Abstract
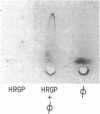
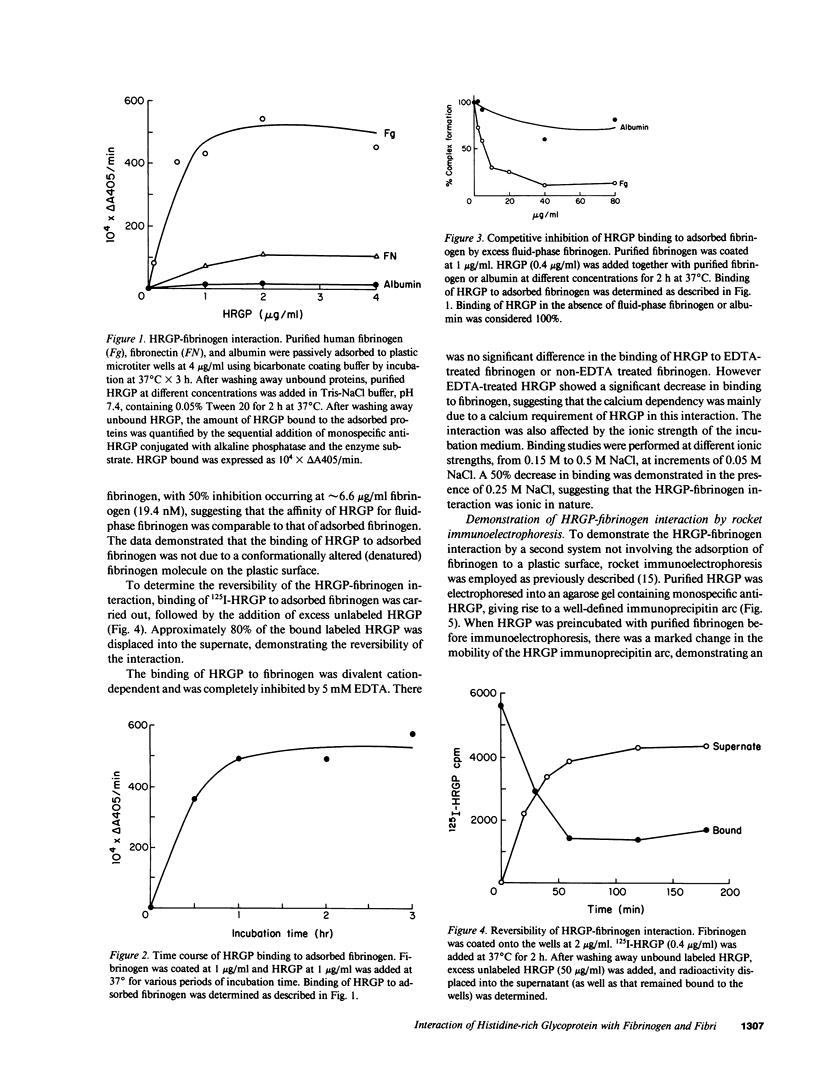
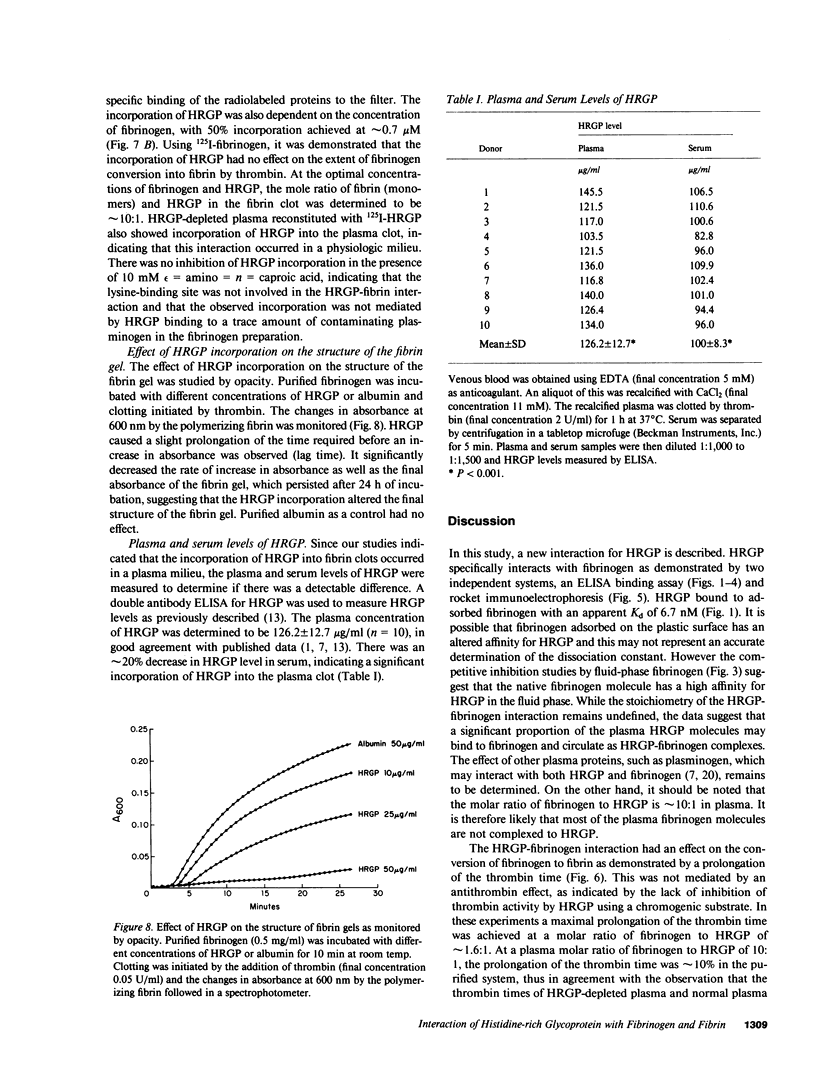
Histidine-rich glycoprotein (HRGP) is a human plasma and platelet protein of apparently diverse biological functions. In this study a new interaction for HRGP is described. HRGP specifically interacts with fibrinogen as demonstrated by two independent systems. Using an enzyme-linked immunosorbent assay it was demonstrated that HRGP bound to adsorbed fibrinogen in a concentration-dependent and saturable manner, with an apparent dissociation constant (Kd) of 6.7 nM. The binding was specific, reversible, and not mediated by a conformationally altered adsorbed fibrinogen molecule. The interaction was divalent cation-dependent and ionic in nature. The HRGP-fibrinogen interaction was also demonstrated using rocket immunoelectrophoresis. The HRGP-fibrinogen interaction had an effect on the kinetics of conversion of fibrinogen to fibrin as demonstrated by a prolongation of the thrombin time. HRGP also became incorporated into fibrin clots in a concentration-dependent and saturable manner, with an apparent Kd of 0.25 microM. The incorporation of HRGP into fibrin clots occurred in a plasma milieu as demonstrated by the direct incorporation of radiolabeled HRGP into plasma clots and by a significant decrease in serum HRGP levels as compared with plasma levels. HRGP prolonged the lag time phase of fibrin gel formation, and decreased the rate of turbidity rise, as well as the final absorbance of fibrin gels. Since the extent of fibrin polymerization was not influenced by the presence of HRGP, these data suggest that fibrin is distributed over more, but thinner, fibrils in the presence of HRGP. In addition to its potential effect on fibrin polymerization, the HRGP-fibrin interaction may play a role in the cell-cell interactions of platelets and macrophages.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bale M. D., Westrick L. G., Mosher D. F. Incorporation of thrombospondin into fibrin clots. J Biol Chem. 1985 Jun 25;260(12):7502–7508. [PubMed] [Google Scholar]
- Carr M. E., Jr, Hermans J. Size and density of fibrin fibers from turbidity. Macromolecules. 1978 Jan-Feb;11(1):46–50. doi: 10.1021/ma60061a009. [DOI] [PubMed] [Google Scholar]
- Colvin R. B., Dvorak H. F. Fibrinogen/fibrin on the surface of macrophages: detection, distribution, binding requirements, and possible role in macrophage adherence phenomena. J Exp Med. 1975 Dec 1;142(6):1377–1390. doi: 10.1084/jem.142.6.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis C. G., Brown K. L., Credo R. B., Domanik R. A., Gray A., Stenberg P., Lorand L. Calcium-dependent unmasking of active center cysteine during activation of fibrin stabilizing factor. Biochemistry. 1974 Aug 27;13(18):3774–3780. doi: 10.1021/bi00715a024. [DOI] [PubMed] [Google Scholar]
- Garman A. J., Smith R. A. The binding of plasminogen to fibrin: evidence for plasminogen-bridging. Thromb Res. 1982 Aug 1;27(3):311–320. doi: 10.1016/0049-3848(82)90078-0. [DOI] [PubMed] [Google Scholar]
- Gonda S. R., Shainoff J. R. Adsorptive endocytosis of fibrin monomer by macrophages: evidence of a receptor for the amino terminus of the fibrin alpha chain. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4565–4569. doi: 10.1073/pnas.79.15.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantgan R. R., Hermans J. Assembly of fibrin. A light scattering study. J Biol Chem. 1979 Nov 25;254(22):11272–11281. [PubMed] [Google Scholar]
- Harpel P. C., Chang T. S., Verderber E. Tissue plasminogen activator and urokinase mediate the binding of Glu-plasminogen to plasma fibrin I. Evidence for new binding sites in plasmin-degraded fibrin I. J Biol Chem. 1985 Apr 10;260(7):4432–4440. [PubMed] [Google Scholar]
- Haupt H., Heimburger N. Humanserumproteine mit hoher Affinität zu Carboxymethylcellulose. I. Isolierung von Lysozym, C1q und bisher unbekannten -Globulinen. Hoppe Seylers Z Physiol Chem. 1972 Jul;353(7):1125–1132. [PubMed] [Google Scholar]
- Heimburger N., Haupt H., Kranz T., Baudner S. Humanserumproteine mit hoher Affinität zu Carboxymethylcellulose. II. Physikalisch-chemische und immunologische Charakterisierung eines histidinreichen 3,8S- 2 -Glykoproteins (CM-Protein I. Hoppe Seylers Z Physiol Chem. 1972 Jul;353(7):1133–1140. [PubMed] [Google Scholar]
- Hoylaerts M., Rijken D. C., Lijnen H. R., Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982 Mar 25;257(6):2912–2919. [PubMed] [Google Scholar]
- Ichinose A., Mimuro J., Koide T., Aoki N. Histidine-rich glycoprotein and alpha 2-plasmin inhibitor in inhibition of plasminogen binding to fibrin. Thromb Res. 1984 Feb 15;33(4):401–407. doi: 10.1016/0049-3848(84)90079-3. [DOI] [PubMed] [Google Scholar]
- Kaminski M., McDonagh J. Studies on the mechanism of thrombin. Interaction with fibrin. J Biol Chem. 1983 Sep 10;258(17):10530–10535. [PubMed] [Google Scholar]
- Leung L. L., Harpel P. C., Nachman R. L., Rabellino E. M. Histidine-rich glycoprotein is present in human platelets and is released following thrombin stimulation. Blood. 1983 Nov;62(5):1016–1021. [PubMed] [Google Scholar]
- Leung L. L., Nachman R. L. Complex formation of platelet thrombospondin with fibrinogen. J Clin Invest. 1982 Sep;70(3):542–549. doi: 10.1172/JCI110646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung L. L., Nachman R. L., Harpel P. C. Complex formation of platelet thrombospondin with histidine-rich glycoprotein. J Clin Invest. 1984 Jan;73(1):5–12. doi: 10.1172/JCI111206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijnen H. R., DeCock F., Collen D. Turnover of human histidine-rich glycoprotein in healthy subjects and during thrombolytic therapy. Thromb Res. 1981 Jul 1;23(1-2):121–131. doi: 10.1016/0049-3848(81)90245-0. [DOI] [PubMed] [Google Scholar]
- Lijnen H. R., Hoylaerts M., Collen D. Heparin binding properties of human histidine-rich glycoprotein. Mechanism and role in the neutralization of heparin in plasma. J Biol Chem. 1983 Mar 25;258(6):3803–3808. [PubMed] [Google Scholar]
- Lijnen H. R., Hoylaerts M., Collen D. Isolation and characterization of a human plasma protein with affinity for the lysine binding sites in plasminogen. Role in the regulation of fibrinolysis and identification as histidine-rich glycoprotein. J Biol Chem. 1980 Nov 10;255(21):10214–10222. [PubMed] [Google Scholar]
- Lijnen H. R., Rylatt D. B., Collen D. Physicochemical, immunochemical and functional comparison of human histidine-rich glycoprotein and autorosette inhibition factor. Biochim Biophys Acta. 1983 Jan 12;742(1):109–115. doi: 10.1016/0167-4838(83)90365-5. [DOI] [PubMed] [Google Scholar]
- Lijnen H. R., Van Hoef B., Collen D. Histidine-rich glycoprotein modulates the anticoagulant activity of heparin in human plasma. Thromb Haemost. 1984 Apr 30;51(2):266–268. [PubMed] [Google Scholar]
- Lucas M. A., Fretto L. J., McKee P. A. The binding of human plasminogen to fibrin and fibrinogen. J Biol Chem. 1983 Apr 10;258(7):4249–4256. [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Morgan W. T. Human serum histidine-rich glycoprotein. I. Interactions with heme, metal ions and organic ligands. Biochim Biophys Acta. 1978 Aug 21;535(2):319–333. doi: 10.1016/0005-2795(78)90098-3. [DOI] [PubMed] [Google Scholar]
- Morgan W. T. Interactions of the histidine-rich glycoprotein of serum with metals. Biochemistry. 1981 Mar 3;20(5):1054–1061. doi: 10.1021/bi00508a002. [DOI] [PubMed] [Google Scholar]
- Morgan W. T. The histidine-rich glycoprotein of serum has a domain rich in histidine, proline, and glycine that binds heme and metals. Biochemistry. 1985 Mar 12;24(6):1496–1501. doi: 10.1021/bi00327a031. [DOI] [PubMed] [Google Scholar]
- Mosher D. F. Cross-linking of cold-insoluble globulin by fibrin-stabilizing factor. J Biol Chem. 1975 Aug 25;250(16):6614–6621. [PubMed] [Google Scholar]
- Niewiarowska J., Cierniewski C. S. Inhibitory effect of fibronectin on the fibrin formation. Thromb Res. 1982 Sep 1;27(5):611–618. doi: 10.1016/0049-3848(82)90308-5. [DOI] [PubMed] [Google Scholar]
- Sakata Y., Aoki N. Cross-linking of alpha 2-plasmin inhibitor to fibrin by fibrin-stabilizing factor. J Clin Invest. 1980 Feb;65(2):290–297. doi: 10.1172/JCI109671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata Y., Mimuro J., Aoki N. Differential binding of plasminogen to crosslinked and noncrosslinked fibrins: its significance in hemostatic defect in factor XIII deficiency. Blood. 1984 Jun;63(6):1393–1401. [PubMed] [Google Scholar]
- Sherman L. A., Lee J. Specific binding of soluble fibrin to macrophages. J Exp Med. 1977 Jan 1;145(1):76–85. doi: 10.1084/jem.145.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia D. Y., Rylatt D. B., Parish C. R. Anti-self receptors. V. Properties of a mouse serum factor that blocks autorosetting receptors on lymphocytes. Immunology. 1982 Feb;45(2):207–216. [PMC free article] [PubMed] [Google Scholar]
- Silverstein R. L., Leung L. L., Harpel P. C., Nachman R. L. Platelet thrombospondin forms a trimolecular complex with plasminogen and histidine-rich glycoprotein. J Clin Invest. 1985 Jun;75(6):2065–2073. doi: 10.1172/JCI111926. [DOI] [PMC free article] [PubMed] [Google Scholar]