Abstract
Portal cavernoma cholangiopathy (PCC) is the presence of typical cholangiographic changes in patients with a portal cavernoma due to chronic portal vein thrombosis, in the absence of other biliary tract diseases. Probably due to biliary stasis related to the cavernoma, there is a high incidence of biliary sludge and calculi in PCC, which trigger symptoms that resolve with appropriate interventions. Persistent and troublesome symptoms are usually due to biliary stenoses or strictures, which may occur with or without biliary calculi and may be short or long, solitary or multifocal, extrahepatic or intrahepatic. Experience with endoscopic interventions in PCC over the last twenty years has shown that it is the procedure of choice for bile duct calculi. Plastic stenting with repeated, timely, stent exchanges is the first line intervention for jaundice or cholangitis due to biliary strictures. If biliary obstruction does not resolve, portosystemic shunt surgery (PSS) or transjugular intrahepatic portosystemic stent shunt (TIPS) is performed to decompress the portal cavernoma. However, for patients with non-shuntable veins or blocked shunts, repeated plastic stent exchanges are the only option though there are reports of the use of biliary self-expandable metal stents in this situation. If symptomatic biliary obstruction persists after successful PSS or TIPS, second stage biliary surgery may be necessary. Recent experience suggests that treating biliary strictures in PCC on the lines of postoperative benign biliary strictures with balloon dilatation and repeated exchanges of plastic stent bundles may be effective therapy. Endoscopic management appears to be associated with an increased frequency of hemobilia, which usually responds to standard management. Recurrent cholangitis with formation of sludge and concretions may be a problem with repeated stent exchanges, especially if patient compliance is poor. In conclusion, the current understanding is that symptomatic PCC is best managed jointly by the endoscopist and surgeon with sequential interventions designed initially to establish and maintain biliary drainage, then to decompress the portal cavernoma and, finally, if required, second stage biliary surgery or endotherapy for biliary strictures. Endoscopic therapy occupies a central role in management before, during and after surgical therapy. Paradigms of endoscopic therapy continue to evolve as knowledge of pathogenesis and natural history improves and newer approaches and techniques are applied.
Keywords: portal hypertensive biliopathy, portal biliopathy, pseudosclerosing cholangitis, portal hypertension, extrahepatic portal venous obstruction
Abbreviations: PCC, portal cavernoma cholangiopathy; PSS, portosystemic shunt surgery; TIPS, transjugular intrahepatic portosystemic shunt; EHVPO, extrahepatic portal venous obstruction; ERCP, endoscopic retrograde cholangiopancreatography
Biliary changes secondary to portal hypertension, especially in portal cavernoma secondary to extrahepatic portal vein obstruction, have long been described in literature under different names by various authors.1 The Indian National Association for Study of Liver (INASL) working party defined portal cavernoma cholangiopathy (PCC) as abnormalities in the extrahepatic biliary system including the cystic duct and gallbladder, with or without abnormalities in the 1st and 2nd generation biliary ducts, in a patient with portal cavernoma in the absence of other causes of these biliary changes like bile duct injury, primary sclerosing cholangitis, cholangiocarcinoma etc.1 Although an early report by Sarin2 described PCC, called portal biliopathy by the authors, in patients with liver cirrhosis and non-cirrhotic portal fibrosis as well as with extrahepatic portal venous obstruction (EHPVO), subsequent reports found that it occurred almost exclusively in patients with EHPVO with a portal cavernoma. The majority of patients with these abnormalities are asymptomatic and are incidentally detected to have biliary abnormalities on cholangiography. A minority of patients present with symptoms of chronic cholestasis with or without biliary pain or acute cholangitis, related most often to the presence of biliary strictures or stones.3 Finding stricture with dilatation at cholangiography is associated with a high risk of developing symptoms of PCC. Symptomatic PCC is a late presentation in the natural history of the condition.4 Asymptomatic patients with PCC do not require any treatment. In symptomatic PCC, treatment is focussed on relief of obstructive jaundice and the management of portal hypertension.5
Endoscopic therapy for portal cavernoma cholangiopathy
The era of endoscopic management in PCC began in 1993, with the first report6 of endoscopic biliary stenting in this condition, almost fifty years after symptomatic PCC was first reported in 1944.7 Twenty years later, endotherapy has come to occupy a central place in the management of symptomatic PCC. Although therapeutic paradigms are still evolving, the debate for primacy between surgeon and endoscopist is giving way to the perception that close co-ordination between them with a careful, calibrated approach is needed to ensure that patients receive optimal therapy.
Evolution of endoscopic therapy in portal cavernoma cholangiopathy
Endoscopic intervention in PCC began as a fire-fighting exercise for managing cholestasis or cholangitis by establishing biliary drainage with plastic stents or nasobiliary drains. The first report of endoscopic extraction of common bile duct stones in PCC8 was soon followed by other small series5,9–13 reporting success without an increase in complications, though some workers reported hemobilia during the procedure.14–17 In many centers, endoscopic management has remained confined to establishing drainage of the obstructed biliary system before surgery, in the expectation that either the obstruction would resolve after a period of endoscopic drainage or that portosystemic shunt surgery (PSS), with or without second-stage biliary surgery, would provide definitive management. However, as the complexity of patients with symptomatic PCC was realized, with biliary strictures, calculi or both being present in the extrahepatic, intrahepatic or both locations, and as the difficulties and limitations of surgical management became clear,11,18–20 most workers accepted that the optimal management of symptomatic PCC required appropriate use of both endoscopic and surgical interventions. When surgery is unsuccessful, the only option available for patients is repeated stent exchanges for prolonged periods or lifelong. When biliary access is difficult, some workers have resorted to the placement of covered removable self-expanding metallic stents but their long-term outcome remains uncertain.10 Recently, good results have been obtained with the use of plastic stent bundles as popularized for postoperative benign biliary strictures (personal data described below).
The practice of endoscopic therapy in portal cavernoma cholangiopathy: a literature summary
Biliary Calculi in Portal Cavernoma Cholangiopathy
Prevalence
Patients with PCC appear to have increased prevalence of biliary calculi (Table 1), though available data come from small series of non-consecutive patients treated in tertiary care centers and may be an overestimate. Eleven series published between 1992 and 20119,10,12,13,18–23 have reported a mean frequency of 26.3% (16–85%) for biliary calculi among a total of 331 patients, including 143 with symptomatic PCC. Prevalence of both, gallbladder (mean 13.6%, range 0–69%) as well as biliary ductal calculi (mean 17.8%, range 0–77%), is increased and prevalence is much higher in symptomatic PCC, being 60.8% overall in 143 symptomatic patients (35.1% for gallstones, 41.2% for choledocholithiasis).5,9–11,18 While increased prevalence of gallstones in PCC remains inadequately explained, it appears that biliary stasis due to collateral compression or stricture formation results in the high prevalence of biliary calculi and that formation of these calculi may precipitate biliary obstruction and trigger symptoms in patients with PCC.9,10
Table 1.
Frequency of Biliary Calculi in Portal Cavernoma Cholangiopathy.
| Author year | Subjects | Duration | Biliary calculi, N (%) | Gallbladder calculi, N (%) | Bile duct calculi, N (%) |
|---|---|---|---|---|---|
| Bayraktar 199221 | Portal vein thrombosis—47 | – | 8 (19) | 0 | 8 (19) |
| Chaudhary 199818 | Symptomatic PCC—9 | – | 2 (22) | 0 | 2 (22) |
| Condat 200322 | Symptomatic PCC—7 | 2 years | 4 (16) | 2 (8) | 2 (8) |
| Sezgin 200312 | Symptomatic PCC—10 | 6 years | 2 (20) | 1 (10) | 1 (10) intrahepatic |
| Dumortier 200313 | Symptomatic PCC—6 | – | 4 (66) | 2 (33) | 4 (66) |
| Khare 200511 | Symptomatic PCC—13 | 10 years | 8 (61) | 6 (46) | 8 (61) |
| Dhiman 200623 | Portal vein thrombosis—53 | – | 11 (20) | 7 (13) | 4 (7) |
| Symptomatic PCC—13 | |||||
| Vibert 200719 | Portal vein thrombosis—64 | 20 years | 11 (58) | 0 | 4 (21) |
| Symptomatic PCC—19 | Intrahepatic, 7 (37) | ||||
| Oo 200910 | Symptomatic PCC—13 | 13 years | 11 (85) | 9 (69) | 10 (77) |
| Agarwal 201120 | Symptomatic PCC—39 | 11 years | 19 (48.7) | 12 (31) | 7 (18) |
| Llop 20119 | PCC—52 | 12 years | 7 (50) | 6 (43) | 2 (14) |
| Symptomatic PCC—14 | |||||
| Saraswat 2013 (personal data) | Symptomatic PCC—20 | 16 years | 10 (50) | 6 (30) | 8 (40) |
| Total | 351 | NA | 97 (27.7) | 51 (14.6) | 67 (19.1) |
| Symptomatic PCC—163 | (59.9) | (31.5) | (41.3) |
PCC, portal cavernoma cholangiopathy; PVT, portal vein thrombosis.
Management
Endoscopic clearance of CBD calculi in patients with PCC was first reported by Bhatia and Sarin in 1995.8 In three years, they managed 4 patients with PCC and choledocholithiasis, two of whom developed cholangitis. Three of the 4 patients underwent ES with extraction of multiple small brown black calculi. No complications were reported, symptoms resolved and patients were well 4–8 months after the procedure. Khare et al11 found bile duct calculi in 8 of their 13 patients, including 5 who had stones above common bile duct strictures. Endoscopic clearance by sphincterotomy followed by repeated sweeps with balloon extractors and Dormia baskets was successful in 5 of the 6 patients in whom it was attempted. Multiple sessions (12 sessions in 3 patients) were necessary for successful clearance in patients with calculi above strictures, who needed biliary balloon dilatation and mechanical lithotripsy. Oo et al10 found biliary calculi or sludge in 10 of 13 patients in their series. However, they reported successful CBD clearance after ES and balloon trawl in only 1 patient, though biliary drainage was achieved in 8. Llop et al9 reported a series of 52 patients with PCC followed over 12 years (1996–2008), including 14 who were symptomatic. Choledocholithaisis in 6 patients could be treated by sphincterotomy and stone extraction in 5, though repeat sphincterotomy was needed in one of them while the 6th patient needed biliary surgery. No calculi were detected in 3 others who presented with cholestasis and cholangitis and could be treated with sphincterotomy followed by ursodeoxycholic acid (UDCA). Interestingly, they also reported 5 patients with abdominal pain and cholestasis who did well on UDCA treatment alone.
Management of Biliary Strictures in Portal Cavernoma Cholangiopathy
Since the first report of biliary stenting for choledochal stenosis in 1993,6 endoscopic management of symptomatic PCC has been reported in at least 87 patients in 17 case series,6,8–13,22,24–32 nine of which involved fewer than 4 patients and the largest included 20 patients (Table 2). Results of endoscopic management for biliary stenoses are summarized below.
Table 2.
Endoscopic Treatment for Portal Cavernoma Cholangiopathy.
| Series | PCC patients | Cholangiogram abnormalities | Endoscopic treatment | Duration of therapy | Complications | Outcome |
|---|---|---|---|---|---|---|
| Bhatia et al 19958 | Symptomatic PCC—4 | Stricture + stone—3; CBDS—4 | ES, extraction, ENBD | 3 years | Nil | Symptoms—resolved; (FU 3–8 months) |
| Condat et al 200322 | Asymptomatic PCC—18; symptomatic PCC—7 | Stricture—2; stricture + stone—1; GS—2, CBDS—1 | Nil; UDCA—4 | NA | NA | Improved on UDCA—3 |
| Sezgin et al 200312 | Symptomatic PCC—10 | Stricture (CHD/CBD) 9; intrahepatic stone 1 | ES, BD, stenting | 3.3 years (range, 1–7) | Hemobilia—1; cholangitis—5, death—1 | Normal ERC in 3 after 1 year; stricture improved—1; 5 on stent exchange |
| Dumortier 200313 | Symptomatic PCC—6 | Stricture—6 (CHD-CBD 5, CHD-cystic 1); CBDS—2 | ES—5, BD—5; stone removal—2; single stent with 6-months exchanges | 10 months (2 days–18 months) | Cholangitis; cholecystitis in 4 | 2 asymptomatic after 2 and 3 years; 4 shunted |
| Khare et al 200511 | Symptomatic PCC—13 | Stricture—11 (CBD—9, CHD—2, CBDS—8) | Stricture—5 (BD 1, stented—4); CBDS—3 (stone extraction—2); CBDS + stricture—5 (BD, stone extraction—2) | NA | Nil Death (postoperative) 1 | CBDS cleared—4/6; strictures stented—11(till shunt—6, after shunt—4); successful surgery—7 |
| Dhiman et al 20075 | Symptomatic PCC—12 | Stricture—7; CBDS—5; choledocha varices—1; Mirizzi's syndrome—1 | PSS 5; ES 3; ES + stricture dilatation—2; serial stent exchange—2 | NA | Cholangitis in patients on serial stent exchange | All asymptomatic FU, 19 months (range 6–132). |
| Oo et al 200810 | Symptomatic PCC—13 | Stricture—13 (intrahepatic—12, CBD—6, CHD—7) CBDS—10 | ERCP stenting | NA | Hemobilia—2; cholangitis—3 | Successful outcome, ERCP alone—6; ERCP + shunt—3; liver transplantation—1; spontaneous—3 |
| Llop et al 20119 | Symptomatic PCC—14 | Stricture—14; CBDS—6; GS—2 | ES + stone extraction—5; UDCA—8 | NA | NA | ES—6; surgery—3 (bilio-enteric anastomosis—1, cholecystectomy—1, failed—1) |
| Saraswat et al 2013 (personal data) | Symptomatic PCC—20 | Strictures—20; CBDS—8; GS—6 | ES + stone extraction—8; stenting—9; dilatation + stent bundles—11 | 18 months (3–188 months) | 130 procedures: cholangitis—40, hemobilia—9 | Asymptomatic 18 months (3–90) after stent removal—12; deaths—2; lost to FU—6 |
ES, endoscopic sphincterotomy; BD, balloon dilatation; CBDS, common bile duct stone; ENBD, endoscopic nasobiliary drainage; FU, follow-up; GS, gallstones; NA, not available; PCC, portal cavernoma cholangiopathy; PSS, portosystemic shunt; CBD, common bile duct; CHD, common hepatic duct; UDCA, ursodeoxycholic acid.
Lohr6 reported a patient with symptomatic PCC who was successfully treated with insertion of a single stent that was exchanged for 3 years. Follow up after stent removal was not reported. From Marseilles, France, Thervet24 reported 2 patients with symptomatic PCC due to idiopathic calcified EHPVO who had a single stent inserted preoperatively. Since definitive biliary surgery was not possible, both were managed with long-term stenting and were alive and well over a 2-year follow up period. In another French report, Perlemuter25 followed 8 patients with symptomatic PCC over 10 years, 4 of whom underwent endotherapy. They reported ‘good outcome’ in 2 of 3 patients treated with sphincterotomy alone and in one after sphincterotomy followed by balloon dilation. From Erlangen-Nuremberg, Mörk26 reported endoscopic management in 2 patients with Bismuth type 1 stricture, one of whom had a single stent inserted for 3 months with ‘good response’ while the second had a single stent placed that was exchanged thrice for cholangitis without long-term relief and underwent PSS. Solmi27 reported a ‘successful outcome’ with plastic stenting in an Italian patient with symptomatic PCC. Hernandez28 reported a Mexican patient with symptomatic PCC due to a dominant stricture in the mid-CBD who underwent preoperative endoscopic biliary drainage followed by the Sugiura procedure. Ajayi29 reported a ‘good outcome’ in a Nigerian patient treated with stent exchanges for 6 months. Layec30 placed a self-expandable metal stent in a French patient with symptomatic PCC and reported a ‘good outcome’ at 18 months with stent in situ. Cantu31 reported that a ‘stent-trial’ with a single stent placed for 3 months in a patient with a common bile duct stricture helped in establishing that obstruction was related to ischemic stricturing after which they resorted to surgical treatment and observed a ‘good response’ at 4 years.
From Turkey, Sezgin et al12 reported endoscopic management of ‘severe’ strictures in 10 patients with symptomatic PCC seen over 7 years. Repeated exchanges of plastic stents after sphincterotomy were done for a mean period of 3.3 years (range 1–7 years); four of them also needed balloon dilatation with nasobiliary drains and extraction of sludge or stones. Hemobilia was seen in only 1 patient though cholangitis occurred in 5. ‘Significant’ improvement in stricturing was noted in 4 patients and stents were removed in 3, who remained asymptomatic for 1 year after that, prompting the authors to conclude that endoscopic management was safe and effective in symptomatic PCC.
Dumortier et al13 reported 6 patients from Lyon, France, with symptomatic PCC who presented with acute cholangitis (3) or cholestasis (3); four also had gallstones. ‘Good’ results were reported with repeated plastic stenting in two patients but it ‘failed’ in four, who underwent PSS that allowed removal of biliary stents. Khare et al11 reported 13 patients with symptomatic PCC managed over a ten year period (1992–2002) from Lucknow, India, who had biliary strictures (5; Group A), choledocholithiasis (3; Group B) or both (5; Group C). Twenty eight endoscopic procedures were performed in 10 of these patients. In Group A, 4 patients underwent plastic stenting for jaundice and cholestasis followed by PSS. A second ERCP done in two of them at the time of stent removal showed stricture resolution. However, till end of follow up, 4 more sessions had been performed in one of these patients in whom the stricture persisted despite PSS, for a total of 10 sessions in this group. Among 8 patients with CBD stones, endoscopic clearance was attempted in 6 patients and was successful in 4 (2 each in groups B and C). In group B, the CBD was cleared in a single session in one patient while the other needed 5 sessions. However, among 4 patients in group C, the initial attempt at clearance failed in all, after which two underwent surgical CBD clearance (PSS followed by CBD exploration in 1, splenectomy with devascularization, partial cholecystectomy and CBD exploration in 1), while PSS failed in the other 2. Endoscopic clearance was achieved in both these patients, though only after repeated attempts using balloon dilatation and mechanical lithotripsy.
Oo et al10 reported a series of 13 patients with symptomatic PCC managed in Birmingham, UK, over 13 years (1992–2005). All had presented with jaundice while 10 also had biliary calculi or debris. Jaundice resolved spontaneously in 3 patients while endoscopic interventions were successful in 6 patients. Sphincterotomy and balloon trawl extracted calculi from the common bile duct in one patient. Endoscopic biliary drainage was achieved in 5 others by repeated plastic stent exchanges (1), followed by placement of a self-expandable metallic stent (3) or after a combined procedure (1). Portosystemic shunting (TIPS in 2, meso-caval shunt in 1) was followed by relief of jaundice in 3 patients. Though 3 patients were considered for orthotopic liver transplantation, 2 were not found suitable while one died on the wait-list of septic complications. The authors recommended repeated plastic stent exchange for biliary drainage, reserving metal stent placement for those with successful but difficult endoscopic biliary drainage and PSS for those who did not improve after endoscopic biliary drainage.
Endoscopic Management of Portal Cavernoma Cholangiopathy as a Benign Biliary Stricture
Biliary obstruction in PCC is due to biliary stenoses with or without associated biliary calculi. Fixed, high grade biliary obstruction in symptomatic PCC may be due to extrinsic compression by a large collateral, a fibrotic cavernoma or mural fibrosis. The latter two situations may be regarded as benign biliary stricture. Encouraging long-term results, comparable with those of surgery, have been reported with aggressive endoscopic management of postoperative benign biliary strictures by placing bundles of plastic stents and exchanging them at periodic intervals for 12–15 months32,33 (Figure 1).
Figure 1.
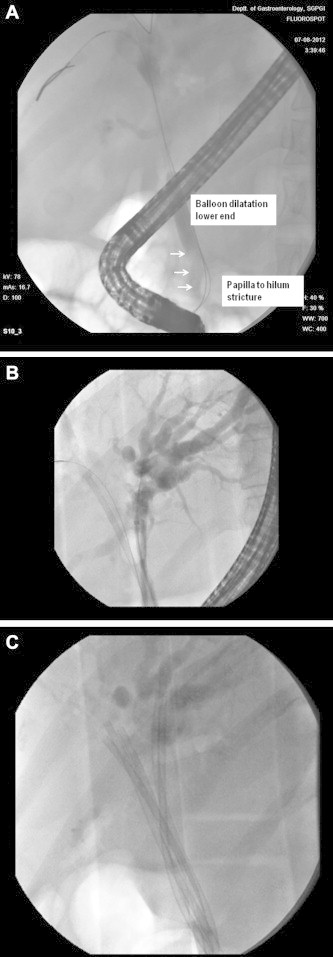
Endoscopic treatment of advanced portal cavernoma cholangiopathy. A. Long stricture from lower bile duct to hilum with controlled radial expansion balloon dilataion of lower end (arrow). B. Hilar stricture with left hepatic duct ‘hand sign; two 10F stents in left hepatic duct, one stent and a guide wire in right hepatic duct. C. Hilar stricture with left hepatic duct hand sign and five 10F stents, 2 in right hepatic duct and 3 in left hepatic duct.
A group of patients with symptomatic PCC has been treated on the lines of postoperative benign biliary stricture at Lucknow (personal data). Between 1994 and 2010, 130 sessions of ERCP were performed for 20 patients with symptomatic PCC, which included 11 patients (101 ERCP sessions) in whom biliary strictures were treated on the lines of postoperative benign biliary stricture. Stricture dilatation using balloon dilators (6–15 mm) was followed by the insertion of 10F plastic stent bundles (median 4, range 2–8) that were exchanged every 3–4 months till patients had normal blood tests and complete or near-complete resolution of strictures, at which point stents were removed. At the end of treatment, liver functions had normalized in all while the cholangiogram had normalized completely in 8 and partially in 3 patients. Partial improvement was seen when stents had to be removed prematurely for recurrent cholangitis due to sludge. Cholangitis was present at the time of 25 of the 101 (25%) procedures and was usually due to delay in stent exchange, as many patients were irregular during follow up. Severe sepsis occurred only in 2 patients who recovered with medical management. Hemobilia occurred in 7 of 101 procedures (7%) and was considered a major complication in 2 episodes, necessitating multiple blood transfusions. Two patients died before completion of therapy due to variceal bleeding (1) and concomitant gallbladder cancer (1). Two have not followed up 8 and 11 months after successful completion of therapy. The remaining 7 patients were asymptomatic at a median follow up of 18 months after stent removal (range 2–90 months). Thus, not only was endotherapy safe and effective in treating cholangitis but prolonged periods of stenting with bundles of plastic stents led to stricture resolution or regression followed by long asymptomatic periods.
Role of Ursodeoxycholic Acid in Symptomatic Portal Cavernoma Cholangiopathy
Several workers have reported the concomitant use of ursodeoxycholic acid (UDCA) in patients with symptomatic PCC undergoing endoscopic therapy. Perlemuter et al25 used UDCA in 5 of 8 patients with liver fibrosis or secondary biliary cirrhosis (SBC) on liver biopsy. Condat et al22 used UDCA in 3 of 4 patients with cholestasis who underwent endoscopic sphincterotomy and reported no recurrence of symptoms while on therapy. Llop et al9 used it in 10 of 14 patients with symptomatic PCC, including 5 patients with abdominal pain and cholestasis treated with UDCA alone, in two patients with stricture but no calculi and in 3 of 6 with choledochal stones after sphincterotomy and ductal clearance. They reported ‘disappearance of symptoms and improvement in liver tests’ in all treated patients during follow up.
However, other workers have used UDCA sparingly or not at all. Khare et al11 did not use UDCA at all while Oo et al10 have reported the use of UDCA in only 1 of their 13 patients with symptomatic gallstones and found that symptoms did not recur. However, they have also reported ‘spontaneous’ regression of symptoms of biliary obstruction without any specific therapy in at least 3 of their 13 patients. Thus, in the absence of appropriate controls, it is difficult to be sure that the observed benefit is due to UDCA and not part of the natural history of PCC or due to spontaneous passage of sludge and small calculi.
Self-expandable Metallic Stents for Symptomatic Portal Cavernoma Cholangiopathy
There is very limited experience with the placement of self-expandable metallic stents in the biliary system for symptomatic PCC.10,30 Oo et al10 have reported their experience of placing bare stents in three patients who had undergone repeated plastic stent exchanges, were not candidates for PSS or surgery had been unsuccessful, and in whom biliary access was difficult. While initial experience with self-expandable metallic stents was satisfactory, obviating the need for frequent exchange of plastic stents, at least one patient developed stent occlusion after 6 years and had to be salvaged with regular exchange of plastic stents through the metal stent. Layec et al30 reported their experience with placement of a covered removable self-expandable metallic stent in a patient with symptomatic PCC. While the initial placement and course were uneventful, they encountered torrential bleeding along with a bile leak during attempted removal three months later and had to re-deploy a fresh covered metal stent to treat the complication. The patient was alive and well 18 months after the event albeit with the covered metal stent in situ.
Problems with endoscopic management
Bleeding During Endotherapy
The risk of hemobilia has been a major worry during endoscopic management of PCC. An early report in a single patient14 raised apprehensions of bleeding from bile duct varices during ES. Hemobilia was reported during endoscopic therapy in 3 patients treated in Italy, leading the authors to recommend surgical management over endotherapy.15 Intra-choledochal varices, masquerading as filling defects,5,34,35 may be the source of bleeding during a balloon trawl or a basket sweep of the bile duct for calculi. Hemobilia was reported in 3 patients during balloon sweeps for extraction of biliary calculi and attributed to the balloon extractor squeezing intra-choledochal varices.16 Others did not implicate any one procedure, reporting minor episodes of hemobilia during balloon sweeps (2), Dormia sweeps (2) and removal of stent or nasobiliary catheter (2), which were controlled with terlipressin infusion.17 Recently, covered removable self-expanding metal stents have been placed for symptomatic PCC10 but at least one report30 documented torrential bleeding after stent removal, which could only be controlled with reinsertion of another covered metal stent.
However, fears of excessive bleeding during endotherapy for PCC have not been substantiated by other reports and the overall frequency of hemobilia reported in published series is low.8–13 None of the series have reported excessive bleeding during ES, suggesting that it is safe in patients with PCC with no more risk than in patients without PCC. Bhatia et al8 reported uneventful ES and stone extraction in 3 patients with symptomatic PCC and choledocholithiasis. Hemobilia during removal of a plastic stent was reported in 1 of 10 patients treated by Sezgin et al.12 Khare et al11 did not encounter significant hemobilia in any of 28 procedures done in 10 patients undergoing endotherapy in their series of 13 patients. These included ES, repeated sweeps with balloon extractors and Dormia baskets as well as the use of biliary balloon dilators in 3 and mechanical lithotripsy in 2 patients. Oo et al10 reported hemobilia in 2 (4%) of 49 procedures performed in 12 patients. In a series of 14 patients with symptomatic PCC, Llop et al9 did not encounter hemobilia during ES, stone extraction and biliary stenting in any of 8 patients undergoing endotherapy. Thus, it appears that, though hemobilia may be more common during endoscopic procedures for PCC than for other indications, overall it is infrequent and may respond to terlipressin infusion.17
Cholangitis
Not only is cholangitis a common indication for endoscopic interventions, it is also the commonest complication during the course of endotherapy. Cholangitis was reported in 5 of 10 patients managed with repeated stent exchanges in France,12 in 4 of 6 patients managed by Dumortier et al13 and in 3 of 13 patients (3 of 49 procedures; 6%) reported by Oo et al.10 In the series of 13 patients with symptomatic PCC reported by Khare et al,11 none of the 10 patients who underwent endoscopic management developed cholangitis. However, recent experience from the same center noted that cholangitis was present at the time of 40 of 130 procedures (30%) in 20 patients managed with repeated stent exchanges over a period of 16 years and this was attributed to inability of patients to report for timely stent exchanges.
Cholangitis may be encountered in patients who undergo repeated endoscopic procedures over a prolonged period of time, as is the case in patients with non-shuntable veins or failed surgical procedures. Cholangitis may be due to incomplete clearance of calculi and debris above strictures or, due to delay in planned stent exchanges in poorly compliant patients. If treatment is delayed, cholangitis may result in serious complications such as cholangitic liver abscesses, ruptured abscesses and sepsis.
Endoscopic management in portal cavernoma cholangiopathy: principles and strategy
It would not be an exaggeration to state that endoscopic therapy is the mainstay of management in symptomatic PCC and is the sheet anchor around which the surgeon works to provide benefits of successful PSS. Rarely does a patient with symptomatic PCC undergo PSS without requiring even a single endoscopic procedure. Indications for endoscopic therapy for symptomatic PCC are summarized in Table 3.
Table 3.
Indications for Biliary Endoscopy in Portal Cavernoma Cholangiopathy.
| Before portosystemic shunt | Peri-operative | After portosystemic shunt |
|---|---|---|
| Cholangitis, cholangitic abscesses | Treat or prevent cholangitis, jaundice or cholestasis | Blocked portosystemic shunt surgery |
| Jaundice, cholestasis | Failed shunt or devascularization | |
| No shuntable vein | Persistent obstruction despite patent shunt |
Who Should Treat?
Optimal management of symptomatic and complicated PCC needs close co-ordination between skilled endoscopic and surgical teams working in tandem to tackle challenges in the biliary and the portal vascular tree of each patient. The patient, the endoscopist and the surgeon must realize that management proceeds in phases and should not be left incomplete. The key is close follow up in each patient till biliary obstruction is resolved, the patient is asymptomatic once again and remains so without further interventions.
Whom to Treat?
The threshold for initiating endoscopic intervention should be high. It should be avoided in asymptomatic PCC with only cholangiographic changes or minor biochemical abnormalities. Endoscopic intervention should be undertaken as part of a plan developed in concert with the surgical team, in a motivated patient prepared to go through the three phases of therapy needed for complete treatment of symptomatic PCC.
How to Treat?
In the symptomatic patient with biliary obstruction with or without calculi, sphincterotomy and biliary drainage, with or without stone extraction, constitute the first phase in the management. Biliary sludge and calculi often precipitate symptoms of biliary obstruction and clearance of the bile duct may provide prolonged relief. UDCA may be beneficial in this setting but controlled data are awaited. Sphincterotomy in PCC is not associated with an increased risk of bleeding and the use of Dormia baskets and balloon extractors is safe. Though hemobilia is reported, and may be alarming at times, it responds to conservative management and most workers find it no more troublesome than in patients without PCC. Experience with advanced endoscopic techniques for ‘difficult’ biliary calculi such as large balloon sphincteroplasty and cholangioscopy with intraductal lithotripsy using laser or electrohydraulic probes has not been reported in symptomatic PCC. Application of these techniques is likely to make even ‘intractable’ biliary calculi in PCC amenable to endoscopic therapy.
Portal decompression by PSS or TIPSS constitutes the second phase of management of symptomatic PCC. Whether all patients with shuntable veins should undergo elective PSS as soon as symptoms are controlled11,19,20 or it should be considered only if symptoms recur after multiple endoscopic session,9 has been debated. While the latter approach is reasonable for patients at an early stage in the natural history of the disease, when prolonged relief may be obtained by clearing biliary calculi or treating sludge and microcalculi with sphincterotomy and UDCA, most patients with advanced changes, complicated PCC and fixed biliary tract obstruction will have prompt recurrence of symptoms with removal of stents and will need PSS.
Benefits from PSS include regression of changes seen in early cholangiopathy,35,36 complete regression of biliary obstruction in about 60–88% of patients11,19,20,35,37 with further interventions needed for persistent biliary obstruction in only 25–30%.20 The remaining patients usually remain asymptomatic, even though regression of cholangiographic changes is incomplete, suggesting arrest or slowing-down of progression after PSS. Some workers have observed that need for endotherapy, as also frequency of hemobilia in those who do need endotherapy, decreases after successful shunt surgery.11 Thus, it appears reasonable to undertake PSS in all patients with symptomatic PCC who have shuntable veins as soon as possible after initial stabilization.
The third phase of management in symptomatic PCC after successful PSS is to determine which patients are in need of further therapy for unresolved biliary obstruction. Once PSS has been performed, close follow up is required to judge whether there is complete response in biliary obstruction, with resolution of clinical, biochemical and cholangiographic abnormalities, or incomplete response, with clinical improvement but persistence of cholangiographic and/or biochemical abnormalities, and to assess shunt patency. If the patient remains asymptomatic, even an incomplete response is acceptable. If biliary obstruction persists even after PSS with a patent shunt, ‘second-stage’ surgery on the bile ducts is usually recommended. However, endoscopic options, such as reverting to periodic plastic stent exchanges according to either a standard regime or an aggressive ‘benign biliary stricture protocol’ or the use of self-expandable metallic stents, need to be weighed against the risks of ‘second-stage’ surgery. In case PSS is not possible due to extensive thrombosis and absence of a shuntable vein or if the shunt blocks, the patient must be rescued with continued endoscopic stenting. In general, placement of metal stents is not recommended in patients with benign diseases who are expected to have prolonged survival. Even the short-term use of removable stents maybe fraught with problems and should be considered with care, on a case-by-case basis. However, it appears to be a useful maneuver for the control of torrential hemobilia from the extrahepatic bile duct.
Conclusions
-
1.
The diagnosis of PCC must be suspected in every patient with EHPVO and confirmed with appropriate imaging. For all patients with PCC, the stage in the natural history of PCC at which they are should be established.
-
2.
Those with symptomatic PCC should have endotherapy as first line therapy for biliary pain, cholestasis and cholangitis since it is safe and effective for clearing bile duct calculi and for controlling cholangitis and cholestasis.
-
3.
All patients with PCC are candidates for portal decompression with PSS or TIPSS, which should be done as soon as possible after the diagnosis is made. It might be the only intervention likely to change the natural history of PCC. Endotherapy may be required to bridge the patient with symptomatic or complicated PCC to PSS.
-
4.
Endotherapy may have to be used as definitive long-term strategy in patients who are unfit for PSS, in whom PSS is not possible due to non-shuntable veins, who show no response to PSS and in those with blocked PSS which cannot be redone. Percutaneous externo-internal biliary drainage has also been used by European workers when endotherapy is not possible as after bilio-enteric anastomosis.
-
5.
When endotherapy is used for definitive management of symptomatic PCC, the options are repeated exchanges with bundles of plastic stents or the placement of covered removable self-expanding metallic stents. Presently, adequate data are not available for either option. Whilst preliminary data suggest that repeated exchanges with bundles of plastic stents for 12–18 months, as used for postoperative benign biliary stricture, is an effective strategy, the place for covered, removable self-expanding metallic stents in the management of symptomatic PCC is being explored presently.
Conflicts of interest
All authors have none to declare.
References
- 1.Chawla Y., Agrawal S. Portal cavernoma cholangiopathy – history, definition and nomenclature. J Clin Exp Hepatol. 2013 doi: 10.1016/j.jceh.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chandra R., Tharakan A., Kapoor D., Sarin S.K. Comparative study of portal biliopathy in patients with portal hypertension due to different etiologies. Indian J Gastroenterol. 1997;15(suppl 2):A59. [abstract] [Google Scholar]
- 3.Duseja A. Portal cavernoma cholangiopathy (PCC) – clinical characteristics. J Clin Exp Hepatol. 2013 doi: 10.1016/j.jceh.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar M. Natural history and prognosis of portal cavernoma cholangiopathy. J Clin Exp Hepatol. 2013 doi: 10.1016/j.jceh.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhiman R.K., Behera A., Chawla Y.K., Dilawari J.B., Suri S. Portal hypertensive biliopathy. Gut. 2007;56:1001–1008. doi: 10.1136/gut.2006.103606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lohr J.M., Kuchenreuter S., Grebmeier H., Hahn E.G., Fleig W.E. Compression of the common bile duct due to portal vein thrombosis in polycythemia vera. Hepatology. 1993;17:586–592. doi: 10.1002/hep.1840170410. [DOI] [PubMed] [Google Scholar]
- 7.Fraser J., Brown A.K. A clinical syndrome associated with a rare anomaly of vena portal system. Surg Gynecol Obstet. 1944;78:520–524. [Google Scholar]
- 8.Bhatia V., Jain A.K., Sarin S.K. Choledocholithiasis associated with portal biliopathy in patients with extrahepatic portal vein obstruction. Management with endoscopic sphincterotomy. Gastrointest Endosc. 1995;42:178–181. doi: 10.1016/s0016-5107(95)70080-3. [DOI] [PubMed] [Google Scholar]
- 9.Llop E., de Juan C., Seijo S. Portal cholangiopathy: radiological classification and natural history. Gut. 2011;60:853–860. doi: 10.1136/gut.2010.230201. [DOI] [PubMed] [Google Scholar]
- 10.Oo Y.H., Olliff S., Haydon G., Thorburn D. Symptomatic portal biliopathy: a single centre experience from the UK. Eur J Gastroenterol Hepatol. 2009;21:206–213. doi: 10.1097/MEG.0b013e3283060ee8. [DOI] [PubMed] [Google Scholar]
- 11.Khare R., Sikora S.S., Srikanth G. Extrahepatic portal venous obstruction and obstructive jaundice: approach to management. J Gastroenterol Hepatol. 2005;20:56–61. doi: 10.1111/j.1440-1746.2004.03528.x. [DOI] [PubMed] [Google Scholar]
- 12.Sezgin O., Oguz D., Altintas E., Saritas U., Sahin B. Endoscopic management of biliary obstruction caused by cavernous transformation of the portal vein. Gastrointest Endosc. 2003;58:602–608. doi: 10.1067/s0016-5107(03)01975-8. [DOI] [PubMed] [Google Scholar]
- 13.Dumortier J., Vaillant E., Boillot O. Diagnosis and treatment of biliary obstruction caused by portal cavernoma. Endoscopy. 2003;35:446–450. doi: 10.1055/s-2003-38779. [DOI] [PubMed] [Google Scholar]
- 14.Tighe M., Jacobson I. Bleeding from bile duct varices as unexpected hazard during therapeutic ERCP. Gastrointest Endosc. 1996;43:250–252. doi: 10.1016/s0016-5107(96)70327-9. [DOI] [PubMed] [Google Scholar]
- 15.Mutignani M., Shah S.K., Bruni A., Perri V., Costamagna G. Endoscopic treatment of extrahepatic bile duct strictures in patients with portal biliopathy carries a high risk of hemobilia: report of 3 cases. Dig Liver Dis. 2002;34:587–591. doi: 10.1016/s1590-8658(02)80093-7. [DOI] [PubMed] [Google Scholar]
- 16.Sharma M., Ponnusamy R.P. Is balloon sweeping detrimental in portal biliopathy? A report of 3 cases. Gastrointest Endosc. 2009;70:171–173. doi: 10.1016/j.gie.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Tyagi P., Puri A.S., Sharma B.C. Balloon sweep in portal biliopathy. Gastrointest Endosc. 2010;71:885–886. doi: 10.1016/j.gie.2009.08.014. [Lett] [DOI] [PubMed] [Google Scholar]
- 18.Chaudhary A., Dhar P., Sarin S.K. Bile duct obstruction due to portal biliopathy in extrahepatic portal hypertension: surgical management. Br J Surg. 1998;85:326–329. doi: 10.1046/j.1365-2168.1998.00591.x. [DOI] [PubMed] [Google Scholar]
- 19.Vibert E., Azoulay D., Aloia T. Therapeutic strategies in symptomatic portal biliopathy. Ann Surg. 2007;246:97–104. doi: 10.1097/SLA.0b013e318070cada. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agarwal A.K., Sharma D., Singh S., Agarwal S., Girish S.P. Portal biliopathy: a study of 39 surgically treated patients. HPB (Oxford) 2011;13:33–39. doi: 10.1111/j.1477-2574.2010.00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bayraktar Y., Balkanci F., Kayhan B., Ozenç A., Arslan S., Telatar H. Bile duct varices or “pseudocholangiocarcinoma sign” in portal hypertension due to cavernous transformation of the portal vein. Am J Gastroenterol. 1992;87:1801–1806. [PubMed] [Google Scholar]
- 22.Condat B., Vilgrain V., Asselah T. Portal cavernoma-associated cholangiopathy: a clinical and MR cholangiography coupled with MR portography imaging study. Hepatology. 2003;37:1302–1308. doi: 10.1053/jhep.2003.50232. [DOI] [PubMed] [Google Scholar]
- 23.Dhiman R.K., Chawla Y., Duseja A. Portal hypertensive biliopathy (PHB) in patients with extrahepatic portal venous obstruction (EHPVO) J Gastroenterol Hepatol. 2006;21:A504. [abstract] [Google Scholar]
- 24.Thervet L., Faulques B., Pissas A. Endoscopic management of obstructive jaundice due to portal cavernoma. Endoscopy. 1993;25:423–425. doi: 10.1055/s-2007-1009120. [DOI] [PubMed] [Google Scholar]
- 25.Perlemuter G., Bejanin H., Fritsch J. Biliary obstruction caused by portal cavernoma: a study of 8 cases. J Hepatol. 1996;25:58–63. doi: 10.1016/s0168-8278(96)80328-x. [DOI] [PubMed] [Google Scholar]
- 26.Mörk H., Weber P., Schmidt H., Goering R.M., Scheurlen M. Cavernous transformation of the portal vein associated with common bile duct strictures: report of two cases. Gastrointest Endosc. 1998;47:79–83. doi: 10.1016/s0016-5107(98)70305-0. [DOI] [PubMed] [Google Scholar]
- 27.Solmi L., Rossi A., Conigliaro R., Sassatelli R., Gandolfi L. Endoscopic treatment of a case of obstructive jaundice secondary to portal cavernoma. Ital J Gastroenterol Hepatol. 1998;30:202–204. [PubMed] [Google Scholar]
- 28.Hernandez I.G., Sandoval M.W., Méndez E.L., Calleros J.H., Tapia A.L., Uribe M. Biliary stricture caused by portal biliopathy: case report and literature review. Ann Hepatol. 2005;4:286–288. [PubMed] [Google Scholar]
- 29.Ajayi A.O., Chandrasekar T.S.C., Hammed A.H. Portal biliopathy in a 13-year-old Asian girl: a case report and review of literature. Ann Afr Med. 2009;8:185–188. doi: 10.4103/1596-3519.57244. [DOI] [PubMed] [Google Scholar]
- 30.Layec S., D'Halluin P.N., Pagenault M., Bretagne J.F. Massive hemobilia during extraction of a covered self-expandable metal stent in a patient with portal hypertensive biliopathy. Gastrointest Endosc. 2009;70:555–556. doi: 10.1016/j.gie.2009.04.051. [DOI] [PubMed] [Google Scholar]
- 31.Cantu P., Bezzio C. Role of a short-term stent-trial in a patient with biliary stricture and portal hypertensive biliopathy: long-term outcome result. Dig Dis Sci. 2011;56:1242–1244. doi: 10.1007/s10620-010-1372-5. [DOI] [PubMed] [Google Scholar]
- 32.Costamagna G., Pandolfi M., Mutignani M., Spada C., Perri V. Long-term results of endoscopic management of postoperative bile duct strictures with increasing numbers of stents. Gastrointest Endosc. 2001 Aug;54(2):162–168. doi: 10.1067/mge.2001.116876. [DOI] [PubMed] [Google Scholar]
- 33.Costamagna G., Tringali A., Mutignani M. Endotherapy of postoperative biliary strictures with multiple stents: results after more than 10 years of follow-up. Gastrointest Endosc. 2010 Sep;72(3):551–557. doi: 10.1016/j.gie.2010.04.052. [DOI] [PubMed] [Google Scholar]
- 34.Kim S., Chew F.S. Choledochal varices. Am J Roentgenol. 1988;150:578–580. doi: 10.2214/ajr.150.3.578. [DOI] [PubMed] [Google Scholar]
- 35.Dhiman R.K., Puri P., Chawla Y. Biliary changes in extrahepatic portal venous obstruction: compression by collaterals or ischemic? Gastrointest Endosc. 1999;50:646–652. doi: 10.1016/s0016-5107(99)80013-3. [DOI] [PubMed] [Google Scholar]
- 36.Dhiman R.K., Chhetri D., Behera A., Duseja A., Chawla Y., Singh P. Management of biliary obstruction in patients with portal hypertensive biliopathy (PHB) J Gastroenterol Hepatol. 2006;21:A505. [abstract] [Google Scholar]
- 37.Chattopadhyay S., Govindasamy M., Singla P. Portal biliopathy in patients with non-cirrhotic portal hypertension: does the type of surgery affect outcome? HPB (Oxford) 2012;14:441–447. doi: 10.1111/j.1477-2574.2012.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


