Abstract
Flow cytometry was conducted to evaluate genome size diversity among African diploid species of the Coffea genus. The study included 15 species and six new taxa from Congolese and Cameroonian forest regions which have yet to be botanically characterized. Between‐population differences were also recorded in some cases. These evaluations using an internal standard were highly correlated with previous results obtained with an external standard, but differences of up to 18 % existed for some species, involving stoichiometric errors. Consequently, genome size variation between species and within species are discussed as true genome size differences or stoichiometric errors. Environmental and phenotypic correlations with genome size are also discussed.
Key words: Coffea, genome size, flow cytometry, interspecific diversity, intraspecific diversity
INTRODUCTION
The Coffea genus (Rubiaceae family) includes two subgenera, Baracoffea and Coffea. Coffee trees sensu stricto belong to the subgenus Coffea. In the wild, they are endemic to intertropical forest zones in Africa, Madagascar, Mauritius, Comoros and Réunion (Chevallier, 1929, 1938, 1942, 1947; Leroy, 1963; Charrier, 1978; Bridson and Vercourt, 1988; Anthony, 1992; Stoffelen, 1998). Systematists have described over 80 species, including two cultivated species, C. arabica L. and C. canephora Pierre. All taxa are diploid with the same chromosome number (2n = 2x = 22), except C. arabica (2n = 4x = 44).
A first flow cytometry evaluation of genome size, using propidium iodide (PI) as dye and Oryza sativa ssp. japonica as external standard, was carried out on 12 diploid species of the subgenus Coffea (Cros et al., 1995). This first evaluation revealed a broad genome size range, from 0·95 pg in C. racemosa Lour., an East African species, up to 1·78 pg in C. humilis Chev., a West African species (Cros et al., 1995). However, several African Coffea species and taxa have not yet been evaluated, in particular new taxa collected in the Congo and Cameroon (Anthony et al., 1985; De Namur et al., 1987).
Since then, an accurate flow cytometry method has been developed to determine small genome size differences (0·025 pg) in coffee trees (Barre et al., 1996). The method differs from the former by: (a) the use of an internal standard, Petunia hybrida (2·85 pg; Marie and Brown, 1993); (b) a statistically defined sample size; and (c) a full randomization or Fisher’s block experimental design. We used this new method to assess genome size diversity from a more complete set of Coffea species. Fifteen African species, some of them including several populations (C. canephora Pierre, C. congensis Froehner, C. brevipes Hiern) or subspecies (C. liberica ssp. liberica Hiern., C. l. ssp. Dewevrei De Wild. et Dur.) were studied. Six new taxa from Congolese and Cameroonian forest regions which have yet to be botanically characterized were also evaluated.
Some genome size differences are artefactual due to the presence of cytosolic compounds (Noirot et al., 2000, 2002; Price et al., 2000). In coffee trees, caffeine increases dye accessibility, while chlorogenic acids (CGA) decrease it (Noirot et al., 2003). As caffeine and CGA contents vary between trees, this leads to pseudo‐variations in genome size within and between species. This also induces genome size differences when using an internal or external standard. In the first case, leaves of standard and target are chopped together, thus releasing cytosolic compounds of both species in the buffer; while in the second case, leaves are chopped separately and cytosolic compounds of the target cannot act on standard nuclei and vice versa. Such induced differences between internal and external standardization are described within a back‐cross progeny (Noirot et al., 2003).
Consequently, in the present study, the genome sizes of new undescribed taxa along with those of botanically characterized species were compared with those obtained using external standard (Cros et al., 1995). Variations in genome size between species and within species are then discussed while taking the stoichiometric error into account.
MATERIALS AND METHODS
Plant material
Table 1 gives the geographical origin of all species and populations. All trees were maintained in tropical conditions in a glasshouse in Montpellier. Five plants were evaluated for most species or populations. Each plant was represented by four extracts obtained from two leaves. The internal standard was Petunia hybrida (2·85 pg; Marie and Brown, 1993).
Table 1.
Geographical origin of species, taxa and populations
| Species, taxa and populations | Geographical origin |
| C. brevipes | Cameroon (Mt Cameroon) |
| C. brevipes | Cameroon (Kumba‐Loum) |
| C. canephora | Cameroon |
| C. canephora | Côte d’Ivoire |
| C. canephora | Central African Republic |
| C. canephora | Congo |
| C. congensis | Cameroon |
| C. congensis | Central African Republic |
| C. congensis | Congo |
| C. costatifructa | Tanzania |
| C. eugenioides | Kenya |
| C. heterocalyx | Cameroon |
| C. humilis | Côte d’Ivoire |
| C. kapakata | Angola |
| C. liberica ssp. liberica | Côte d’Ivoire |
| C. liberica ssp. Dewevrei | Central African Republic |
| C. liberica Koto | Cameroon |
| C. pocsii | Tanzania |
| C. pseudozanguebariae | Kenya |
| C. racemosa | Tanzania |
| C. salvatrix | Tanzania |
| C. sessiliflora | Kenya |
| C. stenophylla | Côte d’Ivoire |
| C. sp. Bakossi | Congo (Bakossi) |
| C. sp. Congo | Congo |
| C. sp. Mayombe | Congo |
| C. sp. Ngongo 2 | Congo (Ngongo) |
| C. sp. Ngongo 3 | Congo (Ngongo) |
| C. sp. Moloundou | Congo (Souanké) |
| C. sp. Moloundou | Cameroon (Moloundou) |
| C. sp. Nkoumbala | Cameroon (Nkoumbala) |
Underlined species were evaluated by Cros et al. (1995).
Sample preparation and cytometric measurements
Nuclei were extracted by chopping the leaves (Galbraith et al., 1983) in a slightly modified (0·5 % Triton X‐100, pH 9) lysis buffer (LB) described by Dolezel (1989). The addition of mercaptoethanol in the buffer avoided polyphenol oxidation. Leaf samples (4 cm2) were chopped for about 30 s at constant rate of chopping in a Petri dish containing 2 ml of lysis buffer. The solution was filtered through nylon cloth (50 µm mesh size) and then kept on ice for at least 2 h (this incubation time is very important in Coffea species). Several preparations were pooled when a large volume of extract was required. This extraction method and buffer gave high nuclear fluorescence stability, i.e. less than 2 % variation after 6 h incubation.
Nuclei were stained with PI (95–98 % Sigma P 4170). In all experiments, including those involving dilution, a saturating final concentration of 330 µg ml–1 (Barre et al., 1996) was used.
A FACScan cytometer (Becton Dickinson, Franklin lakes, NJ, USA) with an argon laser (15 mW) at 488 nm and an emission pulse area of 585 nm ± 22 nm was used. FL2 area and FL2 width were measured for 1024 channels. A window was defined for each sample so that only G1 nuclei were monitored. Five hundred nuclei were counted for each measurement. The zero offset of the analogue‐to‐digital converter was checked with Petunia nuclei (Barre et al., 1996). There were no gain fluctuations in the amplifier system. The voltage was maintained at a constant high level throughout each experiment. The processing order was fully randomized within each experiment day.
Statistical analysis
A nested ANOVA model was used to compare populations and trees within populations in species represented by more than one population. For other species, a one‐way ANOVA was applied, whereby each tree was represented by a mean of four evaluations. Modes of standardization were compared using the linear regression model. All analyses were carried out using the Statistica Software package (5.1 version, 1997 for Microsoft Windows).
RESULTS
Genome size comparison between populations within species
Three species, C. canephora, C. congensis and C. brevipes, and a botanically uncharacterized taxon, C. sp. Moloundou, were represented by several populations (2–4). Another species, C. liberica, included two subspecies, C. l. ssp. liberica and C. l. ssp. Dewevrei, and one population, C. sp. Koto.
A nested‐ANOVA was carried out within each species to test the between‐population factor and the between‐tree (within population) factor. Three types of results were recorded: (1) in C. canephora and C. sp. Moloundou, there were no differences between populations nor any differences between trees within populations; (2) in C. brevipes, there were no differences between populations, but there were differences between trees within populations; and (3) for C. congensis and C. liberica, significant differences were noted for both factors (Table 2).
Table 2.
Genome size comparisons between populations and trees within populations in five diploid species of the Coffea genus
| Species | Population or subspecies | DNA content | Pop F test | Tree F test |
| C. canephora | Cameroon | 1·429a | F1,8 = 0·80 | F16,20 = 1·37 |
| (1·425–1·438) | P = 0.51 | P = 0.24 | ||
| Côte d’Ivoire | 1·431a | |||
| (1·325–1·521) | ||||
| Central African Republic | 1·440a | |||
| (1·423–1·455) | ||||
| Congo | 1·448a | |||
| (1·432–1·469) | ||||
| C. congensis | Central African Republic | 1·477a | F2,12 = 6·90 | F12,15 = 4·86 |
| (1·455–1·510) | P = 0·010 | P = 0·0026 | ||
| Congo | 1·478a | |||
| (1·460–1·495) | ||||
| Cameroon | 1·509b | |||
| (1·499–1·521) | ||||
| C. liberica | Côte d’Ivoire (subsp. liberica) | 1·396a | F1,12 = 17·9 | F12,15 = 8·25 |
| (1·358–1·425) | P = 0·0003 | P = 0·0001 | ||
| Central African Republic (subsp. Dewevrei) | 1·406a | |||
| (1·395–1·414) | ||||
| Cameroon (Koto) | 1·511b | |||
| (1·419–1·547) | ||||
| C. sp. Moloundou | Cameroon (Moloundou) | 1·432c | F1,8 = 0·87 | F8,10 = 1·18 |
| (1·404–1·453) | P = 0·38 | P = 0·40 | ||
| Congo (Souanké) | 1·444c | |||
| (1·423–1·461) | ||||
| C. brevipes | Cameroon (Kumba‐Loum) | 1·519b | F1,8 = 0·16 | F8,10 = 7·49 |
| (1·502–1·545) | P = 0·60 | P = 0·002 | ||
| Cameroon (Mt Cameroon) | 1·523b | |||
| (1·496–1·544) |
The DNA content column includes mean and between‐tree range in parenthesis (below).
The results of the Newmann–Keuls multiple mean comparison test within species are indiced by superscript letters.
The Pop F test column gives ANOVA results for the between‐population factor; the subscript numbers following of the F are degrees of freedom. The Tree F test column gives ANOVA results for the between‐tree factor.
C. congensis, a Cameroonian population, had a slighly larger mean genome size (2C = 1·51 pg) than other populations (2C = 1·48 pg) from the Central African Republic and the Congo, but this difference represented only 2 %.
In C. liberica, the two subspecies C. l. ssp. liberica and C. l. ssp. Dewevrei, had the same genome size (2C = 1·40 pg). These two subspecies differed from the Koto population collected in Cameroon (2C = 1·51 pg). In this case, the difference in genome size was about 8 %.
Genome size of new botanically uncharacterized Coffea taxa
All of these new taxa are native to Cameroon and the Congo (Brazzaville). As the genome sizes of the two C. sp. Moloundou populations did not differ, that of Souanké was retained for the ANOVA (Table 3). The genome size of these new taxa varied significantly (F5,24 = 20·2; P < 0·0001) from 2C = 1·31 pg for C. sp. Ngongo 2 to 1·44 pg in C. sp. Moloundou. Three groups could be defined according to the genome size: group A, including only C. sp. Ngongo 2; group B, with three taxa (C. sp. Congo, C. sp. Nkoumbala and C. sp. Mayoumbe) and 2C = 1·36–1·37 pg; group C, also with three taxa, C. sp. Ngongo 3, C. sp. Bakossi and C. sp. Moloundou, and 2C = 1·42 – 1·44 pg.
Table 3.
Nuclear DNA content in new uncharacterized taxa
| Taxons | DNA content | Range |
| C. sp. Ngongo 2 | 1·308a | 1·295–1·320 |
| C. sp. Congo | 1·358b | 1·309–1·417 |
| C. sp. Nkoumbala | 1·366b | 1·346–1·400 |
| C. sp. Mayombe | 1·369b | 1·350–1·399 |
| C. sp. Ngongo 3 | 1·417c | 1·383–1·445 |
| C. sp. Bakossi* | 1·430 | – |
| C. sp. Moloundou Souanké | 1·444c | 1·423–1·461 |
Letters indicate multiple mean comparison results using the Newman–Keuls test.
The F test (df1 = 4, df2 = 5) concern between‐tree differences within populations.
* Represented by only one tree
Genome size in botanically characterized species
A population choice was applied in the analysis to obtain a balanced design when species were represented by more than one population. The selected population generally had a mean genome size close to that of the whole species. A random choice was made for species represented by two populations. Consequently, C. brevipes was represented by the population from Mount Cameroon, C. canephora by the population from the Central African Republic, C. congensis by the population from the Congo. C. liberica was an exception since it was represented by the two subspecies C. l. ssp. Dewevrei and C. sp. Koto population. Lastly, as the within species variation increased along with the genome size, a logarithmic transformation was applied to the data before performing the one‐way ANOVA.
Genome size ranged from 2C = 1·04 pg in C. racemosa to 1·76 pg in C. humilis. Between‐species variations were highly significant (F14,60 = 518; P < 0·0001) and represented 99 % of the total variance. In these conditions, it was not surprising that each species differed from the others when the genome size difference was above 0·027 pg (Table 4).
Table 4.
Nuclear DNA content in African coffee species
| Species | DNA content | Range |
| C. racemosa | 1·035a | 1·030–1·039 |
| C. pocsii | 1·083b | 1·059–1·141 |
| C. sessiliflora | 1·109c | 1·080–1·128 |
| C. pseudozanguebariae | 1·131cd | 1·115–1·133 |
| C. costatifructa | 1·150d | 1·129–1·162 |
| C. salvatrix | 1·221e | 1·200–1·248 |
| C. stenophylla | 1·286f | 1·267–1·314 |
| C. kapakata* | 1·323 | – |
| C. eugenioides | 1·364g | 1·344–1·384 |
| C. liberica ssp. Dewevrei | 1·406h | 1·395–1·414 |
| C. canephora RCA | 1·440i | 1·423–1·455 |
| C. congensis CR | 1·478j | 1·460–1·495 |
| C. liberica Koto | 1·511k | 1·419–1·547 |
| C. brevipes Mt Cameroon | 1·523k | 1·496–1·544 |
| C. heterocalyx | 1·737l | 1·718–1·751 |
| C. humilis | 1·764l | 1·732–1·792 |
Species are arranged in increasing DNA amounts.
Letters indicate multiple mean comparison results using the Newman–Keuls test.
The F test (df1 = 4, df2 = 5) concern between‐tree differences within populations.
* Represented by only one tree
Most species generally had a specific genome size. Exceptions concerned: (a) the group of three species— C. sessiliflora (2C = 1·11 pg), C. pseudozanguebariae (2C = 1·13 pg) and C. costatifructa (2C = 1·15 pg), where the nuclear DNA content increased over a gradient by 0·02 pg step–1 (the smallest genome size of this set, C. sessiliflora, did not differ significantly from that of C. pseudozanguebariae, and the latter did not differ from that of C. costatifructa, but the C. sessiliflora and C. costatifructa genome sizes differed significantly); (b) C. brevipes and C. l. Koto; and (c) C. humilis and C. heterocalyx.
C. kapakata, represented by only one accession from Angola, was not processed in the ANOVA. The genome size of this accession was 2C = 1·32 pg, i.e. midway between C. stenophylla and C. liberica.
Correlation with previous evaluations
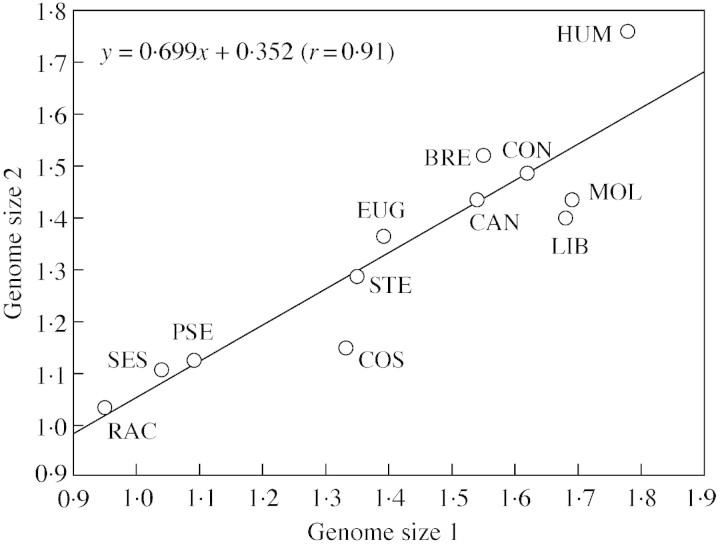
The genome size range in the subgenus Coffea was similar to that observed by Cros et al. (1995) using external standardization. For the 12 diploid species assessed in both studies, there was a high and significant correlation (r = 0·91; P < 0·0001) between evaluations (Fig. 1). Nevertheless, the slope (b = 0·7) and intersect (a = 0·35) differed from the expected b = 1 and a = 0, respectively. Some species, i.e. C. pseudozanguebariae, C. brevipes, C. eugenioides and C. humilis, were close to the theoretical regression line y = x. In contrast, the difference between standardization methods was greater for C. liberica (18 %), C. sp. Moloundou (16 %), and C. costatifructa (14·5 %). For these species, internal standardization led to a smaller genome size.
Fig. 1. Relationship between evaluations by Cros et al. (1995) (genome size 1) and current evaluations (genome size 2).
DISCUSSION
Various impacts of stoichiometric error on genome size evaluations
The genome size range in the subgenus Coffea was similar to that observed by Cros et al. (1995) using external standardization and a strong correlation between the two evaluations was recorded. Nevertheless, the slope and intersect differed from the expected results. The standardization mode (internal in the present case) could be responsible for these differences through nucleus– cytoplasm interactions (Noirot et al., 2000, 2002). Indeed, basic assumptions concerning genome size estimation using flow cytometry do not apply when dye accessibility to DNA is not identical for standard and target nuclei (Noirot et al., 2003).
External standardization became unreliable when a marked difference was noted for C. liberica (18 %), C. sp. Moloundou (16 %), and C. costatifructa (14·5 %). For these species, internal standardization led to a smaller genome size. This could correspond to higher dye accessibility of Petunia nuclei DNA due to caffeine release in the buffer during chopping. In all cases, internal standardization should have led to lower bias (lowering the bias does not mean eliminating it), since the standard and target nuclei conditions were the same with regards to the antagonistic effects of caffeine and phenols. Consequently, they should be quite similar with respect to the extent of dye accessibility to DNA. Note that both standardization modes could be considered reliable when internal–external differences are not significant, as for C. pseudozanguebariae, C. eugenioides, C. brevipes and C. humilis.
Internal standardization lowers the bias but does not eliminate it completely. The stoichiometric error obtained when using internal standardization is about 5 % in coffee trees (Noirot et al., 2002). This is, however, much lower than differences between standardization mode. This also means that a difference of less than 5 % between all modes would not reflect a true DNA content variation. This state of uncertainty concerns between‐tree variations determined within C. brevipes, C. congensis and C. liberica. The latter case confirms previous results, where between‐tree variations were due to stoichiometric error (Noirot et al., 2002).
Caffeine and chlorogenic acid, two compounds abundant in coffee leaves and fruits, could explain intraspecific variations in genome size (Noirot et al., 2003), and also interspecific variations. Indeed, the caffeine content ranges from 0 in C. pseudozanguebariae to 2·54 % dry matter basis in C. canephora. Similarily, the chlorogenic acid content is 1·1 and 11·3 % in these two species, respectively (Charrier and Berthaud, 1975; Anthony et al., 1993; Ky et al., 2001). Such variations could lead to pseudo‐differences in genome size between species. Nonetheless, stoichiometric error cannot explain the entire genome size range observed within the genus.
Are between‐species and between‐population genome size variations valid speciation criteria in coffee trees?
The accuracy of the method permitted us to separate most species by their genome size when there was a difference of at least 0·03 pg. Genome size could be a species‐specific feature and exceptions seemed to be due to a lack of power of either the experiment (replicate number) or to the Newmann–Keuls test (this test is less powerful than the F test). In both cases, this problem could be overcome by using a slightly higher replicate number.
An absence of difference between populations was recorded within C. canephora, C. brevipes and C. sp. Moloundou. The higher genome size determined for the C. liberica Koto population could suggest speciation. This hypothesis is supported by specific morphological traits: the Koto trees have long‐shaped leaves with spatuled acumen and large, black fruits (fruits are red and smaller in the two other C. liberica subspecies), with thick, fibrous pulp (Anthony, 1992). Similarly, based on the species‐specific genome size hypothesis, the Congolese C. congensis pop ulation could have been undergoing a speciation process, but stoichiometric error might also have been a key factor.
The species‐specific genome size hypothesis would confirm the pre‐classification—on the basis of morphological traits—of the collected accessions into new taxa. This was the case for C. sp. Ngongo 2, whose genome size differed from that of all other uncharacterized taxa.
Conversely, an absence of genome size differences did not imply that taxa could be considered as belonging to only one species. For example, the genome size did not differ between C. sp. Ngongo 3 and C. sp. Moloundou, but they differed markedly with respect to their morphological traits and mating system (allogamous vs. autogamous). In summary, the genome size differences noted here indicate a potential change in species status, while nevertheless keeping in mind that minor differences could reflect a stoichiometric error. In contrast, a similarity in genome size is not sufficient evidence of a lack of speciation between taxa in coffee trees.
Is genome size variation correlated with adaptive traits in coffee trees?
Correlations between genome size and abiotic factors are well known, but are often reversed. For example, genome size increases with elevation in Dasypyrum villosum (Caceres et al., 1998) but not in Artemisia genus (Torrell and Vallès, 2001).
In the Coffea subgenus, genome size generally increases from East to West Africa (Cros et al., 1995). Geographical gradients have already been observed in the UK, i.e. southern plant species generally have a larger genome size than northern species (Grime and Mowforth, 1982). In coffee trees, the east–west gradient was confirmed by our study on a larger sample. Cros et al. have shown that the gradiant is related to rainfall, opposing xerophytic species from East Africa, like C. racemosa, C. sessiliflora, C. salvatrix and C. pseudozanguebariae (Chevallier, 1947), to West African species such as C. humilis that are adapted to the sempervirens forest (Chevallier, 1947). This hypothesis is further supported by two other examples focused within a country.
In the Central African Republic, three species, C. congensis (2C = 1·48 pg), C. canephora (2C = 1·45 pg) and C. l. ssp. Dewevrei (2C = 1·41 pg) differ in adaptative terms (Berthaud and Guillaumet, 1978). C. congensis is strictly limited to sempervirens forests that are periodically flooded by the Oubangui River. C. canephora is found from the humid sempervirens forest to the semi‐deciduous forest, but always in well drained soils. In the first type of forest, C. canephora could be close to C. congensis on the edges of flooded zones. C. l. ssp. Dewevrei grows in forest galleries from the semi‐decidious forest to the savannah zone and is sympatric to C. canephora in semi‐deciduous forests. In this example, the genome size, despite low between‐species variations, seems to be positively correlated with the soil water deficit. The low genome size variation could also explain the ecological overlapping of these three species, i.e. the sympatry encountered for C. congensis and C. canephora, and also for C. canephora and C. l. Dewevrei. Note the absence of sympatry between the two extremes, i.e. C. congensis and C. l. ssp. Dewevrei (Berthaud and Guillaumet, 1978).
Another example of a relationship between water deficit and genome size was observed in Côte d’Ivoire, where four species, i.e. C. humilis, C. canephora, C. l. ssp. liberica and C. stenophylla, can be collected in the wild. C. humilis, the species with the largest genome size (2C = 1·76 pg), grows in the most humid forest of Côte d’Ivoire (Taï forest, along the Liberian border), whereas C. stenophylla with the lowest genome size (2C = 1·29 pg) is particularily well adapted to long cold dry seasons (up to 6 months) (Portères, 1962)—the two other species grow under intermediate water deficit conditions.
A correlation does not reflect causality, and other contrasts should be taken into account to differentiate East and West African species. In West African species, fructification time is longer (10 months vs. 2 months in East African species), red fruits are more frequent, caffeine content is higher (Anthony et al., 1993) and flower numbers per node are higher. This set of gradients is highly colinear. Consequently, it is difficult to attribute causality between genome size and each of these traits. In addition, there are always some exceptions: C. stenophylla with a low genome size has a long fructification time (10 months), C. sp. Bakossi produces black fruits, but has a genome size (1·43 pg) typical of a red fruit species, and C. heterocalyx, a species with large genome size, has few flowers per node.
Consequently, it appears important to undestand the role of genome size variation on fitness during speciation before attibuting a causality relationship between any adaptative traits and genome size variation.
Supplementary Material
Received: 7 November 2002;; Returned for revision: 21 May 2003. Accepted: 3 July 2003
References
- AnthonyF.1992.Les ressources génétiques des caféiers: collecte, gestion d’un conservatoire et évaluation de la diversité génétique, série TDM 81. Paris: ORSTOM Press. [Google Scholar]
- AnthonyF, Clifford MN, Noirot M.1993. Biochemical diversity in the genus Coffea L.: chlorogenic acids, caffeine, and mozambioside contents. Genetic Resources and Crop Evolution 40: 61–70. [Google Scholar]
- AnthonyF, Couturon E, de Namur C.1985. Les caféiers sauvages du Cameroun: résultats d’une mission de prospection effectuée par l’ORSTOM en 1983. Proceedings of the International Congress of ASIC 11: 495–505. [Google Scholar]
- BarreP, Noirot M, Louarn J, Duperray C, Hamon S.1996. Reliable flow cytometric estimation of nuclear DNA content in coffee trees. Cytometry 24: 32–38. [DOI] [PubMed] [Google Scholar]
- BerthaudJ, Guillaumet JL.1978. Les caféiers sauvages en Centrafrique. Résultats d’une mission de prospection (Janvier–Février 1975). Café Cacao Thé 22: 171–186. [Google Scholar]
- BridsonDM, Vercourt B.1988.Coffea and Psilanthus In: Polhill RM, ed. Flora of Tropical East Africa: Rubiaceae, Part 2. Rotterdam: Balkema, 703–727. [Google Scholar]
- CaceresME, De Pace C, Scarascia Mugnozza GT, Kotsonis P, Ceccarelli M, Cionini PG.1998. Genome size variations within Dasypyrum villosum: correlations with chromosomal traits, environmental factors and plant phenotypic characteristics and behaviour in reproduction. Theoretical and Applied Genetics 96: 559–567. [Google Scholar]
- CharrierA.1978.La structure génétique des caféiers spontanés de la région malgache (Mascaro coffea). Leurs relations avec les caféiers d’origine africaine (Eucoffea), série Mémoires 87. Paris: ORSTOM Press. [Google Scholar]
- CharrierA, Berthaud J.1975. Variation de la teneur en caféine dans le genre Coffea. Café Cacao Thé 19: 251–264. [Google Scholar]
- ChevallierA.1929. Les caféiers du globe, fasc. I. Généralités sur les caféiers. In: Lechevallier P, ed. Encyclopédie biologique, Tome V. Paris. [Google Scholar]
- ChevallierA.1938. Essai d’un regroupement systématique des caféiers sauvages de Madagascar et des iles Mascareignes. Revue Botanique appliquée et Agriculture tropicale 18: 825–843. [Google Scholar]
- ChevallierA.1942. Les caféiers du globe, fasc II. Iconographie des caféiers sauvages et cultivés et des Rubiacées prises pour des caféiers. In: Lechevallier P, ed. Encyclopédie biologique, Tome XXII. Paris. [Google Scholar]
- ChevallierA.1947. Les caféiers du globe, fasc III. Systématique des caféiers et faux‐caféiers. Maladies et insectes nuisibles. In: Lechevallier P, ed. Encyclopédie biologique, Tome XXVIII. Paris. [Google Scholar]
- CrosJ, Combes MC, Chabrillange N, Duperray C, Monnot des Angles A, Hamon S.1995. Nuclear DNA content in the subgenus Coffea (Rubiaceae): inter‐ and intra‐specific variation in African species. Canadian Journal of Botany 73: 14–20. [Google Scholar]
- DeNamurC, Couturon E, Sita P, Anthony F.1987. Résultats d’une mission de prospection des caféiers sauvages du Congo. Proceedings of the International Congress of ASIC 12: 397–404. [Google Scholar]
- DolezelJ, Binarova P, Lucretti S 1989. Analysis of nuclear DNA content in plant cells by flow cytometry. Biologia Plantarum 31: 113–120. [Google Scholar]
- GalbraithDW, Harkins KR, Maddox JM, Ayres NM, Sharma DP, Firozabady E.1983. Rapid flow cytometry analysis of the cell in intact plant tissues. Science 220: 1049–1051. [DOI] [PubMed] [Google Scholar]
- GrimeJP, Mowforth MA.1982. Variation in genome size – an ecological interpretation. Nature 299: 151–153. [Google Scholar]
- KyCL, Louarn J, Dussert S, Guyot B, Hamon S, Noirot M.2001. Caffeine, trigonelline, chlorogenic acids and sucrose diversity in wild Coffea arabica L. and C. canephora P. accessions. Food Chemistry 75: 223–230. [Google Scholar]
- LeroyJF.1963. Sur les caféiers sauvages des iles Mascareignes. Compte‐Rendus de l’Académie des Sciences, Paris 256: 2897–2899. [Google Scholar]
- MarieD, Brown SC.1993. A cytometric exercise in plant DNA histogram. Biology of the Cell 78: 41–51. [DOI] [PubMed] [Google Scholar]
- NoirotM, Barre P, Duperray C, Louarn J, Hamon S.2003. Effects of caffeine and chlorogenic acid on propidium iodide accessibility to DNA: consequences on genome size evaluation in coffee tree. Annals of Botany 92: 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NoirotM, Barre P, Louarn J, Duperray C, Hamon S.2000. Nucleus‐cytosol interactions – a source of stoichiometric error in flow cytometric estimation of nuclear DNA content in plants. Annals of Botany 86: 309–316. [Google Scholar]
- NoirotM, Barre P, Louarn J, Duperray C, Hamon S.2002. Consequences of stoichiometric error for nuclear DNA content evaluation in Coffea liberica var. dewevrei using DAPI and propidium iodide. Annals of Botany 89: 385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PortèresR.1962. Caféiers de la république de Guinée. Café Cacao Thé 6: 3–18. [Google Scholar]
- PriceHJ, Hodnett G, Johnston JS.2000. Sunflower (Helianthus annuus) leaves contain compounds that reduce nuclear propidium iodide fluorescence. Annals of Botany 86: 929–934. [Google Scholar]
- StoffelenP.1998. Coffea and Psilanthus (Rubiaceae) in tropical Africa: a systematic and palynological study, including a revision of the West and Central African species. Leuven: Katholieke Universiteit. [Google Scholar]
- TorrellM, Vallès J.2001.Genome size in 21 Artemisia L. species (Asteraceae, Anthemideae): systematic, evolutionary, and ecological implications. Genome 44: 231–238. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.