Abstract
In patients with portal cavernoma cholangiopathy (PCC), appearance and location of collateral channels depends on extent and location of occlusive thrombus in the porto-mesenteric venous system. If the porto-mesenteric venous system is occluded near the formation of portal vein, blood tends to flow through collateral channels that form varices in and around the common bile duct. Though endoscopic ultrasound (EUS) is considered the investigative modality of choice for evaluating common bile duct obstruction, its role in evaluating collateral pathways in and around the common bile duct is poorly defined. This article reviews the anatomy, genesis and appearance of these collateral pathways in PCC. EUS identifies different layers of the common bile duct (CBD) wall and, in PCC, where varices are in close contact with or part of these different layers, can establish the relationship between them. Thus, EUS appears to be the investigation of choice for tracing the origin and course of collaterals in PCC. Careful study of varices in the common bile duct wall prior to ERCP for bile duct stones or biliary strictures may help to plan the procedure and to manage anticipated complications such as hemobilia.
Keywords: endoscopic ultrasound, portal cavernoma cholangiopathy, common bile duct
Abbreviations: PCC, portal cavernoma cholangiopathy; EUS, endoscopic ultrasound; CBD, common bile duct; PCD, paracholedochal venous plexus; ECD, epicholedochal venous plexus; TIPSS, transjugular intrahepatic portosystemic shunt; CDUS, color Doppler US
Both porto-systemic and porto-portal collaterals can develop in response to chronic occlusion of the portal venous system. The location and extent of occlusive thrombus in the porto-mesenteric venous system determine the type of collaterals that eventually develop and the direction of collateral flow.1 Portal vein thrombosis may extend for a variable distance into the superior mesenteric vein and can involve or spare the drainage site of the pancreaticoduodenal venous tributaries. If portal vein thrombosis extends beyond the level of the first significant tributary communicating with the portal vein, mesenteric decompression predominantly occurs in a hepatofugal direction into gastroesophageal varices. However, if portal vein thrombosis spares the drainage of pancreaticoduodenal venous tributaries, mesenteric decompression provides hepatopetal portal flow through pancreaticoduodenal veins (Figure 1a and b).2
Figure 1.
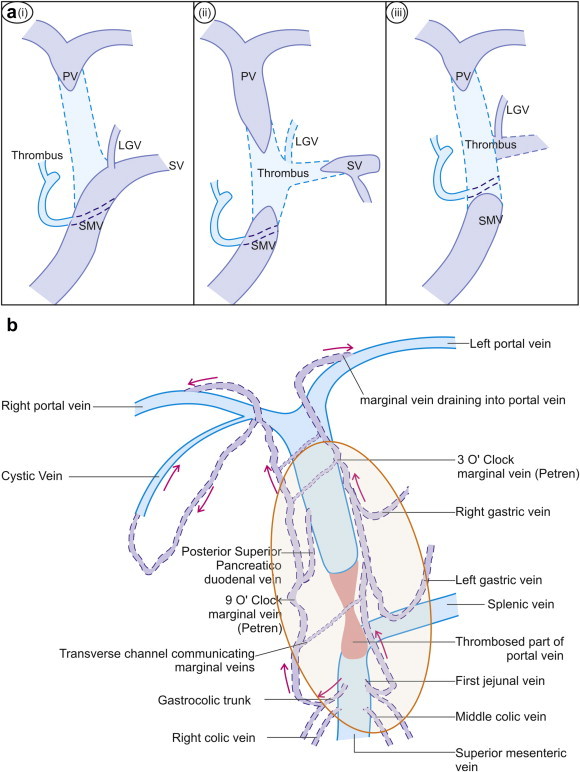
a: The extent of thrombosis in the porto mesenteric axis may decide the formation of collaterals. Generally the patency of pancreaticoduodenal veins is required for formation of portal cavernoma as shown in figure (a, i & ii). However, the patency of left gastric vein generally decides the presence or absence of associated collaterals going towards the esophagogastric junction (a, i & ii). If the origin of pancreaticoduodenal veins is obliterated by the presence of thrombus the chances of development of collaterals are less likely (a, iii). b: The paracholedochal plexus consists of 3 o'clock and 9 o'clock marginal veins lying parallel to the CBD, which are interconnected with transverse channels. Near the hilum these marginal veins enter into branches of portal vein. The marginal veins are connected to right gastric vein, cystic vein, left gastric vein, posterior superior pancreaticoduodenal vein, gastrocolic trunk, 1st jejunal vein, and occasionally to 2nd order tributaries of superior mesenteric vein.
Hepatopetal flow through porto-portal collaterals can obstruct the common bile duct (CBD) by forming huge varices that is, a portal cavernoma, around the biliary tree. Portal cavernoma cholangiopathy (PCC) refers to abnormalities of the biliary tract in such patients.3 Pancreaticoduodenal venous flow which comes via tributaries of the portal vein from the head of pancreas, duodenum and stomach is drained by two groups of veins near the CBD, the paracholedochal venous plexus (PCD) of Petren, also called the parabiliary venous plexus, and the epicholedochal venous plexus (ECD) of Saint, also called the peribiliary venous plexus.4–6 The paracholedochal venous plexus of Petren probably represents obliterated right umbilical vein and apparently has an embryological origin independent of the portal vein and is related to the accessory portal venous system.7
Normally, venous drainage of CBD is bidirectional. The paracholedochal plexus comprises of two veins, the 3 o'clock and 9 o'clock marginal veins, that run parallel to the CBD in the hepatoduodenal ligament, but not in contact with the CBD wall (Figure 1), and are interconnected with multiple large transverse channels.8–11 Near the hilum, these marginal veins can enter the hepatic substance, join the right and left branches of portal vein or join the hilar venous plexus. Just below the hilum, the marginal veins are connected to the patent part of portal vein and its tributary, the right gastric vein. The cystic vein always joins the 9 o'clock marginal vein and the right gastric vein always connects with the 3 o'clock marginal vein.10,11 Inferiorly the marginal veins and the venous plexus are connected to the patent part of portal vein through its 1st order tributaries, the left gastric and posterior superior pancreaticoduodenal veins, to the patent part of superior mesenteric vein through its 1st order tributaries, the gastrocolic trunk, 1st jejunal and right colic veins, or occasionally through 2nd order tributaries (Figure 1, Video 1).1,4,6
Supplementary video related to this article can be found at http://dx.doi.org/10.1016/j.jceh.2013.08.015.
The following is the supplementary video related to this article:
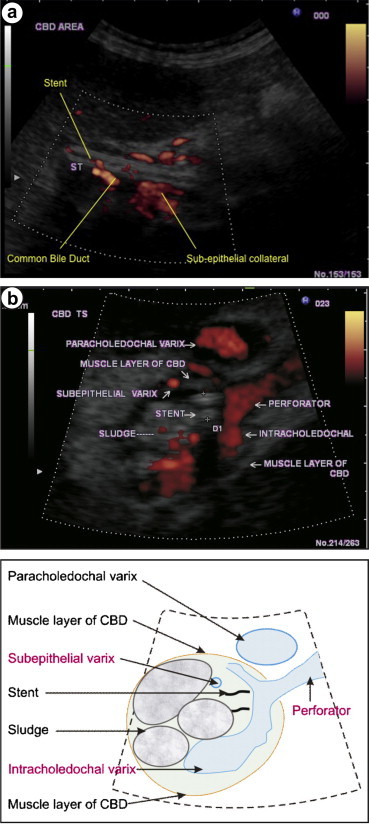
In a normal person the paracholedochal plexus is made of two parallel veins on left and right side of CBD. The collaterals formed from PCD plexus can be called as paracholedochal varices till they lie away from the fibro muscular layer and pericholedochal varices if they lie adjacent to the fibro muscular layer. The para and pericholedochal collaterals can perforate the muscular layer of the CBD and act as a link between veins outside the muscular wall of the CBD and those inside the muscular wall. These Perforators can either run in the subepithelial layer of the CBD as subepithelial varices or lie more freely inside the CBD as intracholedochal varices when they bulge into CBD lumen.
The ECD venous plexus is a continuation of vessels from the ECD arterial plexus, which is supplied by the marginal arteries and consists of a mesh of vessels about 1 mm in diameter. Normally, the ECD venous plexus is an extrinsic plexus, which lies in intimate contact with the outer aspect of fibro muscular layer of the CBD wall and extends cephalad on both right and left hepatic ducts.3 The ECD venous plexus blood flow predominantly goes into the hilar plexus, which is a network around the confluence of the right and left hepatic ducts. The hilar venous plexus can drain into marginal veins of PCD venous plexus, communicate with the veins of caudate lobe and segment IV or drain into sinusoids (Figure 2).8,9 The hilar plexus can also directly drain into nearby branches of portal vein via peribiliary venules (Figure 2).12 Scanning electron microscopy of corrosion casts of the microvascular arrangement of CBD wall in animals and humans has provided evidence for the presence of two more intrinsic vascular plexuses: the subepithelial and intramural venous plexuses which are connected to the ECD and PCD venous plexuses (Figure 3).
Figure 2.
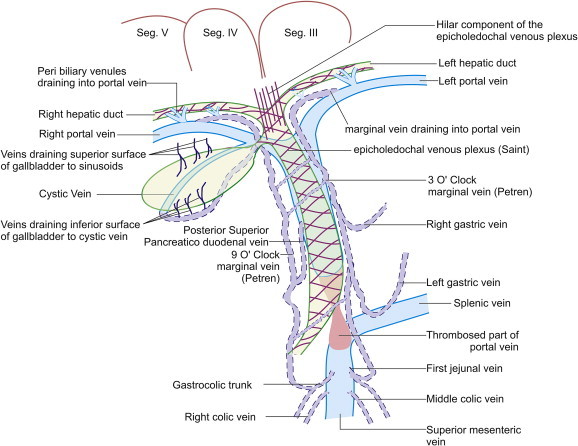
The ECD venous plexus is a mesh of vessels of about 1 mm in diameter which lies in intimate contact with the outer aspect common bile duct wall and extends into hilar plexus and to the right and left hepatic ducts. The hilar venous plexus can drain into marginal veins, communicate with the veins of caudate lobe and segment IV or drain into portal vein via peribiliary venules.
Figure 3.
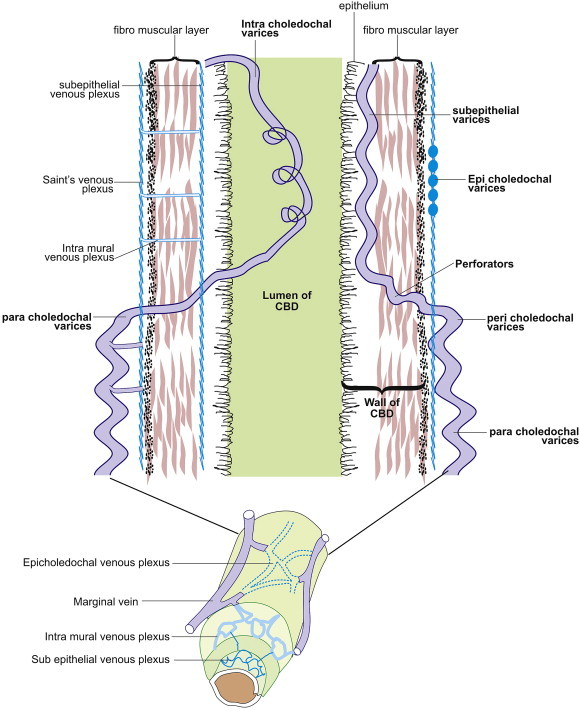
CBD wall has three layers: fibro muscular layer, subepithelial layer and epithelium. Paracholedochal varices lie away from the fibro muscular layer. Pericholedochal varices lie adjacent to the fibro muscular layer. Perforators go through the muscular layer of the CBD and are connected to subepithelial varices beneath the epithelium or intracholedochal varices inside the CBD. The epicholedochal varices are present mainly on the surface of CBD.
Mapping of individual feeding and draining veins to PCD venous plexuses has not been done in PCC. In a recent series, the first large collateral vein that provided pancreaticoduodenal venous inflow in PCC was the gastrocolic trunk and the posterior superior pancreaticoduodenal vein was the important outflow collateral of the patent part of portal vein above the block.2 Posterior superior pancreaticoduodenal vein, gastrocolic trunk, right gastric vein and cystic veins have been shown as either inflowing or outflowing channels to the PCD venous plexuses or marginal veins. Mori et al13 have suggested that the posterior superior pancreaticoduodenal vein is an important hepatopetal collateral. Denys et al14 showed that cystic veins in the gallbladder wall act as a collateral pathway. Couinaud6 also found that cystic vein was an important collateral pathway in 41% cases and veins from inferior surface of gallbladder drain into the cystic vein which then drains into 9 o'clock marginal vein.
Classifying collaterals
The term ‘choledochal varices’ is used in a broad sense for all varices around CBD and the term ‘intrapancreatic venous collaterals’ for all varices around the intrapancreatic part of CBD. When portal vein thrombosis extends inferiorly into the superior mesenteric vein, peri- and intra-pancreatic venous collaterals can form around the intrapancreatic or retropancreatic part of CBD depending on the location of the block. The distal bile duct lies either in a groove along the back of pancreatic head or outside the parenchyma in a retropancreatic (18%) or intrapancreatic (82%) location. Intrapancreatic venous collaterals can mimic cystic pancreatic neoplasm but use of color Doppler allows differentiation from tumor (Figure 4a and b, Video 2). The presence of intrapancreatic venous collaterals may be responsible for inducing compressive/ischemic changes in some cases of pancreatic ductopathy. Use of the term “portal double ductopathy” has been suggested to describe involvement of both systems.15 PCD collaterals can compress the suprapancreatic part of CBD where it lies freely in the hepatoduodenal ligament near the anterior margin of foramen of Winslow.
Figure 4.
a & b: Sometimes peri and intrapancreatic venous collaterals can form around the intrapancreatic part of CBD and they can mimic cystic pancreatic neoplasm but use of color Doppler allows differentiation from tumor. In this case use of color Doppler shows the presence of bile duct hidden between the collaterals. The collaterals cause pericholedochal extrinsic compression over the CBD wall but the LFT were normal. This case suggests asymptomatic portal double ductopathy with normal LFT and is predisposed to cholangiopathy (video).
Supplementary video related to this article can be found at http://dx.doi.org/10.1016/j.jceh.2013.08.015.
The following is the supplementary video related to this article:
The intrapancreatic venous collaterals can mimic cystic pancreatic neoplasm but use of color Doppler allows differentiation from tumor.
Initial attempts to classify the varices around CBD were on the basis of location on or outside the wall. Varices in the CBD wall were called CBD wall varices (probably related to intramural ECD collaterals) and varices outside the CBD wall were called PCD collaterals (probably related to PCD venous plexus).6,7 However if the CBD wall is taken as a landmark for differentiation of varices in a manner analogous to the gut wall, varices can be classified in relation to three layers of CBD wall, namely fibro muscular layer, subepithelial layer and epithelium lining the CBD lumen.1 Collaterals formed from PCD plexus can be called paracholedochal varices when they lie away from the fibro muscular layer and pericholedochal varices if they lie adjacent to the fibro muscular layer. The para- and peri-choledochal collaterals can perforate the muscular layer of the CBD and link veins outside the muscular wall of the CBD with those inside this wall. These perforating vessels or perforators can either run in the subepithelial layer of the CBD as subepithelial varices or lie more freely inside the CBD as intracholedochal varices when they bulge into the CBD lumen.16 Varices in the subepithelial layer without a tumorous bulge may cause luminal narrowing or make the wall irregular but once they prolapse into the lumen they are more mobile and can be mistaken for a stone. Varices in a subepithelial location carry the risk of shearing during removal but once they are free to move they are at risk of getting squeezed as well as of getting caught during ERCP. These prolapsing intracholedochal varices are still covered with the epithelium of CBD and they can be considered analogous to hematocystic spot or red sign on an esophageal varix. Subepithelial varices do not originate from the epicholedochal varices, which are present mainly on the surface of CBD, and probably represent overflow and congestion because of diversion of blood from the PCD venous plexus to the ECD venous plexus. Suggested EUS criteria for identifying paracholedochal, pericholedochal, epicholedochal, intracholedochal and subepithelial varices and perforators are given below (Figure 3).
-
(a)
Paracholedochal: varices at a distance from the fibro muscular layer.
-
(b)
Pericholedochal: large varices (>1 mm) which lie outside and adjacent to the fibro muscular layer. The course of these varices can often be followed by real time color Doppler EUS.
-
(c)
Epicholedochal: small varices (<1 mm) which lie outside and adjacent to the fibro muscular layer.
-
(d)
Perforators: a varix clearly going through the fibro muscular layer of CBD wall with possible narrowing at the point of entry or exit through the wall.
-
(e)Intracholedochal:
-
i.Varices at <1 mm distance from stent or stone.
-
ii.Varices with CBD wall on both sides.
-
iii.Varices in lumen after perforating CBD wall when followed by real time color Doppler EUS.
-
i.
-
(f)
Subepithelial varices: varix clearly demonstrated in the middle of CBD wall.
Role of imaging for different type of collaterals
Detailed discussion of MRCP, CT and ERCP finding is beyond the scope of this review and only a few important points are highlighted. A comparative evaluation of EUS, ERCP and MRI in PCC has not been done till date. EUS is operator dependent and changes on EUS are difficult to interpret, MRI may not be widely available and ERCP may not always be required. Further, findings in PCC may change over time and, unless examination with all three modalities is done simultaneously, comparison may not be possible.
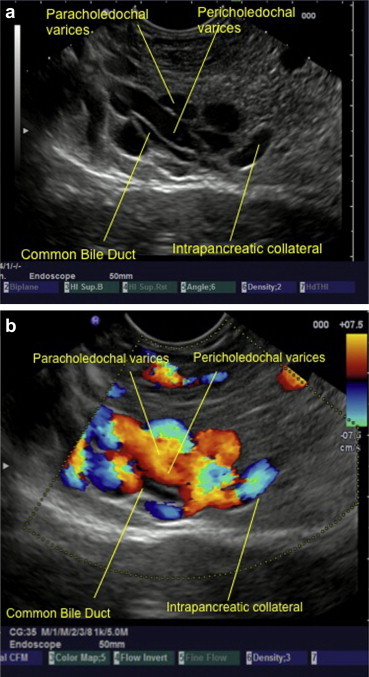
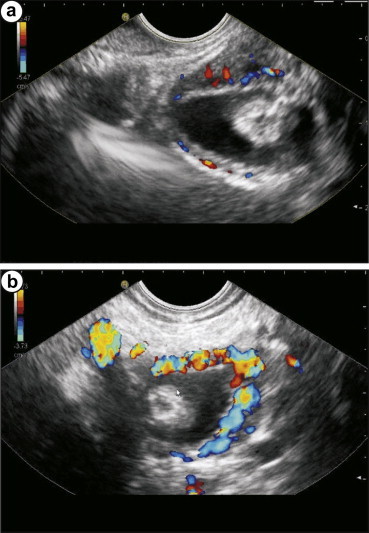
Dilated paracholedochal collaterals may cause extrinsic compression and protrusion into the thin and pliable CBD, resulting in scalloped or smooth indentations on the duct wall that are best demonstrated on ERCP or MRCP images. Dilated ECD collaterals may make the normally smooth intraluminal surface of the CBD irregular.8 On MRI, PCD collaterals and ECD collaterals are identified separately.17 PCD collaterals and gallbladder varices appear as low signal intensity channels on T2-weighted images and as enhancing tortuous collaterals on dynamic 3D gradient-echo images. ECD collaterals appear as dot like enhancing structures in the bile duct wall.17,18 Ultrasonography has many shortcomings. High level of echoes in the porta hepatis may obscure the biliary system and CBD may be hidden behind multiple collaterals, seen as anechoic tubular and fibrotic structures.19 The use of ultrasonography and EUS detects large PCD and ECD collaterals while smaller collaterals have been detected by color Doppler US (CDUS) (Figure 5a, b & Video 3), color Doppler EUS and intraductal EUS.18,20,21 On EUS biliary varices are defined as multiple, large, serpiginous, anechoic vascular channels in and/or around the extrahepatic biliary tract20 (Figure 6a and b). Intraductal sonography of biliary varices associated with EHPVO has shown presence of varices in mid and lower part of CBD.22 MRCP and EUS have been recommended to assess collaterals in symptomatic PCC with obstructive jaundice before ERCP.17,23
Figure 5.
a & b Ultrasonography may obscure the biliary system and CBD may be hidden behind multiple collaterals .The use of color Doppler ultrasound is able to detect large PCD and ECD collaterals and perforators which come to lie inside the CBD (video).
Figure 6.
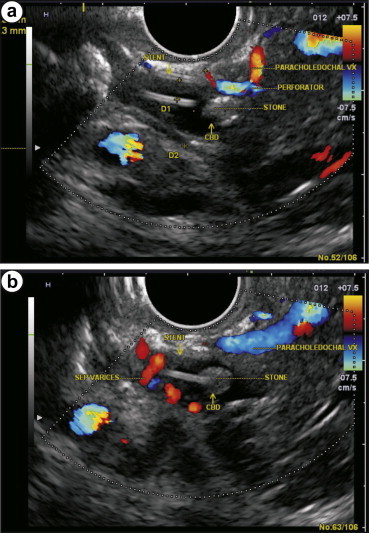
a & b On endoscopic ultrasound (EUS) biliary varices are defined as multiple, large, serpiginous, anechoic vascular channels in and/or surrounding the extrahepatic biliary tracts. In this image the presence of stent near the stone allows detection of varices inside the CBD (a and b).
Supplementary video related to this article can be found at http://dx.doi.org/10.1016/j.jceh.2013.08.015.
The following is the supplementary video related to this article:
Sometimes the use of color Doppler abdominal ultrasound is able to detect large PCD and ECD collaterals.
Endoscopic sphincterotomy has been shown to be a safe procedure in extrahepatic obstruction of the portal vein but hemobilia has been reported during stone or stent removal or stricture dilatation.24,25 Bleeding associated with stone removal or stricture dilatation during ERCP in PCC has been assumed to be due to PCD collaterals causing varicoid biliary abnormality seen during ERCP and MRCP.17 During stent removal tearing of adhesions between the stent and the biliary mucosa has been suggested as the cause of hemobilia.25 Since PCD collaterals are extracholedochal, it is logical to look for intracholedochal and subepithelial varices as an explanation for bleeding during removal of CBD stone in PCC (Figure 7a and b, Video 4 Figure 8 & Video 5 & Figure 9). Balloon sweeps during stone removal may be detrimental in PCC, especially at the lower end, as intracholedochal varices first get squeezed by the inflated balloon and then rupture at the lower end when the balloon is pulled out into duodenum.26 Hemobilia occurs as intracholedochal varices and subepithelial varices rupture because of the shearing effect of the balloon, basket or stent.26
Figure 7.
a & b (video) The para and pericholedochal collaterals can perforate the muscular layer of the CBD and these perforators can either run in the subepithelial layer of the CBD as subepithelial varices. The subepithelial varices should not be confused with the epicholedochal varices, which are present mainly on the surface of CBD.
Figure 8.

Contrast enhanced color Doppler ultrasound shows CBD with subepithelial varices (video).
Figure 9.
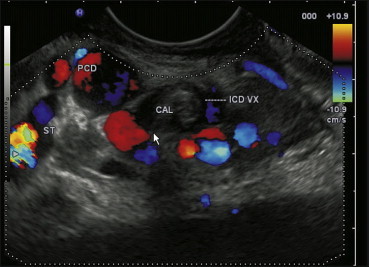
Paracholedochal varices are seen which can be followed via perforators to a location inside the CBD as intracholedochal collaterals. A stone is also seen.
Supplementary video related to this article can be found at http://dx.doi.org/10.1016/j.jceh.2013.08.015.
The following is the supplementary video related to this article:
The Para Choledochal collaterals are present in an extracholedochal location. These varices can perforate through the fibromuscular layer of common bile duct and appear as intracholedochal and subepithelial varices.
The following is the supplementary video related to this article:
The use of contrast during EUS and ultrasound may be able to show the presence of subepithelial varices much better.
Place for endoscopic ultrasound in management of portal cavernoma cholangiopathy
In patients with a clinical picture of biliary tract obstruction, EUS with Doppler should be performed to determine whether obstruction is due to bile duct varices, stones, strictures, or a tumor.19 Identification of choledochal varices as filling defects by EUS may be easy if an endoscopic sphincterotomy has not been done; after sphincterotomy air may create confusion (Table 1). Patients with asymptomatic PCC, who have radiological evidence of compression of CBD can be followed up clinically.27 Comparative evaluation of imaging modalities is given in Table 1.
Table 1.
A Comparative Evaluation of Different Modalities of Imaging for Portal Cavernoma Cholangiopathy.
| Sr. No. | Collaterals | EUS | MRCP | ERCP |
|---|---|---|---|---|
| 1. | Paracholedochal | Well demonstrated away from the surface of CBD. The course of collateral may be followed by real-time color Doppler endoscopic ultrasound | Well demonstrated as low signal intensity on T2 weighted images or as tortuous collaterals on dynamic 3D gradient-echo images but differentiation from peri-choledochal collaterals is not possible | Scalloped or smooth indentiation has been suggested to be due to the presence of para/peri-choledochal collaterals near the CBD wall |
| 2. | Pericholedochal | Well demonstrated near the surface of CBD | ||
| 3. | Epicholedochal | Difficult to differentiate from pericholedochal collaterals | Demonstrated on MRCP as dot like enhancing structures | Not demonstrated |
| 4. | Perforators | Well demonstrated and may be followed up from a para/pericholedochal location to sub-epithelial/intra-choledochal location at the position of perforation through the wall layer of CBD | Not demonstrated on MRCP | Not demonstrated on ERCP |
| 5. | Sub-epithelial | Well demonstrated, may be sheared during ERCP | Demonstrated but differentiation between intra-choledochal & peri-choledochal collaterals is difficult | Demonstrated as irregularities along the wall of CBD |
| 6. | Intra-choledochal | Well demonstrated as filling defect and may be mistaken for stones. Can be caught by basket or squeezed by balloon | Demonstrated but differentiation between intra-choledochal & peri-choledochal collaterals is difficult | Demonstrated as filling defects in the lumen of CBD which can be mobile for a limited extent during manipulation in ERCP |
ERCP may be terminated or postponed because of excessive bleeding, though pharmacotherapy with terlipressin has been shown to be effective.24 Intracholedochal varices and perforators provide a satisfactory explanation for hemobilia after sphincterotomy and stone extraction by balloon or basket.28,29 For half a century, controlled hypotension with various drugs has been used to reduce bleeding and the need for blood transfusions in different types of surgery.30 Lowering portal pressure with Transjugular Intrahepatic Portosystemic Shunt (TIPSS)28 has been shown to result in disappearance of the pseudocholangiocarcinoma sign on ERCP, suggesting that intracholedochal varices can disappear at low intravariceal pressures. Suppressing intracholedochal varices with controlled hypotension has allowed successful completion of biliary procedures.31 Thus, a role for EUS is proposed before proceeding to therapeutic ERCP in PCC (Figure 10). EUS offers crucial information which cannot be obtained otherwise. It pinpoints the precise location of the varices, distinguishes intracholedochal varices from stones and sludge, differentiates between fibrotic strictures from collateral compression as the cause of ductal narrowing and helps in detecting malignancy masquerading as PCC.
Figure 10.
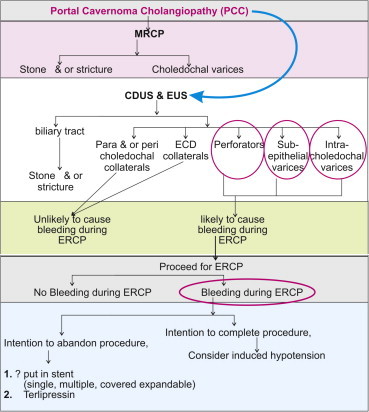
The possible role of EUS in portal cavernoma cholangiopathy.
Conclusion
ERCP remains the gold standard for imaging PCC, though noninvasive MRC is replacing ERCP as the diagnostic procedure of choice. EUS is likely to have a place in the algorithm for management of symptomatic PCC for detailed evaluation at the time of diagnosis of PCC and before ERC. It appears to be the investigation of choice in tracing the origin, caliber, entry, and course of varices outside and through the CBD. Layer-wise localization of varices is best done by color Doppler EUS and is important because subepithelial and intracholedochal varices can cause hemobilia during ERCP. Intracholedochal varices and probably even subepithelial varices can temporarily disappear during controlled hypotension with nitroglycerin infusion which may allow completion of therapeutic ERCP in PCC.31,32
Conflicts of interest
All authors have none to declare.
References
- 1.Sharma M., Rameshbabu C.S. Collateral pathways in portal hypertension. J Clin Exp Hepatol. 2012;2:338–352. doi: 10.1016/j.jceh.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walser E.M., Runyan B.R., Heckman M.G. Extrahepatic portal biliopathy: proposed etiology on the basis of anatomic and clinical features. Radiology. 2011;258:146–153. doi: 10.1148/radiol.10090923. [DOI] [PubMed] [Google Scholar]
- 3.Dhiman R.K., Behera A., Chawla Y.K., Dilawari J.B., Suri S. Portal hypertensive biliopathy. Gut. 2007;56:1001–1008. doi: 10.1136/gut.2006.103606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saint J.H. The epicholedochal venous plexus and its importance as a means of identifying the common duct during operation on extrahepatic biliary tract. Br J Surg. 1961;48:489–498. doi: 10.1002/bjs.18004821104. [DOI] [PubMed] [Google Scholar]
- 5.Petren T. The veins of the extrahepatic biliary system and their pathologic anatomic significance. Vert Anat Ges. 1932;41:139–143. [Google Scholar]
- 6.Couinaud C. The parabiliary venous system. Surg Radiol Anat. 1988;10:311–316. doi: 10.1007/BF02107904. [DOI] [PubMed] [Google Scholar]
- 7.Novellas S., Chevallier P., Peroux J.L., Bruneton J.N. Rare localization of a portal cavernoma in the wall of the common bile duct. Clin Imaging. 2004;28:132–134. doi: 10.1016/S0899-7071(04)00002-6. [DOI] [PubMed] [Google Scholar]
- 8.Vellar I.D. Preliminary study of the anatomy of venous drainage of the intrahepatic and extrahepatic bile ducts and its relevance to the practice of hepatobiliary surgery. ANZ J Surg. 2001;71:418–422. doi: 10.1046/j.1440-1622.2001.02150.x. [DOI] [PubMed] [Google Scholar]
- 9.Northover J.M., Terblanche J. A new look at the arterial supply of the bile duct in man and its surgical implications. Br J Surg. 1979;66:379–384. doi: 10.1002/bjs.1800660603. [DOI] [PubMed] [Google Scholar]
- 10.Skandalakis J.E. vol. II. PMP; Athens: 2004. pp. 1095–1150. (Surgical Anatomy: the Embryologic and Anatomic Basis of Modern Surgery). [Google Scholar]
- 11.Deltenre P., Valla D.C. Ischemic cholangiopathy. Semin Liver Dis. 2008;28:235–246. doi: 10.1055/s-0028-1085092. [DOI] [PubMed] [Google Scholar]
- 12.Cho K.J., Lunderquist A. The peribiliary vascular plexus: the microvascular architecture of the bile duct in the rabbit and in clinical cases. Radiology. 1983;147:357–364. doi: 10.1148/radiology.147.2.6836115. [DOI] [PubMed] [Google Scholar]
- 13.Mori H., Miyake H., Aikawa H. Dilated posterior superior pancreaticoduodenal vein: recognition with CT and clinical significance in patients with pancreaticobiliary carcinomas. Radiology. 1991;181:793–800. doi: 10.1148/radiology.181.3.1947099. [DOI] [PubMed] [Google Scholar]
- 14.Denys A., Hélénon O., Lafortune M. Thickening of the wall of the bile duct due to intramural collaterals in three patients with portal vein thrombosis. AJR. 1998;171:455–456. doi: 10.2214/ajr.171.2.9694474. [DOI] [PubMed] [Google Scholar]
- 15.Bayraktar Y. Portal ductopathy: clinical importance and nomenclature. World J Gastroenterol. 2011;17:1410–1415. doi: 10.3748/wjg.v17.i11.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma M., Pathak A. Intracholedochal varices in portal hypertensive biliopathy. Eur J Radiol Extra. 2009;72:e119–e123. [Google Scholar]
- 17.Ozkavukcu E., Erden A., Erden I. Imaging features of portal biliopathy: frequency of involvement patterns with emphasis on MRCP. Eur J Radiol. 2009;71:129–134. doi: 10.1016/j.ejrad.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Umphress J.L., Pecha R.E., Urayama S. Biliary stricture caused by portal biliopathy diagnosis by EUS with Doppler US. Gastrointest Endosc. 2004;60:1021–1024. doi: 10.1016/s0016-5107(04)02216-3. [DOI] [PubMed] [Google Scholar]
- 19.Harmanci O., Bayraktar Y. How can portal vein cavernous transformation cause chronic incomplete biliary obstruction? World J Gastroenterol. 2012;18:3375–3378. doi: 10.3748/wjg.v18.i26.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palazzo L., Hochain P., Helmer C. Biliary varices on endoscopic ultrasonography. Endoscopy. 2000;32:520–524. doi: 10.1055/s-2000-9009. [DOI] [PubMed] [Google Scholar]
- 21.Ikeura T., Matsushita M., Sakao M. Characteristic intraductal ultrasonographic features of portal biliopathy. Dig Endosc. 2008;20:213–216. [Google Scholar]
- 22.Takamatsu M., Furutake M., Hisa T., Ueda M. Obstructive jaundice caused by a portal cavernoma. Jpn J Radiol. 2010;28:754–758. doi: 10.1007/s11604-010-0480-7. [DOI] [PubMed] [Google Scholar]
- 23.Chevallier P., Denys A., Novellas S., Schmidt S., Schnyder P., Bruneton J.N. Magnetic resonance cholangiography features of biliary abnormalities due to cavernous transformation of the portal vein. Clin Imaging. 2006;30:190–194. doi: 10.1016/j.clinimag.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 24.Layec S., D'Halluin P.N., Pagenault M., Bretagne J.F. Massive hemobilia during extraction of a covered self-expandable metal stent in a patient with portal hypertensive biliopathy. Gastrointest Endosc. 2009;70:555–556. doi: 10.1016/j.gie.2009.04.051. [DOI] [PubMed] [Google Scholar]
- 25.Mutignani M., Shah S.K., Bruni A., Perri V., Costamagna G. Endoscopic treatment of extrahepatic bile duct strictures in patients with portal biliopathy carries a high risk of haemobilia: report of 3 cases. Dig Liver Dis. 2002;34:587–591. doi: 10.1016/s1590-8658(02)80093-7. [DOI] [PubMed] [Google Scholar]
- 26.Sharma M., Ponnusamy R.P. Is balloon sweeping detrimental in portal biliopathy? A report of 3 cases. Gastrointest Endosc. 2009;70:171–173. doi: 10.1016/j.gie.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Shindoh J., Hasegawa K., Kokudo N. Asymptomatic dilatation of the intrahepatic biliary tree due to thrombosed pericholedochal varices (portal biliopathy) Clin Gastroenterol Hepatol. 2011;9:e14–e15. doi: 10.1016/j.cgh.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 28.Görgül A., Kayhan B., Dogan I., Unal S. Disappearance of pseudocholangiocarcinoma sign after TIPPS. Am J Gastroenterol. 1996;91:150–154. [PubMed] [Google Scholar]
- 29.Sharma M., Pathak A. Perforators of common bile duct wall in portal hypertensive biliopathy (with videos) Gastrointest Endosc. 2009;70:1041–1043. doi: 10.1016/j.gie.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 30.Degoute C.S. Controlled hypotension: a guide to drug choice. Drugs. 2007;67(7):1053–1076. doi: 10.2165/00003495-200767070-00007. [DOI] [PubMed] [Google Scholar]
- 31.Sharma M., Babu C.S., Dhiman R.K., Chawla Y. Induced hypotension in the management of acute hemobilia during therapeutic ERCP in a patient with portal biliopathy (with videos) Gastrointest Endosc. 2010;72:1317–1319. doi: 10.1016/j.gie.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 32.Sharma M., Verma S. Nitroglycerin induced hypotension in management of bleeding due to endoscopic sphincterotomy. Gut. 2004;53(suppl VI):A158. [abstract] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In a normal person the paracholedochal plexus is made of two parallel veins on left and right side of CBD. The collaterals formed from PCD plexus can be called as paracholedochal varices till they lie away from the fibro muscular layer and pericholedochal varices if they lie adjacent to the fibro muscular layer. The para and pericholedochal collaterals can perforate the muscular layer of the CBD and act as a link between veins outside the muscular wall of the CBD and those inside the muscular wall. These Perforators can either run in the subepithelial layer of the CBD as subepithelial varices or lie more freely inside the CBD as intracholedochal varices when they bulge into CBD lumen.
The intrapancreatic venous collaterals can mimic cystic pancreatic neoplasm but use of color Doppler allows differentiation from tumor.
Sometimes the use of color Doppler abdominal ultrasound is able to detect large PCD and ECD collaterals.
The Para Choledochal collaterals are present in an extracholedochal location. These varices can perforate through the fibromuscular layer of common bile duct and appear as intracholedochal and subepithelial varices.
The use of contrast during EUS and ultrasound may be able to show the presence of subepithelial varices much better.


