Abstract
Casuarina glauca develops proteoid (cluster) roots in response to Fe deficiency. This study set out to investigate the possible involvement of ethylene in the initiation and/or the morphogenesis of cluster roots (CR). For this purpose, the effect of Ag+ added as silver thiosulfate, an inhibitor of ethylene action has been studied in plants growing hydroponically. No CR formation was observed in these growth conditions. Inhibition of ethylene biosynthesis by aminoethoxyvinylglycine, 1‐ aminoisobutyric acid, aminoxyacetic acid or cobalt chloride also eliminated the positive effect of Fe deficiency on CR formation in C. glauca. CR were not formed in Fe‐ deficient roots in the presence of ethylene inhibitors, suggesting a role for ethylene in the morphological responses to Fe deficiency. Interestingly, treatment of Casuarina plants with the ethylene precursor 1‐aminocyclopropane‐1‐carboxylic acid stimulated significantly the formation of CR, even if plants are supplied with Fe. However, this stimulation did not reach the level of CR obtained in Fe‐deficient plants. These results suggest that an ethylene‐mediated signalling pathway is involved in CR formation process in C. glauca.
Key words: Ethylene, Casuarina, cluster roots, ACC, ethylene inhibitors, Fe deficiency
INTRODUCTION
Cluster roots (CR) are one of the great plant adaptations to nutrient acquisition in terms of structure and function (Skene, 1998). Their anatomy, structure, function and nutritional control are well known. However, to date little is known about mechanisms that control their formation.
Plant growth regulators (hormones) have been previously implicated in proteoid root development, although there is little concrete evidence to support this hypothesis (Lamont et al., 1984; Dinkelaker et al., 1995; Gilbert et al., 1997; Neumann et al., 2000). In this context, Lamont (2003) reported that auxin and other hormones mediate CR production, and Diem et al. (2000) suggested a possible involvement of ethylene in their initiation and/or their morphogenesis.
The aim of the present work was to study the relationship between CR formation and ethylene in C. glauca growing hydroponically, using a precursor of ethylene biosynthesis and inhibitors of ethylene biosynthesis and perception.
MATERIALS AND METHODS
Pre‐germinated seeds of Casuarina glauca (Siebe ex Spreng.) collected around Rabat city (Morocco) were cultivated on autoclaved sand and irrigated by a nutrient solution (Broughton and Dilworth, 1971) containing (µm): CaCl2 (1000), KH2PO4 (500), MgSO4 (250), K2SO4 (250), H3BO3 (2), MnSO4 (1), ZnSO4 (0·5), CuSO4 (0·2), CoSO4 (0·1), Na2MoO4 (0·1) and supplemented with 500 µm KNO3. Experiments were carried out in a culture chamber at 26/20 °C day and night temperature, 14‐h day length and a relative humidity of 75 %.
Three weeks after sowing, the uniform C. glauca seedlings were removed from the sand and transferred to water culture in capped plastic pots, five seedlings per pot. Each pot contained 700 ml of nutrient solution. The nutrient solution was renewed weekly. After a 2‐week acclimatization in the complete nutrient solution, they were then assigned to various treatments. Two pots (ten replicate plants) were used for each treatment. Seedlings were harvested after 8 weeks of treatment.
Experiment 1: effect of iron
The effect of iron (FeCl3) on CR formation by C. glauca was studied. Plants were grown under iron‐deficient (0 µm) and ‐sufficient (100 µm) conditions.
Experiment 2: indirect effect of ethylene
To examine the effect of ethylene on root development, C. glauca plants were grown under iron‐deficient and/or ‐sufficient conditions in the presence of ethylene inhibitors and ethylene stimulators. Aminoethoxyvinylglycine (AVG), 1‐aminoisobutyric acid (AIB), aminoxyacetic acid (AOA) and cobalt chloride (CoCl2) are known to block ethylene biosynthesis while silver thiosulfate (STS) inhibits ethylene action. Since these inhibitors have been widely used to investigate the roles of ethylene in lateral and adventitious root development, it was thought important to determine how they impact upon proteoid root development. The interaction of Fe deficiency and/or Fe sufficiency with concentrations of stimulators and inhibitors of ethylene was studied.
Experiment 2‐1: effect of ethylene stimulators
This experiment was conducted to examine the effect of exogenous ethylene under both Fe deficiency (0 µm) and Fe sufficiency (100 µm) on CR formation. The ethylene biosynthesis precursor 1‐aminocyclopropane‐1‐carboxylic acid (ACC) was used at 1 µm.
Experiment 2‐2: effect of ethylene inhibitors
This experiment was carried out to examine under Fe deficiency the effect of ethylene biosynthesis inhibitors AVG (2 µm, 10 µm), AIB (10 mm), AOA (10 µm, 20 µm) and CoCl2 (10 µm, 100 µm) and the ethylene action inhibitor STS (50 µm, 200 µm) on CR formation. To determine the extent to which ethylene stimulators and inhibitors impact upon CR formation, the number of CR formed in hydroponically grown seedlings was determined at regular intervals.
STATISTICAL ANALYSIS
Experimental data were subjected to analysis by using the ‘Statistica’ (version 5, 97 edition) computer program. t‐Tests were applied to determine significance of difference in CR numbers between treatments, with the lowest level considered significant being P < 0·05.
RESULTS
Control
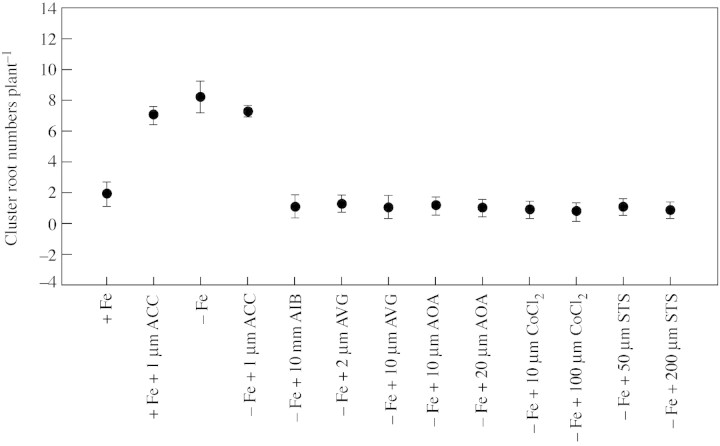
Under Fe‐deficient conditions, C. glauca seedlings had 8·2 CR per plant, compared with 1·9 CR per plant found in control plants grown under Fe‐sufficient conditions for 8 weeks (Table 1).
Table 1.
Effect of exogenous application of ethylene inhibitors and stimulator on cluster root formation in Casuarina glauca plants
| Weeks | + Fe | ACC + Fe | – Fe | ACC – Fe | AIB | AVG (2 µm) | AVG (10 µm) | AOA (10 µm) | AOA (20 µm) | CoCl2 (10 µm) | CoCl2 (100 µm) | STS (50 µm) | STS (200 µm) |
| 0 | 0·8 a | 0·8 a | 0·8 a | 0·8 a | 0·8 a | 0·8 a | 0·8 a | 0·8 a | 0·8 a | 0·8 a | 0·8 a | 0·8 a | 0·8 a |
| 1 | 1·7 a | 4·0 bc | 4·8 cde | 4·3 c | 1·0 a | 1·0 a | 1·0 a | 1·1 a | 1·0 a | 0·9 a | 0·9 a | 1·0 a | 0·9 a |
| 2 | 1·7 a | 4·9 cde | 7·3 f | 5·1 de | 1·1 a | 1·2 a | 1·1 a | 1·2 a | 1·1 a | 1·0 a | 0·9 a | 1·2 a | 1·0 a |
| 3 | 1·7 a | 5·1 de | 7·5 f | 5·4 de | 1·1 a | 1·2 a | 1·1 a | 1·2 a | 1·1 a | 1·0 a | 0·9 a | 1·2 a | 1·0 a |
| 4 | 1·8 a | 5·5 de | 7·7 f | 5·8 de | 1·1 a | 1·2 a | 1·1 a | 1·2 a | 1·1 a | 1·0 a | 0·9 a | 1·2 a | 1·0 a |
| 5 | 1·9 ab | 5·8 e | 8·2 f | 6·7 f | 1·1 a | 1·2 a | 1·1 a | 1·2 a | 1·1 a | 1·0 a | 0·9 a | 1·2 a | 1·0 a |
| 6 | 1·9 ab | 6·1 ef | 8·2 f | 7·3 f | 1·1 a | 1·2 a | 1·1 a | 1·2 a | 1·1 a | 1·0 a | 0·9 a | 1·2 a | 1·0 a |
| 7 | 1·9 ab | 6·8 f | 8·2 f | 7·3 f | 1·1 a | 1·3 a | 1·1 a | 1·2 a | 1·1 a | 1·0 a | 0·9 a | 1·2 a | 1·0 a |
| 8 | 1·9 ab | 7·0 f | 8·2 f | 7·3 f | 1·1 a | 1·3 a | 1·1 a | 1·2 a | 1·1 a | 1·0 a | 0·9 a | 1·2 a | 1·0 a |
Results are expressed as number of CR per plant.
Means followed by different superscript letters indicate significant differences according to the t‐test at P < 0·05; n = 10.
Action of ACC
Applying the ethylene precursor ACC (1 µm) exogenously to Fe‐deficient plants did not increase the formation of CR compared with those with no ACC treatment. However, applying ACC to Fe‐sufficient plants stimulated significantly, from the first week, the formation of CR, although this stimulation did not reach the level of CR obtained in Fe‐deficient plants. In Fe‐deficient plants, as well as in ACC‐treated ones, the number of CR increased continuously during the first 3 weeks (Table 1).
Action of ethylene inhibitors
The addition of either ethylene synthesis inhibitors AVG, AIB, AOA or CoCl2 or of STS, an ethylene action inhibitor, to the nutrient solution lacking Fe, completely stopped the formation of CR after the first week (Table 1). Moreover, the number of CR segments formed on root systems was dramatically reduced (Fig. 1). The percentage of plants developing CR is given in Table 2. This percentage varied with treatment: it was highest in the control (–Fe) and ACC‐treated plants and lowest in the presence of iron (without ACC) or ethylene inhibitors. Indeed, almost all the plants with ACC treatment formed CR, while only 30 % of those treated with ethylene inhibitors did so.
Fig. 1. Effect of exposure, for 8 weeks, to ethylene stimulator and inhibitors on cluster root numbers in Casuarina glauca. Error bars represent standard errors of the mean (n = 10).
Table 2.
Percentage of Casuarina glauca plants with cluster roots (8 weeks of treatment)
| Treatment | + Fe | ACC + Fe | – Fe | ACC – Fe | AIB | AVG (2 µm) | AVG (10 µm) | AOA (10 µm) | AOA (20 µm) | CoCl2 (10 µm) | CoCl2 (100 µm) | STS (50 µm) | STS (200 µm) |
| % plants with CR | 40 | 100 | 90 | 100 | 20 | 40 | 20 | 30 | 30 | 30 | 20 | 40 | 30 |
DISCUSSION
The formation of CR is the consequence of a series of interactions between plants and their environment. Many studies have shown the role of nutritional imbalance on CR formation but the signalling for CR development and metabolism remains obscure. Developmental processes are likely to be mediated through hormonal signals (Watt and Evans, 1999) and changes in hormone concentrations could be the link between development and internal nutrient concentrations. Ethylene, chemically the simplest plant hormone, may be a global regulator of root responses to soil nutrient availability, as suggested by Lynch and Brown (1997). However, the effects of ethylene on CR development remain unknown (Watt and Evans, 1999).
Precursor of ethylene biosynthesis
As a precursor of ethylene biosynthesis, ACC, when applied to plants stimulates ethylene evolution in the roots (Van Dijck et al., 1998). In our conditions, the application of ACC to Fe‐sufficient plants, but not to Fe‐deficient ones, increases the number of CR, suggesting that ethylene can enhance CR formation in C. glauca. This result is concordant with the findings of Romera et al. (1997) about the stimulation of all Fe‐deficiency responses by ACC in several plant species but discordant with those of Waters and Blevins (2000), who found that plant roots with ACC did not induce CR formation in Cucurbita pepo L.
A role for ethylene can also be inferred from the fact that root appearance under Fe stress can be mimicked by supplementing the medium with the ethylene precursor ACC (Romera and Alcantara, 1994; Landsberg, 1996), suggesting a role for ethylene in the morphological responses to Fe deficiency (Romera and Alcantara, 1994; Landsberg, 1996; Schmidt et al., 2000). However, no additional CR formation was induced by ACC treatment in Fe‐deficient plants. Since Fe deficiency is supposed to result in an enhancement of ethylene production (Lynch, 1998; Romera et al., 1999), this could be explained by the production of ethylene in the absence of Fe, independently of the presence of ACC (1 µm). Furthermore, the absence of responsiveness to ACC application could be related to insufficient treatment duration and/or the concentration used. In this regard, Lai et al. (2000) reported that during weeks 2 and 3, only flasks supplemented with 4 and 8 µm ACC remained significantly higher than the control in ethylene levels.
Inhibitors of ethylene biosynthesis and perception
During the first weeks, a slight CR formation occurred despite addition of inhibitors. According to Lai et al. (2000), this is probably because ethylene levels were not changed significantly by treatments with AVG (0, 0·5 and 8 µm) or CoCl2 (0·1, 5 and 50 µm) in weeks 1, 2 and 3. After 3 weeks of treatment, the number of CR remained constant (Table 1), suggesting that ethylene inhibitors stopped CR formation. The stimulatory effect of iron stress on CR formation (Arahou and Diem, 1997) could be eliminated by the addition of AIB, AVG, AOA and CoCl2. Interestingly, Ag+ (STS) caused a significant decrease in CR numbers in Casuarina roots (Table 1).
Blocking ethylene biosynthesis or perception by using AIB, AVG, AOA, CoCl2 or Ag+ prevents the production of CR. However, no significant difference regarding the extent of inhibition under various inhibitor treatments is observed (Table 1). The morphology of the root system did not change with the addition of inhibitors, but a slight thickening of the roots occurred at high concentrations. The significant fluctuation in numbers of CR in C. glauca by application of inhibitors is in apparent contradiction with the observations of Gilbert et al. (2000), working on Lupinus albus L. seedlings who did not observe any effect on CR numbers in the presence of ethylene inhibitors (AVG and STS) applied to the nutrient solution or applied to the leaves of –P and +P plants. This apparent difference could be explained by the duration of AVG and STS treatments (2 weeks) in the experimentation of Gilbert et al. (2000), which was not sufficient to induce changes in CR formation. Moreover, while the AVG concentration (10 µm) used by Gilbert et al. (2000) was similar to ours (8 µm), that of STS was ten‐fold lower (10 µm).
During the analysis of CR formation kinetics (Table 1), the response of Casuarina differed between inhibitors and stimulators of ethylene. The treatment of Fe‐sufficient seedlings with the ethylene precursor ACC triggers the development of CR, and blocking either ethylene biosynthesis or perception causes a reduction in the frequency of CR. These results suggest that in iron‐deficient plants, ethylene would have a role (either directly or in the signal transduction pathway) in the formation of CR in C. glauca, while the findings of Gilbert et al. (2000) suggest that the formation of CR does not depend on ethylene concentration but rather depends on auxin. However, it is interesting to note that these authors, working on L. albus, studied ethylene in relation to P deficiency while our research on C. glauca examined the link existing between ethylene and Fe deficiency.
Due to the highly differentiated morphology of CR, hormonal interactions during the development of these root structures are likely to be quite complex (Neumann et al., 2000). On the other hand, ethylene has been suggested to increase sensitivity of roots to auxin (Visser et al., 1996). Thus, an increase in auxin concentration and increased sensitivity to auxin as a result of increased ethylene production may be the stimulus for CR formation in Fe‐deficient plants as suggested by Waters and Blevins (2000) on Cucumis sativus L.
The results presented here demonstrate the importance of ethylene in mediating CR formation in response to iron deficiency in C. glauca. However, ethylene is not the sole regulating factor in CR formation, and acts synergistically with another regulating factor (or factors), which may represent a further pathway in which ethylene acts. Ethylene could, therefore, mediate changes in root morphology via both changes in synthesis and changes in tissue responsiveness as suggested by Borch et al. (1999).
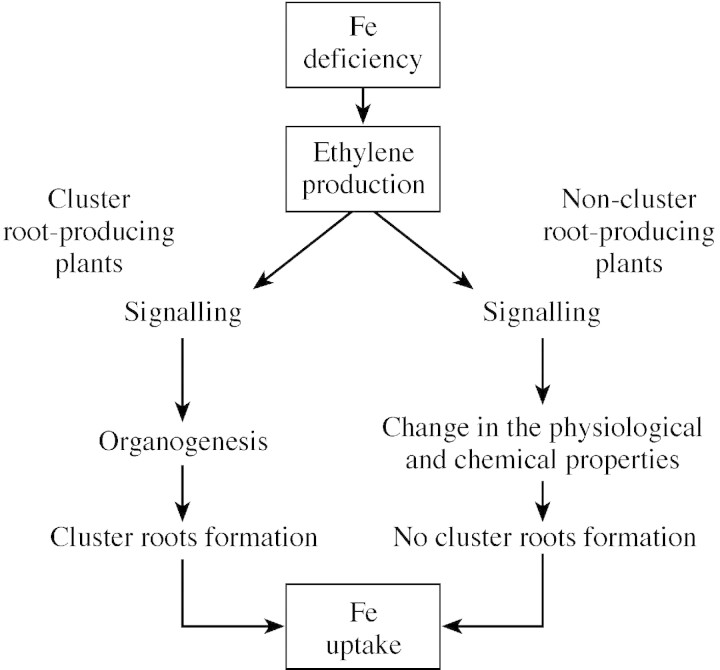
After considering the results of this work and the observations of Romera et al. (1999), it is suggested that the action of ethylene, as a regulating agent of plant responses to Fe deficiency, varies according to whether the plant is able to produce CR or not (Fig. 2). This hormone may (a) induce changes in the chemical properties of roots of non‐cluster root‐producing plants, as reported by Romera et al. (1999) (in this case, ethylene acts as a signal to trigger the expression of genes responsible for physiological and chemical changes to improve Fe uptake), or (b) be implicated in the signalling process leading to CR formation that is an alternative to enhance the plant’s ability to acquire Fe in species that are able to produce them (the authors’ findings). Thus, ethylene is involved in the regulation of morphological changes associated with the adaptation to low Fe as hypothesized by Diem et al. (2000). In favour of this last suggestion, studies have demonstrated that ethylene plays an important role in growth and organogenesis (Kumar et al., 1997) and in the modification of root architecture by affecting root gravitropism (Lee et al., 1990; Lynch and Brown, 1997) root extension, root elongation, radial expansion and aerenchyma formation (Reid, 1995; Dolan, 1997; Clark et al., 1999). Van Bruaene et al. (1998) provided evidence that ethylene is, at least in part, involved in the reorientation of cell expansion from the longitudinal to the radial direction.
Fig. 2. Action of ethylene as a regulating agent of plant responses to Fe deficiency.
The formation of CR seems to be the exclusive property of plant species presenting great morphological root plasticity. According to the observations of Fernandez‐Lopez et al. (1998) and Zhang and Forde (1998), it is suggested that species producing CR should contain genes responsible for a phenotypic plasticity, which could modify the architecture of the plant root system and adapt it to the nutritional characteristics of the soil. The action of ethylene can also be variable because the production of CR probably depends on the close relationship between plasticity of the root development and nutritional imbalance.
CONCLUSION
In this work, three observations must be highlighted: (1) the confirmation of CR formation by Casuarina glauca when Fe is removed from the nutrient solution, (2) the stimulation of CR formation by the addition of the ethylene precursor ACC in the presence of Fe, and (3) the prevention of CR formation by inhibitors of ethylene production or action under Fe deficiency.
These observations suggest that ethylene is implicated in CR formation under Fe deficiency and it appears that ethylene‐stimulated CR formation is a necessary, but not sufficient condition for the up‐regulation. Nevertheless, knowledge regarding how and in which step ethylene induced CR formation is still quite limited. Further experiments, especially the measurement of ethylene production during CR formation under Fe deficiency, are required.
Supplementary Material
Received: 28 May 2003;; Returned for revision: 6 July 2003. Accepted: 25 July 2003; Published electronically: 10 September 2003
References
- ArahouM, Diem HG.1997. Iron deficiency induces cluster (proteoid) root formation in Casuarina glauca Plant and Soil 196: 71–79 [Google Scholar]
- BorchK, Bouma TJ, Lynch JP, Brown KM.1999. Ethylene: a regulator of root architectural responses to soil phosphorus availability. Plant, Cell and Environment 22: 425–431. [Google Scholar]
- BroughtonWT, Dilworth MJ.1971. Control of leghaemoglobin synthesis in snake beans. Biochemistry Journal 125: 1075–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ClarkDG, Gubrium EK, Barrett EJ, Nell TA, Klee HJ.1999. Root formation in ethylene‐insensitive plants. Plant Physiology 121: 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiemHG, Duhoux E, Zaid H, Arahou M.2000. Cluster roots in Casuarinaceae: role and relationship to soil nutrient factors. Annals of Botany 85: 929–936 [Google Scholar]
- DinkelakerB, Hengler C, Marschner H.1995. Distribution and function of proteoid roots and other root clusters. Botanical Acta 108: 183–200. [Google Scholar]
- DolanL.1997. The role of ethylene in the development of plant form. Journal of Experimental Botany 48: 201–210. [Google Scholar]
- Fernandez‐LopezM, Goormachtig S, Gao M, D’Haeze W, Van Montagu M, Holsters M.1998. Ethylene‐mediated phenotypic plasticity in root nodule development on Sesbania rostrata Proceedings of the National Academy of Sciences USA 95: 12724–12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GilbertGA, Knight JD, Vance CP, Allan DL.2000. Proteoid root development of phosphorus deficient lupin is mimicked by auxin and phosphonate. Annals of Botany 85: 921–928. [Google Scholar]
- GilbertGA, Knight JD, Vance CP, Allan DL.1997. Does auxin play a role in the adaptations of white lupin roots to phosphate deficiency? Plant Physiology 114: S31. Abstract no. 67. [Google Scholar]
- KumarPP, Lakshmanan P, Thorpe TA.1997. Regulation of morphogenesis in plant tissue culture by ethylene (review article). In vitro Cellular and Developmental Biology Plant 34: 94–103. [Google Scholar]
- LaiCC, Yeh SD, Yang JS.2000. Enhancement of papaya axillary shoots proliferation in vitro by controlling the available ethylene. Botanical Bulletin of Academia Sinica 41: 203–212. [Google Scholar]
- LamontBB.2003. Structure, ecology and physiology of root clusters – a review. Plant and Soil 248: 1–19. [Google Scholar]
- LamontBB, Brown G, Mitchell DT.1984. Structure, environmental effects on their formation, and function of proteoid root in Leucadendron laureolum (Proteaceae). New Phytologist 97: 381–390. [Google Scholar]
- LandsbergEC.1996. Hormones regulation of iron‐stress response in sunflower roots: a morphological and cytological investigation. Protoplasma 194: 69–80. [Google Scholar]
- LeeJS, Chang WK, Evans ML.1990. Effects of ethylene on the kinetics of curvature and auxin redistribution in gravistimulated roots of Zea mays Plant Physiology 94: 1770–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LynchJ.1998. Root architecture and phosphorus acquisition efficiency in common bean. In: Flores HE, Lynch JP, Eissenstst D, eds. Radical biology: advances and perspectives on the function of plant roots. Rockville, MD: American Society of Plant Physiologists, 81–91. [Google Scholar]
- LynchJ, Brown KM.1997. Ethylene and plant responses to nutritional stress. Physiologia Plantarum 100: 613–619. [Google Scholar]
- NeumannG., Massanneau A, Langlade N, Dinkelaker B, Hengler C, Romheld V, Matrinoria E.2000. Physiological aspects of cluster root function and development in phosphorus deficient white lupin (Lupinus albus L.). Annals of Botany 82: 909–919. [Google Scholar]
- ReidMS.1995. Ethylene in plant growth, development, and senescence. In: Davies PJ, ed. Plant hormones Dordrecht: Kluwer Academic Publications, 486–508. [Google Scholar]
- RomeraFJ, Alcantara E.1994. Iron‐deficiency stress responses in cucumber (Cucumis sativus L.) roots. Plant Physiology 105: 1133–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RomeraFJ, Alcantara E, De la Guardia MD.1997. Iron deficiency stimulates the production of ethylene by roots of strategy I plants. Ninth International Symposium on Iron Nutrition and Interaction in Plants, Universitat Hohenhein, Stuttgart, Germany. Abstract no. S4‐1. [Google Scholar]
- RomeraFJ, Alcantara E, De la Guardia MD.1999. Ethylene production by Fe‐deficient roots and its involvement in the regulation of Fe‐deficiency stress responses by strategy I plants. Annals of Botany 83: 51–55. [Google Scholar]
- SchmidtW, Schikora A, Pich A, Bartels M.2000. Hormones induce a Fe‐deficiency‐like root epidermal cell pattern in the Te‐inefficient tomato mutant fer. Protoplasma 213: 67–73. [Google Scholar]
- SkeneKR.1998. Clusters root: some ecological considerations. Journal of Ecology 86: 1060–1064. [Google Scholar]
- VanBruaeneN, Van Caeneghem W, Van Der Streaten D, Van Oostveldt P.1998. Root hair development in Arabidopsis thaliana A confocal microscopy study. Med. Fuc. andbouww. University of Gent, Gent, Belgium, 63a/3a, 845–851. [Google Scholar]
- VanDijckRM, De Proft, De Greef J.1998. Role of ethylene and cytokinins in the initiation of lateral shoot growth in bromeliads. Plant Physiology 86: 836–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VisserEJW, Cohen JD, Barendse GWM, Blom CWPM, Voesenek LACJ.1996. An ethylene mediated increase in sensitivity to auxin induces adventitious root formation in flooded Rumex palustris Sm. Plant Physiology 112: 1687–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WatersBM, Blevins DG.2000. Ethylene production, cluster root formation, and localization of iron (III) reducing capacity in Fe deficient squash roots. Plant and Soil 225: 21–31. [Google Scholar]
- WattM, Evans JR.1999. Proteoid roots: physiology and development. Plant Physiology 121: 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZhangH, Forde BG.1998. An Arabidopsis MADS box gene that controls nutrient‐induced changes in root architecture. Science 279: 407–409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.