Abstract
Background
To examine whether lifetime DSM-IV diagnosis of major depressive disorder (MDD), including age at onset and number of episodes, is associated with brain atrophy in older persons without dementia.
Methods
Within the population-based AGES-Reykjavik Study 4,354 persons (mean age 76±5 years, 58% women) without dementia had a 1.5Tesla brain MRI. Automated brain segmentation total and regional brain volumes were calculated. History of MDD, including age at onset and number of episodes, and MDD in the past 2 weeks was diagnosed according to DSM-IV criteria using the MINI International Neuropsychiatric Interview.
Results
Of the total sample, 4.5% reported a lifetime history of MDD; 1.5% had a current diagnosis of MDD (including 75% with a prior history of depression) and 3.0% had a past but no current diagnosis (remission). After adjusting for multiple covariates, compared to participants never depressed, those with current MDD (irrespective of past) had more global brain atrophy (B=−1.25%; 95%CI −2.05 to −0.44%), including more gray and white matter atrophy in most lobes as well as more atrophy of the hippocampus and thalamus. Participants with current, first onset, MDD also had more brain atrophy (B=−1.62%; 95%CI −3.30 to 0.05%), while those remitted did not (B=0.06%; 95%CI −0.54 to 0.66%).
Conclusion
In older persons without dementia, current MDD, irrespective of prior history, but not remitted MDD, was associated with widespread gray and white matter brain atrophy. Prospective studies should examine whether MDD is a consequence of or contributes to brain volume loss and development of dementia.
INTRODUCTION
Major depressive disorder (MDD) is a serious illness with severe impact on daily functioning. Because of its chronic course, associated impaired functioning and high health care costs MDD accounts for a considerable part of the population burden of disease (Mathers and Loncar 2006). Many studies in younger populations indicate that compared to healthy controls, patients with MDD have structural brain abnormalities on magnetic resonance images (MRI) (Campbell and MacQueen 2004, 2006; Geuze et al. 2005; Konarski et al. 2008; Koolschijn et al. 2009; Lorenzetti et al. 2009; Videbech and Ravnkilde 2004). Recent studies characterizing MDD in more detail suggest smaller volumes of brain regions may occur especially in patients with longer duration of depression, greater severity, and in those with repeated episodes (Konarski et al. 2008; Lorenzetti et al. 2009; McKinnon et al. 2009); some studies did not find an association between depression severity and brain volumes (Koolschijn et al. 2009; van Tol et al. 2010).
The majority of extant studies on MDD and structural brain changes have examined specific brain regions, in particular the hippocampus, and have been conducted in relatively young populations. Less is known of the association between MDD and brain volumes in older people(Konarski et al. 2008). Increased understanding of the relation between MDD and brain abnormalities in older people is important, because older people may be especially vulnerable to the adverse consequences of brain abnormalities, in particular adverse cognitive outcomes. Indeed, loss of total brain volume in older people is a risk factor for cognitive decline and dementia (Ikram et al. 2010; Jack et al. 2005), and older people with a history of depression may be at increased risk for Alzheimer’s disease (Byers and Yaffe 2011; Geerlings et al. 2008; Ownby et al. 2006). From this, it has been hypothesized that depression is a causal risk factor for AD {Byers, 2011 #291; Jorm, 2001 #1004}. However, because of the long preclinical phase of AD, the direction of association is still not well understood; several other hypotheses may explain the relation between depression, brain atrophy and dementia, including depression being a prodomal phase of dementia (Jorm 2001). Recently, results from two population-based studies with long follow-up periods showed that depressive symptoms increased the risk for dementia many years before dementia diagnosis (Saczynski et al. 2010) and that increasing number of depressive episodes increased dementia risk (Dotson et al. 2010), suggesting that depression may contribute to development of dementia. However, these studies did not have brain MRI and it is unclear whether brain volume loss was underlying these associations. In another population-based study, history of depressive symptoms increased risk for dementia, but this was not explained by smaller hippocampal or amygdalar volumes (Geerlings et al. 2008).
Clarification is needed for several issues related to depression and brain volumes in older populations. Because the majority of older people with MDD will also have a history of MDD, it is important to make a distinction between first onset and a history with early onset or multiple episodes so that the distinction can be made between late-life MDD resulting from, or contributing to, brain atrophy. Further, it is unclear whether MDD leads to smaller brain volume only in the acute state and reverses in remission (Ahdidan et al. 2011; Geerlings et al. 2012; Hsieh et al. 2002; MacQueen et al. 2008).
Previous studies that examined the association of MDD later in life with total brain volume had a case-control design and relatively small sample size (Ashtari et al. 1999; Ballmaier et al. 2004; Konarski et al. 2008; Kumar et al. 1998; Sheline et al. 1996). Relatively few studies examined this relationship in the general elderly population and these studies used depressive symptoms as opposed to a formal diagnosis of MDD to assess depression (Dotson et al. 2009; Geerlings et al. 2012; Goveas et al. 2011). To our knowledge there are no studies that had lifetime DSM-IV diagnoses of MDD and MRI measures on a population-based cohort of older people.
We investigate the associations of lifetime DSM-IV diagnoses of MDD with total brain volume on MRI in a large population-based study of older people without dementia. We hypothesize that if MDD contributes to brain atrophy, an early onset and repeated episodes of MDD will be associated with smaller brain volume, whereas if MDD is a sequale of brain atrophy, current MDD will be associated with smaller brain volume.
METHODS
Participants
Study participants are from the Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study, a population-based cohort study originating from the Reykjavik study, as fully described elsewhere (Harris et al. 2007). Briefly, from 2002 through 2006, 5764 persons, randomly chosen from survivors of the Reykjavik Study, were examined for the AGES-Reykjavik Study. As part of comprehensive assessments at the Reykjavik research center participants answered questionnaires and underwent clinical examinations, had blood drawn, cognitive testing, and brain MRI. The AGES-Reykjavik Study was approved by the Icelandic National Bioethics Committee (VSN: 00-063), the Icelandic Data Protection Authority, Iceland, and by the Institutional Review Board for the National Institute on Aging, National Institutes of Health, USA. Written informed consent was obtained from all participants.
MRI Protocol
All participants without contraindications were eligible for a brain MRI scan on a study-dedicated 1.5T Signa Twinspeed system (General Electric Medical Systems, Waukesha, WI). The image protocol included an axial T1-weighted 3-dimensional spoiled gradient echo sequence (time to echo (TE) 8ms; repetition time (TR) 21ms; flip angle (FA) 30°; field of view (FOV) 240mm; matrix 256×256; slice thickness 1.5mm); a fluid attenuated inversion recovery (FLAIR) sequence (TE 100ms; TR 8000ms; inversion time 2000ms; FA 90°; FOV 220mm; matrix 256×256); a proton density/T2-weighted fast spin echo (FSE) sequence (TE1 22ms; TE2 90ms; TR 3220ms; echo train length 8; FA 90°; FOV 220mm; matrix 256×256); and a T2*-weighted gradient echo type echo planar (GRE-EPI) sequence (TE 50ms; TR 3050ms; flip angle (FA) 90°; field of view (FOV) 220mm; matrix 256×256). The FLAIR, PD/T2 and T2* sequences were acquired with 3mm thick interleaved slices. All images were acquired to give full brain coverage and slices were angled parallel to the anterior commissure–posterior commissure line.
Brain segmentation
The intracranial volume (ICV) and the brain parenchyma compartments were segmented automatically with an AGES-Reykjavik Study modified algorithm described previously (Sigurdsson et al. 2012). The pipeline is based on a multispectral tissue segmentation method that estimated volumes for 4 tissue classes: gray and white matter regions, white matter lesions (WML) and cerebral spinal fluid (CSF). These 4 classes were summed to obtain total intracranial volume (ICV). Total brain volume was defined as the sum of gray matter, normal white matter, and WML volumes and was expressed relative to ICV as the brain parenchymal fraction, an indicator of global brain atrophy. Calculation of regional tissue volumes was based on a regional probabilistic atlas, created from a large sample of the AGES cohort (N=314), which was warped non-linearly to the T1–weighted images of each study participant.
Cerebral infarcts, identified by trained radiographers, were defined as defects in the brain parenchyma with associated hyperintensity on T2 and FLAIR images with a maximal diameter of at least 4mm. For infarcts in the cerebellum and brain stem or infarcts with cortical involvement, no size criterion was required.
Dementia diagnosis
Dementia ascertainment was a 3-step protocol as described previously (Harris et al. 2007). All participants were screened using the Mini-Mental State Examination (MMSE) (Folstein et al. 1975) and the Digit Symbol Substitution test. Those with positive screen results were administered a diagnostic battery of neuropsychological tests and, among them, those with positive screen results were examined by a neurologist and a proxy interview was administered regarding medical history, social, cognitive, and daily functioning changes of the participant. A consensus diagnosis, according to DSM-IV criteria (Association 1994), was made by a panel that included a geriatrician, neurologist, neuropsychologist, and neuroradiologist.
Diagnosis of major depressive disorder
The presence of major depressive disorder (MDD) in the preceding 2 weeks and in the past was assessed according to DSM-IV criteria (Association 1994) using the MINI International Neuropsychiatric Interview (Sheehan et al. 1998). The MINI was administered by 5 trained and standardized health professionals. To rule out possibly unreliable answers to history of depression questions, only participants with no diagnosis of dementia or a MMSE (Folstein et al. 1975) score of >21 were eligible to receive the MINI. In this sample, we applied the following screening criteria to identify persons who may have had past or current episodes of depression: if they had a score ≥6 on the 15-item Geriatric Depression Scale (Yesavage et al. 1982), or a GDS-15 score of 4 or 5 and a positive response to 3 out of 4 of the following anxiety questions “In the past month, have you felt anxious or frightened?”; “Were there times lately that you felt anxious?”; “Are there special situations that make you anxious?”; “Have you ever had attacks of fear or panic?”, or if they reported ever to have had a doctor diagnosis of depression, or if they reported ever to have used antidepressant medications, or if they used antidepressant medication at time of interview as assessed from medication bottles brought to the clinic.
To evaluate the screening properties of our algorithm, 358 consecutive non-demented participants (mean age 76 years, range 66–91 years) were evaluated with the MINI interview from June 2002 until May 2003. For current MDD the sensitivity and specificity were 100% and 64%, respectively; for past MDD sensitivity and specificity were 93% and 66%.
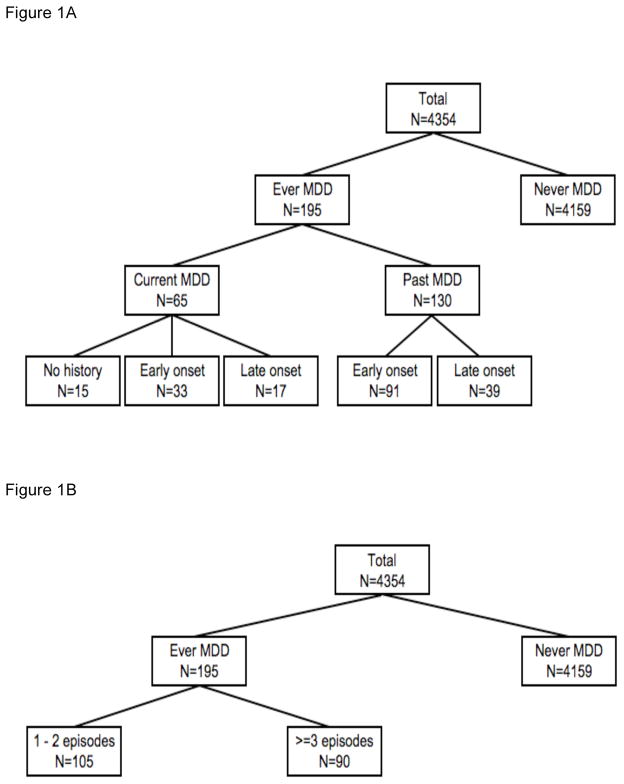
The MINI interview includes questions on age at onset of first MDD episode and number of episodes. In the analyses, participants were first classified as ever vs. never diagnosed with MDD. Next, the ‘ever MDD’ group was divided into past (and not current) MDD and current MDD (irrespective of past); into persons with an age of first onset before age 60 (early onset) and at 60 years or older (late onset); and into persons with 1–2 previous episodes and 3 or more episodes. Third, explorative analyses were performed by further subdividing the current and past MDD groups according to their history and age at onset (Figure 1).
Figure 1.
Figure 1A–B Definition of different depression groups.
Other variables
Age, sex, education (categorized into primary, secondary, and college/university education), smoking history (ever vs. never), current alcohol intake (yes vs. no), and subjective memory complaints (yes vs. no) were assessed via questionnaires. Body-mass index (BMI) was calculated as measured weight (kg) divided by height (m) squared. Systolic and diastolic blood pressure was measured with a standard mercury sphygmomanometer and the mean of two measurements was calculated. Diabetes mellitus was defined as self-reported doctor’s diagnosis of diabetes, use of blood glucose-lowering drugs, or fasting blood glucose level ≥7.0 mmol/L. History of stroke was based on a self-reported doctor’s diagnosis of stroke.
Analytical sample
Of the 5764 persons included, 4614 participants had complete data after postprocessing for brain volume analysis (Sigurdsson et al. 2012). The majority of the 1150 persons without successful brain segmentation did not have an MRI (e.g. contraindications, refusal, scheduling conflicts, home visit), or the MRI had artifacts or did not have all the sequences necessary for brain segmentation. In addition, 260 (5.6%) had a diagnosis of dementia and were excluded from the study sample, leaving 4354 participants for analysis.
Data analyses
We used multiple imputation (Rubin and Schenker 1991) (10 datasets) to address the missing values in the study sample of 4354 persons, using the statistical programme S+ (version 6.0). Data were analyzed using PASW version 17.0 (Chicago, Ill, USA), by pooling the 10 imputed datasets. The percentage of missings on variables varied from 0% to 4.8%.
First, characteristics were calculated according to MDD group (never, past, current). Second, linear regression analyses were used to estimate the associations of ever MDD, and current and past MDD, with brain parenchymal fraction. We also examined the associations of early-onset depression and late-onset depression with brain parenchymal fraction. Similar analyses were performed for the association of number of times MDD (1–2 episodes; 3 or more episodes) with brain parenchymal fraction. In all analyses those with never MDD comprised the reference group. Analyses were adjusted for age, sex and education (model 1), and additionally for MMSE score, subjective memory complaints, smoking history, alcohol intake, BMI, systolic and diastolic blood pressure, diabetes, history of stroke, white matter lesion volume, and presence of infarcts on MRI (model 2). In model 3, additional adjustments were made for current antidepressant use. All analyses were repeated with gray matter fraction and white matter fraction as outcome variables.
To explore in more detail the relative influence of history and age at onset within participants with current and past MDD, we compared the following depression groups to those never depressed: current MDD and no history (first onset), current MDD and early-onset history, current MDD and late-onset history, past MDD and early-onset history, and past MDD and late-onset history.
Finally, we estimated associations of never, past, and current MDD with z-score transformed of regional brain volumes, adjusted for age, sex, education, and intracranial volume.
RESULTS
The mean age of the study population was 76 (SD 5) years and 58% was female. Of the total sample, 95.5% persons never had a diagnosis of MDD; 3.0% had a past but no current diagnosis of MDD and 1.5% had a current diagnosis of MDD (i.e. in the past 2 weeks). Of the persons with current MDD, 75% also had a past history of MDD. Compared to those never depressed and those with a past MDD, persons with current MDD had higher depressive symptom levels, more often used antidepressants, and had more previous depressive episodes (Table 1).
Table 1.
Characteristics of study sample*
| Major depressive disorder | |||
|---|---|---|---|
| Never | Past | Current | |
| Demographics | |||
| Age, years | 76 ± 5 | 74 ± 5 | 74 ± 5 |
| Female, % | 58 | 69 | 66 |
| Education, % | |||
| primary | 23 | 20 | 19 |
| secondary | 50 | 57 | 57 |
| college/university | 27 | 23 | 24 |
| MMSE score | 27 ± 3 | 27 ± 2 | 27 ± 2 |
| Concerns memory is worse, % | 30 | 38 | 60 |
| Vascular factors | |||
| Smoking status, % | |||
| non-smoker | 43 | 40 | 37 |
| former | 45 | 43 | 44 |
| current | 12 | 17 | 19 |
| Alcohol intake now, % | 66 | 62 | 52 |
| BMI, kg/m2 | 27 ± 4 | 28 ± 4 | 28 ± 5 |
| Systolic blood pressure | 143 ± 20 | 138 ± 18 | 139 ± 20 |
| Diastolic blood pressure | 74 ± 10 | 74 ± 10 | 74 ± 10 |
| Diabetes Mellitus, % | 11 | 16 | 18 |
| History of stroke, % | 6 | 7 | 13 |
| MRI parameters | |||
| Infarcts on MRI, % | 31 | 22 | 24 |
| Absolute WML volume, ml | 13 (4 – 45) | 11 (3 – 37) | 15 (6 – 45) |
| Total ICV, ml | 1503 ± 149 | 1470 ± 128 | 1492 ± 153 |
| GMF, % | 45 ± 3 | 46 ± 3 | 45 ± 3 |
| WMF, % | 26 ± 2 | 26 ± 2 | 25 ± 1 |
| BPF, % | 72 ± 4 | 73 ± 4 | 71 ± 4 |
| Depression measures | |||
| GDS score 6+, % | 5 | 19 | 60 |
| GDS score, median (10–90%) | 2 (0–4) | 2 (1–8) | 7 (3–12) |
| Current antidepressant use, % | 11 | 54 | 66 |
| History of MDD, % | 0 | 100 | 75 |
| Age of first onset of MDD, % | |||
| <60 years | 0 | 70 | 53 |
| 60 years or older | 0 | 30 | 47 |
| Number of MDD episodes, % | |||
| 1–2 times | 0 | 58 | 43 |
| 3 or more | 0 | 42 | 57 |
Table is based on the sample before imputation (never MDD n=4050; past MDD n=125; current MM n=62).
Numbers present means ± standard deviations or %, except for WML volume where median and 10–90 percentile is given.
MDD=major depressive disorder; BMI=body mass index; WML=white matter lesion; ICV=intracranial volume; GMF=gray matter fraction; WMF=normal white matter fraction; BPF=brain parenchymal fraction; GDS=Geriatric Depression Scale.
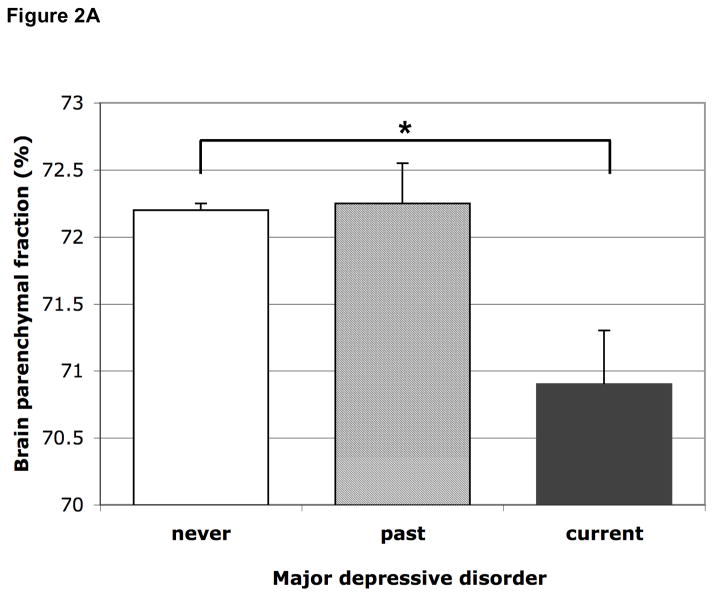
Compared to those never depressed, participants with ever MDD had borderline significantly smaller relative brain volumes, adjusted for age, sex and education, which attenuated in models 2 and 3. When we differentiated between current and past MDD, participants with current MDD had statistically significantly smaller relative brain volume, indicating more global brain atrophy, adjusted for age, sex and education (B=−1.44%; 95%CI −2.26 to −0.63%; p=0.001). After additional adjustment in models 2 and 3, the estimate somewhat attenuated but remained statistically significant. Current MDD was associated with more atrophy in the gray matter and normal white matter (Table 2). Persons with past/not current MDD did not have more brain atrophy than those never depressed (Figure 2A, Table 2).
Table 2.
Results of the linear regression models for the associations of depression groups with relative brain volumes
| Brain parenchymal fraction B (95% CI) |
Gray matter fraction B (95% CI) |
White matter fraction B (95% CI) |
|
|---|---|---|---|
| Never MDD | 0 (reference) | 0 (reference) | 0 (reference) |
| Ever | |||
| Model 1 | −0.48 (−0.97 to 0.02) | −0.35 (−0.78 to 0.08) | −0.18 (−0.42 to 0.07) |
| Model 2 | −0.37 (−0.86 to 0.12) | −0.30 (−0.72 to 0.12) | −0.07 (−0.30 to 0.15) |
| Model 3 | −0.12 (−0.63 to 0.38) | −0.13 (−0.56 to 0.31) | 0.003 (−0.23 to 0.23) |
| Past (and not current) | |||
| Model 1 | 0.005 (−0.61 to 0.62) | −0.12 (−0.66 to 0.41) | 0.14 (−0.17 to 0.44) |
| Model 2 | 0.06 (−0.54 to 0.66) | −0.11 (−0.63 to 0.40) | 0.17 (−0.10 to 0.44) |
| Model 3 | 0.28 (−0.33 to 0.90) | 0.05 (−0.48 to 0.57) | 0.24 (−0.04 to 0.52) |
| Current (including past) | |||
| Model 1 | −1.44 (−2.26 to −0.63)* | −0.82 (−1.52 to −0.13)* | −0.79 (−1.22 to −0.36)* |
| Model 2 | −1.25 (−2.05 to −0.44)* | −0.69 (−1.36 to −0.01)* | −0.56 (−0.95 to −0.17)* |
| Model 3 | −0.97 (−1.79 to −0.15)* | −0.50 (−1.18 to 0.19) | −0.48 (−0.87 to −0.08)* |
| Early onset MDD | |||
| Model 1 | −0.54 (−1.14 to 0.05) | −0.39 (−0.89 to 0.12) | −0.30 (−0.60 to 0.008) |
| Model 2 | −0.50 (−1.08 to 0.09) | −0.33 (−0.82 to 0.16) | −0.16 (−0.44 to 0.11) |
| Model 3 | −0.25 (−0.85 to 0.35) | −0.16 (−0.66 to 0.34) | −0.09 (−0.37 to 0.20) |
| Late onset MDD | |||
| Model 1 | −0.41 (−1.22 to 0.41) | −0.31 (−1.01 to 0.38) | 0.06 (−0.34 to 0.47) |
| Model 2 | −0.22 (−1.03 to 0.59) | −0.29 (−0.97 to 0.39) | 0.07 (−0.30 to 0.43) |
| Model 3 | 0.01 (−0.80 to 0.83) | −0.13 (−0.81 to 0.56) | 0.14 (−0.23 to 0.51) |
| 1–2 times MDD | |||
| Model 1 | −0.19 (−0.84 to 0.47) | −0.11 (−0.67 to 0.45) | −0.06 (−0.39 to 0.27) |
| Model 2 | −0.09 (−0.72 to 0.55) | −0.07 (−0.61 to 0.47) | −0.02 (−0.31 to 0.28) |
| Model 3 | 0.16 (−0.50 to 0.81) | 0.10 (−0.46 to 0.65) | 0.06 (−0.25 to 0.36) |
| 3 or more times MDD | |||
| Model 1 | −0.84 (−1.54 to −0.15)* | −0.63 (−1.23 to −0.04)* | −0.29 (−0.65 to 0.06) |
| Model 2 | −0.76 (−1.45 to −0.08)* | −0.59 (−1.17 to −0.02)* | −0.17 (−0.49 to 0.15) |
| Model 3 | −0.53 (−1.22 to 0.17) | −0.43 (−1.02 to 0.16) | −0.10 (−0.42 to 0.23) |
MDD=major depressive disorder
B (regression coefficient) represents the difference in % brain volume between the respective depression group and participants without a lifetime MDD diagnosis.
Model 1: adjusted for age, sex, and education
Model 2: model 1 with additional adjustment for MMSE score, subjective memory complaints, smoking habits, alcohol intake, BMI, systolic and diastolic blood pressure, diabetes, history of stroke, white matter lesion volume and presence of infarcts on MRI
Model 3: model 2 with additional adjustment for current antidepressant use
p<0.05
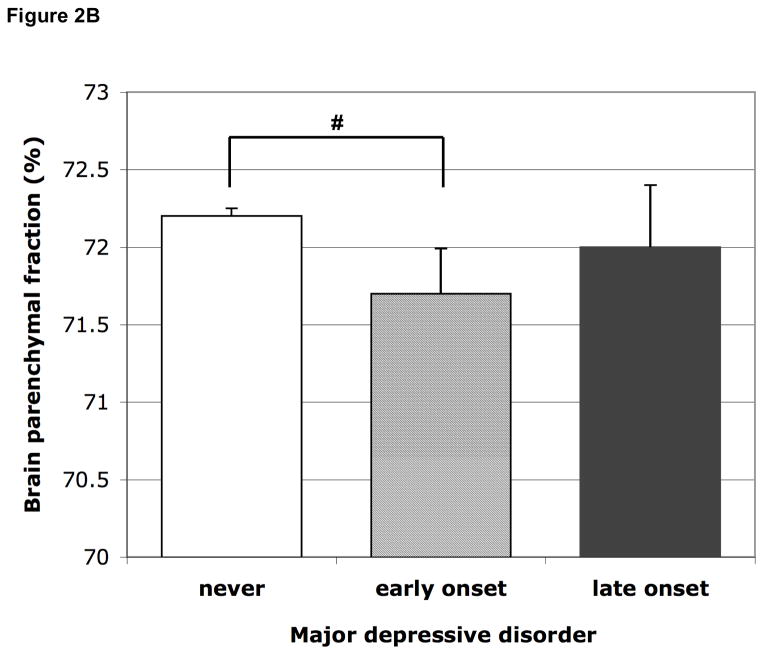
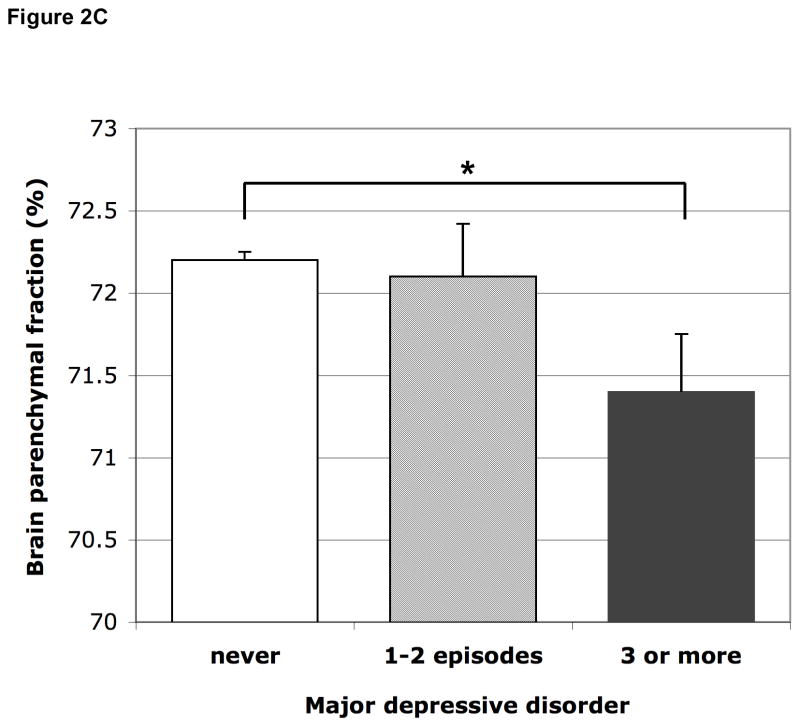
Figure 2.
Figure 2A Mean brain parenchymal fraction according to past and current major depressive disorder. Means are adjusted for age, sex, education, MMSE score, subjective memory complaints, smoking habits, alcohol intake, BMI, systolic and diastolic blood pressure, diabetes, history of stroke, white matter lesion volume and presence of infarcts on MRI. Error bars represent standard errors. * p<0.05
Figure 2B Mean brain parenchymal fraction according to early (first MDD before age 60) and late onset (first MDD at age 60 or older) major depressive disorder. The groups include persons with past as well as current MDD. Means are adjusted for age, sex, education, MMSE score, subjective memory complaints, smoking habits, alcohol intake, BMI, systolic and diastolic blood pressure, diabetes, history of stroke, white matter lesion volume and presence of infarcts on MRI. Error bars represent standard errors. # p=0.095
Figure 2C Mean brain parenchymal fraction according to number of times MDD. Means are adjusted for age, sex, education, MMSE score, subjective memory complaints, smoking habits, alcohol intake, BMI, systolic and diastolic blood pressure, diabetes, history of stroke, white matter lesion volume and presence of infarcts on MRI. Error bars represent standard errors. * p<0.05
Participants with an early onset MDD (<60 years) had borderline significantly smaller relative brain volume than those never depressed in models 1 and 2, which attenuated after adjusting for antidepressant use; those with a late onset did not have smaller relative brain volume (Figure 2B, Table 2). Additional analysis within only the persons with a history of MDD showed no significant differences in total brain volume between late onset vs. early onset MDD (mean difference in relative total brain volume adjusted for age, sex, education was 0.15%; 95% CI −0.90 to 1.19%).
Participants with 1–2 episodes did not have smaller relative brain volume than those never depressed; those with 3 or more episodes had statistically significantly smaller relative brain volume in model 2, which attenuated after adjusting for antidepressant use (Figure 2C, Table 2). Additional analysis within only the persons with a history of MDD showed no significant differences in total brain volume between 3 or more episodes vs. 1–2 episodes (mean difference in relative total brain volume adjusted for age, sex, education was −0.58%; 95% CI −1.58 to 0.42%).
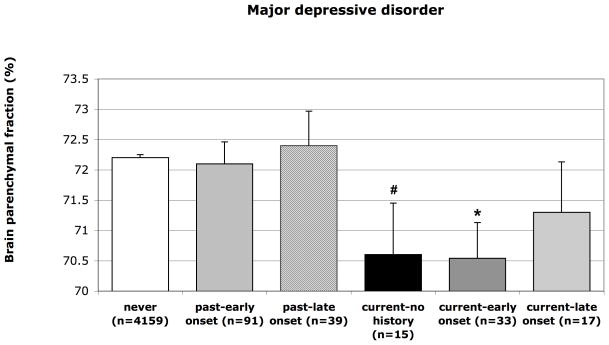
When exploring the relative influence of age at onset within participants with current and past MDD, participants with current MDD with early onset had more brain atrophy than those never depressed in model 1 (B=−1.66%; 95%CI −2.82 to −0.49%; p=0.005) while this association was less strong and not significant for participants with current MDD/late onset (B=−0.87%; 95%CI −2.50 to 0.76%; p=0.29). However, participants with current MDD without a history had moderately more brain atrophy (B=−1.62%; 95%CI −3.30 to 0.05%; p=0.058 (Figure 3). Additional analyses of presence of depressive symptoms as measured with the GDS score (6+ vs. <6) within the group without a lifetime diagnosis of MDD were consistent with this latter finding, where presence of depressive symptoms was associated with more brain atrophy (B (model 2)=−1.03%; 95%CI −1.50 to −0.55%; p<0.0001). Finally, participants with past MDD/early onset (B=−0.09%; 95%CI −0.81 to 0.63%, p=0.81) or those with past MDD/late onset (B=0.18%; 95%CI −0.94 to 1.30%, p=0.75) (Figure 3) did not have smaller brain volumes than those who were never depressed.
Figure 3.
Mean brain parenchymal fraction according to past, current, and age at onset of MDD. Means are adjusted for age, sex, and education. Error bars represent standard errors. * p<0.05; # p=0.058
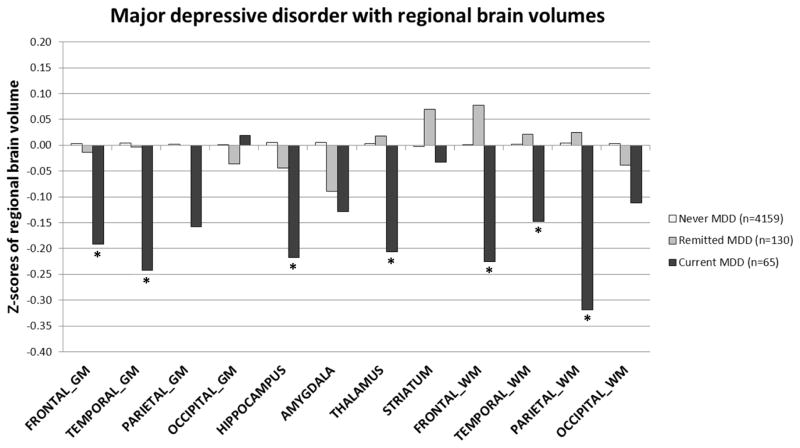
Figure 4 shows the z-scores adjusted for age, sex, education and intracranial volume of brain volume in different regions for persons never depressed, those with past MDD and those with current MDD. As can be seen, compared to participants never depressed, those with current MDD had significantly more atrophy in most of the brain regions, including frontal and temporal gray and white matter, parietal white matter, and the hippocampus and thalamus. When we additionally adjusted for other covariates (model 2), estimates somewhat attenuated and some regions lost statistical significance (frontal gray matter p=0.06, hippocampus p<0.08, and thalamus p=0.11).
Figure 4.
Z-scores of regional brain volumes according to past and current major depressive disorder. Z-scores are adjusted for age, sex, education, and intracranial volume. * p<0.05 compared to never MDD. Unadjusted mean volume in mL (SD) of brain regions: frontal gray matter 213 (22); temporal gray matter 128 (13); parietal gray matter 86 (10); occipital gray matter 87 (11); hippocampus 5.6 (0.6); amygdala 4.8 (0.6); thalamus 15 (1); striatum 20 (2); frontal white matter 137 (18); temporal white matter 63 (9); parietal white matter 71 (10); occipital white matter 53 (8).
DISCUSSION
In a community-based cohort of older people without dementia, we observed that persons with current MDD, but not those in remission, had more global brain atrophy, including more gray and white matter atrophy in the majority of lobes as well as more atrophy of the hippocampus and thalamus. Within those with current MDD, persons with a first onset as well as those with an early onset had more global brain atrophy than those never depressed. Multiple previous episodes were also associated with more global brain atrophy.
To our knowledge, this is the first study in a community-based sample of older people that examined the associations of lifetime DSM-IV diagnoses of MDD with brain volumes on MRI. A major strength and uniqueness of this study is the combination of characteristics that reduced selection bias, information bias, and confounding, which include the population-based design, the exclusion of participants with dementia, the use of structured diagnostic interviews to obtain DSM-IV lifetime depression diagnoses, and the large number of confounders taken into account. Also, the large sample size and the volumetric brain measures increased the precision and power to detect small differences. Furthermore, we were able to examine several depression characteristics, including current and past MDD, age at onset and number of episodes.
A limitation is the cross-sectional design. We tried to distinguish the temporal relationship by differentiating between history of MDD and MDD at time of MRI. Also, we excluded participants with dementia and adjusted for cognitive functioning to decrease the possibility that the association was explained by subclinical dementia.
Of note is the low prevalence of lifetime MDD compared to other studies (Kessler et al. 2005; Kessler et al. 2010). Whereas the low prevalence of current MDD can be explained by the short time period used, i.e. MDD in the past two weeks as opposed to the more commonly used 12-month prevalence, there are several factors, pertinent in general to studies of older persons, that may account for the low prevalence of past MDD. For example, the prevalence may be lower because depression is a risk factor for non-response and for mortality (Penninx et al. 1999; Schoevers et al. 2000) so possibly persons with MDD earlier in life died before the start of AGES-RS. Further, depression and dementia/mild cognitive impairment are highly comorbid (Thomas and O’Brien 2008); as we excluded the participants with dementia and those with MMSE scores <21 we also likely excluded a proportion of persons with depression. Finally, although the sensitivity of our screening-algorithm was very high, most of the depression questions used in the screening algorithm were based on current complaints and to a lesser extent on history of complaints. This may have resulted in identifying a lower proportion of persons with past MDD receiving the the MINI interview. As a result, the reference group (never MDD) will include a proportion of persons who may have had a history of MDD or who had current depressive symptoms or subthreshold depression. Nevertheless, those persons receiving a diagnosis of current or past MDD are likely to be correctly classified. While the comparison between the ever MDD vs. the never MDD group may be somewhat diluted, this will not have affected the comparison to a great extent given the very large size of the reference group; also, we do not think this can explain the difference in brain volume between the current MDD and remitted MDD group.
When we adjusted for current use of antidepressants, associations attenuated. This could suggest medication users had more severe depression and therefore the greatest risk for brain atrophy. It may also suggest antidepressant use is associated with brain atrophy independent of MDD. Antidepressants and in particular SSRI’s are frequently prescribed for other indications than depression, such as anxiety or sleeping problems. A recent population-based study in old persons without dementia showed that use of antidepressants was associated with more brain atrophy, independent of depressive symptom level (Geerlings et al. 2012). More studies are needed with detailed data on type, dose, duration, and prescription indication to determine whether or not antidepressants are harmful for the brain.
Many studies in younger populations found volume reductions of the hippocampus (MacQueen and Frodl 2011) and other specific brain regions thought to be involved in emotion regulation (Kupfer et al. 2012; Lorenzetti et al. 2009; MacQueen and Frodl 2011) in patients with MDD compared to healthy controls. Our findings are not consistent with these previous reports because we observed gray and white matter atrophy in the majority of lobes as well as in the hippocampus and thalamus, suggesting that at older age, atrophy associated with MDD is widespread. From our data we cannot know, however, whether the volume reduction started in specific brain regions at younger age and expanded with older age, or whether a general neurodegenerative process underlies the association with MDD later in life.
Previous population-based studies did not have data on lifetime diagnoses of MDD and findings relied on depressive symptom scales and one or two questions to determine history of depression (Dotson et al. 2009; Geerlings et al. 2008; Geerlings et al. 2012; Goveas et al. 2011). As a result, it is difficult to differentiate between depressive symptoms indicating MDD and depressive symptoms associated with disease and disability, or between a first onset and depression as part of a lifelong history of depressive episodes. If depression is a causal risk factor for brain atrophy and dementia, one would predict that a history of depression, and in particular an early onset and repeated episodes, is associated with more brain atrophy. If depression is a consequence of brain volume loss or a prodrome of dementia, one would predict that depression closest in time of MRI would be associated with more brain atrophy. When we differentiated history within those with current MDD, we observed that those with an early onset had smaller brain volumes, suggesting depression preceded or promoted brain tissue loss. Consistent with this, we also found that multiple episodes were associated with smaller brain volumes. One population-based study examining the bidirectional relation between depression and hippocampal volume loss found that depressive symptoms at baseline predicted faster hippocampal volume loss, but hippocampal volume at baseline was not associated with incident depression, which supports our findings (den Heijer et al. 2011). However, we also found that persons with first onset MDD had smaller brain volumes and consistent with this we also found that a high GDS-15 score in the absence of a lifetime diagnosis of MDD was associated with smaller brain volumes. Although numbers in the subgroups were small this suggests that depression can be both a contributor and a consequence of smaller brain volume.
Older persons with MDD in remission did not have smaller brain volumes than those never depressed. Although this finding is somewhat counterintuitive one explanation could be that MDD is associated with smaller brain volume only in the acute state. Several studies showed that patients with MDD who showed remission at follow-up had larger baseline hippocampal volume than patients with MDD who did not show remission at follow-up (Ahdidan et al. 2011; Hsieh et al. 2002; MacQueen et al. 2008). Also, one study found that patients with current depression had smaller hippocampal volume than patients in remission at the time of the MRI (Caetano et al. 2004). It should be noted that these studies examined hippocampal volume instead of total brain volume and findings may thus not fully comparable. Two studies that examined both hippocampal and total brain volume observed that patients with a history but not current major depression had smaller hippocampal volume, but not total brain volume when compared to healthy controls (Neumeister et al. 2005; Sheline et al. 1996). We did not find an association between past MDD and hippocampal volume, however. Possibly current depression is associated with increased cortisol levels, which may be neurotoxic, and in remitted depression cortisol levels return to normal and atrophy is reversed (Caetano et al. 2004). Few studies in humans however investigated the relation between depression, cortisol, and brain volumes within one study. While higher basal cortisol levels may be associated with smaller hippocampal volume (Knoops et al. 2010) they may not explain the relation between MDD and hippocampal volume (Gerritsen et al. 2011). It should be noted that the findings on depression of the study by Gerritsen et al. are inconsistent with ours, because in their study current MDD was not associated with hippocampal volume while remitted depression was associated with a smaller entorhinal cortex volume (Gerritsen et al. 2011). Further, a history of depression was based on the two core symptoms of MDD and not on a clinical diagnosis (Gerritsen et al. 2011). Clearly, more studies are needed to examine the role of HPA-axis dysregulation in the relation between MDD, brain atrophy, and development of dementia.
In conclusion, in this population-based study of older persons without dementia, current MDD, irrespective of prior history, was associated with widespread gray and white matter brain atrophy, while MDD in remission was not associated with more brain atrophy. Prospective studies should examine whether MDD is a consequence of or contributes to brain volume loss and development of dementia.
Acknowledgments
The Age, Gene/Environment Susceptibility-Reykjavik Study is funded by NIH contract N01-AG-12100, the NIA Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament). Dr Geerlings is funded by a grant from the Netherlands Organization for Scientific Research (NWO: project no. 917-66-311) and the University Medical Center Utrecht (program Internationalisation). The funding sources had no involvement in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Dr Launer (PI) had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- Ahdidan J, Hviid LB, Chakravarty MM, Ravnkilde B, Rosenberg R, Rodell A, Stodkilde-Jorgensen H, Videbech P. Longitudinal MR study of brain structure and hippocampus volume in major depressive disorder. Acta Psychiatrica Scandinavia. 2011;123:211–9. doi: 10.1111/j.1600-0447.2010.01644.x. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Greenwald BS, Kramer-Ginsberg E, Hu J, Wu H, Patel M, Aupperle P, Pollack S. Hippocampal/amygdala volumes in geriatric depression. Psychological Medicine. 1999;29:629–638. doi: 10.1017/s0033291799008405. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, DSM-IV. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Ballmaier M, Sowell ER, Thompson PM, Kumar A, Narr KL, Lavretsky H, Welcome SE, DeLuca H, Toga AW. Mapping brain size and cortical gray matter changes in elderly depression. Biological Psychiatry. 2004;55:382–389. doi: 10.1016/j.biopsych.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Byers AL, Yaffe K. Depression and risk of developing dementia. Nature Reviews Neurology. 2011;7:323–331. doi: 10.1038/nrneurol.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano SC, Hatch JP, Brambilla P, Sassi RB, Nicoletti M, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. Anatomical MRI study of hippocampus and amygdala in patients with current and remitted major depression. Psychiatry Research. 2004;132:141–147. doi: 10.1016/j.pscychresns.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Campbell S, MacQueen G. The role of the hippocampus in the pathophysiology of major depression. Journal of Psychiatry & Neuroscience. 2004;29:417–426. [PMC free article] [PubMed] [Google Scholar]
- Campbell S, MacQueen G. An update on regional brain volume differences associated with mood disorders. Current Opinion in Psychiatry. 2006;19:25–33. doi: 10.1097/01.yco.0000194371.47685.f2. [DOI] [PubMed] [Google Scholar]
- den Heijer T, Tiemeier H, Luijendijk HJ, van der Lijn F, Koudstaal PJ, Hofman A, Breteler MM. A study of the bidirectional association between hippocampal volume on magnetic resonance imaging and depression in the elderly. Biological Psychiatry. 2011;70:191–197. doi: 10.1016/j.biopsych.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Dotson VM, Beydoun MA, Zonderman AB. Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology. 2010;75:27–34. doi: 10.1212/WNL.0b013e3181e62124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson VM, Davatzikos C, Kraut MA, Resnick SM. Depressive symptoms and brain volumes in older adults: a longitudinal magnetic resonance imaging study. Journal of Psychiatry & Neuroscience. 2009;34:367–375. [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Geerlings MI, Brickman AM, Schupf N, Devanand DP, Luchsinger JA, Mayeux R, Small SA. Depressive Symptoms, Antidepressant Use, and Brain Volumes on MRI in a Population-Based Cohort of Old Persons without Dementia. Journal of Alzheimers Disease. 2012 Feb 29; doi: 10.3233/JAD-2012-112009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerlings MI, den Heijer T, Koudstaal PJ, Hofman A, Breteler MM. History of depression, depressive symptoms, and medial temporal lobe atrophy and the risk of Alzheimer disease. Neurology. 2008;70:1258–1264. doi: 10.1212/01.wnl.0000308937.30473.d1. [DOI] [PubMed] [Google Scholar]
- Gerritsen L, Comijs HC, van der Graaf Y, Knoops AJ, Penninx BW, Geerlings MI. Depression, hypothalamic pituitary adrenal axis, and hippocampal and entorhinal cortex volumes-the SMART Medea study. Biological Psychiatry. 2011;70:373–380. doi: 10.1016/j.biopsych.2011.01.029. [DOI] [PubMed] [Google Scholar]
- Geuze E, Vermetten E, Bremner JD. MR-based in vivo hippocampal volumetrics: 2. Findings in neuropsychiatric disorders. Molecular Psychiatry. 2005;10:160–184. doi: 10.1038/sj.mp.4001579. [DOI] [PubMed] [Google Scholar]
- Goveas JS, Espeland MA, Hogan P, Dotson V, Tarima S, Coker LH, Ockene J, Brunner R, Woods NF, Wassertheil-Smoller S, Kotchen JM, Resnick S. Depressive symptoms, brain volumes and subclinical cerebrovascular disease in postmenopausal women: the Women’s Health Initiative MRI Study. Journal of Affective Disorders. 2011;132:275–284. doi: 10.1016/j.jad.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, Thorgeirsson G, Aspelund T, Garcia ME, Cotch MF, Hoffman HJ, Gudnason V. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. American Journal of Epidemiology. 2007;165:1076–1087. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh MH, McQuoid DR, Levy RM, Payne ME, MacFall JR, Steffens DC. Hippocampal volume and antidepressant response in geriatric depression. International Journal of Geriatric Psychiatry. 2002;17:519–525. doi: 10.1002/gps.611. [DOI] [PubMed] [Google Scholar]
- Ikram MA, Vrooman HA, Vernooij MW, den Heijer T, Hofman A, Niessen WJ, van der Lugt A, Koudstaal PJ, Breteler MM. Brain tissue volumes in relation to cognitive function and risk of dementia. Neurobiology of Aging. 2010;31:378–386. doi: 10.1016/j.neurobiolaging.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Shiung MM, Weigand SD, O’Brien PC, Gunter JL, Boeve BF, Knopman DS, Smith GE, Ivnik RJ, Tangalos EG, Petersen RC. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65:1227–1231. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm AF. History of depression as a risk factor for dementia: an updated review. Australian & New Zealand Journal of Psychiatry. 2001;35:776–781. doi: 10.1046/j.1440-1614.2001.00967.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Birnbaum H, Bromet E, Hwang I, Sampson N, Shahly V. Age differences in major depression: results from the National Comorbidity Survey Replication (NCS-R) Psychological Medicine. 2010;40:225–237. doi: 10.1017/S0033291709990213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoops AJ, Gerritsen L, van der Graaf Y, Mali WP, Geerlings MI. Basal hypothalamic pituitary adrenal axis activity and hippocampal volumes: the SMART-Medea study. Biological Psychiatry. 2010;67:1191–1198. doi: 10.1016/j.biopsych.2010.01.025. [DOI] [PubMed] [Google Scholar]
- Konarski JZ, McIntyre RS, Kennedy SH, Rafi-Tari S, Soczynska JK, Ketter TA. Volumetric neuroimaging investigations in mood disorders: bipolar disorder versus major depressive disorder. Bipolar Disorders. 2008;10:1–37. doi: 10.1111/j.1399-5618.2008.00435.x. [DOI] [PubMed] [Google Scholar]
- Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Human Brain Mapping. 2009;30:3719–3735. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Jin Z, Bilker W, Udupa J, Gottlieb G. Late-onset minor and major depression: early evidence for common neuroanatomical substrates detected by using MRI. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7654–7658. doi: 10.1073/pnas.95.13.7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer DJ, Frank E, Phillips ML. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet. 2012;379:1045–55. doi: 10.1016/S0140-6736(11)60602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti V, Allen NB, Fornito A, Yucel M. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. Journal of Affective Disorders. 2009;117:1–17. doi: 10.1016/j.jad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- MacQueen G, Frodl T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Molecular Psychiatry. 2011;16:252–64. doi: 10.1038/mp.2010.80. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Yucel K, Taylor VH, Macdonald K, Joffe R. Posterior hippocampal volumes are associated with remission rates in patients with major depressive disorder. Biological Psychiatry. 2008;64:880–883. doi: 10.1016/j.biopsych.2008.06.027. [DOI] [PubMed] [Google Scholar]
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLOS Medicine. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. Journal of Psychiatry & Neuroscience. 2009;34:41–54. [PMC free article] [PubMed] [Google Scholar]
- Neumeister A, Wood S, Bonne O, Nugent AC, Luckenbaugh DA, Young T, Bain EE, Charney DS, Drevets WC. Reduced hippocampal volume in unmedicated, remitted patients with major depression versus control subjects. Biological Psychiatry. 2005;57:935–937. doi: 10.1016/j.biopsych.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Archives of General Psychiatry. 2006;63:530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninx BW, Geerlings SW, Deeg DJ, van Eijk JT, Van Tilburg W, Beekman AT. Minor and major depression and the risk of death in older persons. Archives of General Psychiatry. 1999;56:889–895. doi: 10.1001/archpsyc.56.10.889. [DOI] [PubMed] [Google Scholar]
- Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Statistics in Medicine. 1991;10:585–598. doi: 10.1002/sim.4780100410. [DOI] [PubMed] [Google Scholar]
- Saczynski JS, Beiser A, Seshadri S, Auerbach S, Wolf PA, Au R. Depressive symptoms and risk of dementia: The Framingham Heart Study. Neurology. 2010;75:35–41. doi: 10.1212/WNL.0b013e3181e62138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoevers RA, Geerlings MI, Beekman AT, Penninx BW, Deeg DJ, Jonker C, van Tilburg W. Association of depression and gender with mortality in old age. Results from the Amsterdam Study of the Elderly (AMSTEL) British Journal of Psychiatry. 2000;177:336–342. doi: 10.1192/bjp.177.4.336. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:3908–13. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson S, Aspelund T, Forsberg L, Fredriksson J, Kjartansson O, Oskarsdottir B, Jonsson PV, Eiriksdottir G, Harris TB, Zijdenbos A, van Buchem MA, Launer LJ, Gudnason V. Brain tissue volumes in the general population of the elderly: the AGES-Reykjavik study. Neuroimage. 2012;59:3862–3870. doi: 10.1016/j.neuroimage.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AJ, O’Brien JT. Depression and cognition in older adults. Current Opinion in Psychiatry. 2008;21:8–13. doi: 10.1097/YCO.0b013e3282f2139b. [DOI] [PubMed] [Google Scholar]
- van Tol MJ, van der Wee NJ, van den Heuvel OA, Nielen MM, Demenescu LR, Aleman A, Renken R, van Buchem MA, Zitman FG, Veltman DJ. Regional brain volume in depression and anxiety disorders. Archives of General Psychiatry. 2010;67:1002–1011. doi: 10.1001/archgenpsychiatry.2010.121. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. American Journal of Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatric Research. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]