilawari J.B., Chawla Y.K. Pseudosclerosing cholangitis in extra-hepatic portal venous obstruction. Gut. 1992;33:272–276. doi: 10.1136/gut.33.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayraktar Y., Balkanci F., Kayhan B., Ozenç A., Arslan S., Telatar H. Bile duct varices or "pseudo-cholangiocarcinoma sign" in portal hypertension due to cavernous transformation of the portal vein. Am J Gastroenterol. 1992;87:1801–1806. [PubMed] [Google Scholar]
- 14.Khuroo M.S., Yattoo G.N., Zargar S.A. Biliary abnormalities associated with extrahepatic portal venous obstruction. Hepatology. 1993;17:807–813. [PubMed] [Google Scholar]
- 15.Bayraktar Y., Balkanci F., Ozenc A. The "pseudo-cholangiocarcinoma sign" in patients with cavernous transformation of the portal vein and its effect on the serum alkaline phosphatase and bilirubin levels. Am J Gastroenterol. 1995;90:2015–2019. [PubMed] [Google Scholar]
- 16.Perlemuter G., Béjanin H., Fritsch J. Biliary obstruction caused by portal cavernoma: a study of 8 cases. J Hepatol. 1996;25:58–63. doi: 10.1016/s0168-8278(96)80328-x. [DOI] [PubMed] [Google Scholar]
- 17.Dhiman R.K., Puri P., Chawla Y. Biliary changes in extrahepatic portal venous obstruction: compression by collaterals or ischemic? Gastrointest Endosc. 1999;50:646–652. doi: 10.1016/s0016-5107(99)80013-3. [DOI] [PubMed] [Google Scholar]
- 18.Malkan G.H., Bhatia S.J., Bashir K. Cholangiopathy associated with portal hypertension: diagnostic evaluation and clinical implications. Gastrointest Endosc. 1999;49:344–348. doi: 10.1016/s0016-5107(99)70011-8. [DOI] [PubMed] [Google Scholar]
- 19.Nagi B., Kochhar R., Bhasin D., Singh K. Cholangiopathy in extrahepatic portal venous obstruction. Radiological appearances. Acta Radiol. 2000;41:612–615. doi: 10.1080/028418500127345992. [DOI] [PubMed] [Google Scholar]
- 20.Sezgin O., Oğuz D., Altintaş E., Saritaş U., Sahin B. Endoscopic management of biliary obstruction caused by cavernous transformation of the portal vein. Gastrointest Endosc. 2003;58:602–608. doi: 10.1067/s0016-5107(03)01975-8. [DOI] [PubMed] [Google Scholar]
- 21.Keizman D., Shalom M.I., Konikoff F.M. An angulated common bile duct predisposes to recurrent symptomatic bile duct stones after endoscopic stone extraction. Surg Endosc. 2006;20:1594–1599. doi: 10.1007/s00464-005-0656-x. [DOI] [PubMed] [Google Scholar]
- 22.Warren B.L. Association between cholangiographic angulation of the common bile duct and choledocholithiasis. S Afr J Surg. 1987;25:13–15. [PubMed] [Google Scholar]
- 23.Chandra R., Kapoor D., Tharakan A., Chaudhary A., Sarin S.K. Portal biliopathy. J Gastroenterol Hepatol. 2001;16:1086–1092. doi: 10.1046/j.1440-1746.2001.02562.x. [DOI] [PubMed] [Google Scholar]
- 24.Ozkavukcu E., Erden A., Erden I. Imaging features of portal biliopathy: frequency of involvement patterns with emphasis on MRCP. Eur J Radiol. 2009;71:129–134. doi: 10.1016/j.ejrad.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Besa C., Cruz J.P., Huete A., Cruz F. Portal biliopathy: a multitechnique imaging approach. Abdom Imaging. 2012;37:83–90. doi: 10.1007/s00261-011-9765-2. [DOI] [PubMed] [Google Scholar]
- 26.Llop E., de Juan C., Seijo S. Portal cholangiopathy: radiological classification and natural history. Gut. 2011;60:853–860. doi: 10.1136/gut.2010.230201. [DOI] [PubMed] [Google Scholar]


