Abstract
Few Neotropical plant species seem to depend on the same animal type both for pollination and seed dispersal, and the known instances refer mostly to birds as the agents in these two phases of a plant reproductive cycle. Dyssochroma viridiflorum (Solanaceae), an epiphyte endemic to the Atlantic rainforest in south‐eastern Brazil, was found to be visited by phyllostomid bats for nectar as well as for fruits, with the pollination and seed dispersal of the plant ensured by these flying mammals. The greenish flowers open at night and are visited by the nectar‐feeding bat Glossophaga soricina, whereas the yellowish‐white fruits are consumed by two species of fruit‐eating bats, Carollia perspicillata and Sturnira lilium. Only clinging visits, an uncommon behavioural pattern for glossophagine bats while feeding on flowers, were recorded. The small seeds of D. viridiflorum are swallowed along with the fruit pulp and later defecated on the bats’ flying pathways. It is suggested that species of Dyssochroma and two other solanaceous bat‐pollinated genera, Merinthopodium and Trianaea, form a derived and bat‐dependent clade within the Juanulloeae.
Key words: Dyssochroma; Solanaceae; reproduction; bat‐pollination; Glossophaga, bat‐dispersal; Carollia; Sturnira; Phyllostomidae; rainforest; south‐eastern Brazil
Introduction
Few Neotropical plant species seem to depend on the same animal type both for pollination and seed dispersal, and the known cases refer mostly to birds as the agents in these two phases of a plant reproductive cycle. For instance, species of Bromeliaceae and Costaceae are pollinated mainly by hummingbirds, and dispersed by passerine birds and small mammals in south‐eastern Brazil (Fischer and Araujo, 1995; Buzato et al., 2000; I. Sazima, pers. obs.). As the distribution of bat flowers and fruits among orders of angiosperms is remarkably concordant (Fleming, 1988) it would be expected that these would be found within the same orders, families, and in certain cases, even genera. Indeed, some species of Cactaceae in Central and northern South America are pollinated and, to some extent, dispersed by phyllostomid bats (e.g. Petit, 1997; Martino et al., 2002). It was observed that Dyssochroma viridiflorum (Sims) Miers, an epiphytic solanaceous plant endemic to the Atlantic rainforest in south‐eastern Brazil, southern South America (Hunziker, 1979; Knapp et al., 1997), also has its flowers pollinated and its seeds dispersed by phyllostomid bats. Dyssochroma belongs in the tribe Juanulloeae, a group composed of six genera of poorly known, rarely collected epiphytic shrubs and small trees distributed over the Neotropics (Knapp et al., 1997). However, Olmstead et al. (1999) and Hunziker (2001) recognize nine genera in this group. Within Juanulloeae (Juanulloinae of Olmstead et al., 1999), species of Dyssochroma, Merinthopodium and Trianaea are recorded as, or supposed to be, pollinated by bats (Vogel, 1958; Baker, 1973; Voss et al., 1980; Dobat and Peikert‐Holle, 1985; Knapp et al., 1997). For present taxonomic allocation of several genera and species presented in earlier papers and referred to herein, see Olmstead et al. (1999) and Hunziker (2001).
Here the floral biology and bat‐pollination, as well as bat‐frugivory and seed dispersal of Dyssochroma viridiflorum is described, and it is predicted that species in the other two bat‐pollinated genera of Juanulloeae, Merinthopodium and Trianaea, may prove to be bat‐dispersed as well.
MATERIALS AND METHODS
The study sites are in the coastal lowlands covered by sub‐humid evergreen broadleaf forest (Eiten, 1970; Sazima et al., 1999) at Picinguaba (about 23°20′S, 44°52′W, 0–10 m a.s.l.) and Praia Dura (about 23°30′S, 45°07′W, at sea level) in Ubatuba, São Paulo, south‐east Brazil. Average annual rainfall at the study sites is 2526 mm, and average annual temperature is 22·7 °C (Sazima et al., 1999; Buzato et al., 2000). The bulk of data on plants and bats was obtained during two nights in March and two nights in April 1998, with supplementary data on flowers and fruits recorded from March 2001 to December 2002. The growth habit, flowering phenology and flower features such as morphology, phases of anthesis, colour, odour, volume and concentration of nectar were recorded (cf. Faegri and Pijl, 1980; Sazima et al., 1999). The phenology was recorded monthly on a pool of 17 individuals to characterize the flowering of the studied population (cf. Newstrom et al., 1994). The internal length of the flower corolla was measured from base to opening (effective length; cf. Wolf et al., 1976). Nectar concentration and volume were measured 2 h after flower opening (visitors excluded from measured flowers), with a pocket refractometer and microlitre syringes, respectively (cf. Kearns and Inouye, 1993). Nectar scent was assessed in situ and out of the flower (flowers with no nectar are odourless). Flower shape nomenclature follows Faegri and Pijl (1980). Fruit and seed features such as morphology, colour, shape, and size were also recorded. Colour names of flowers, fruits and seeds follow Kornerup and Wanscher (1963). Stigma receptivity was tested by the H2O2‐catalase activity method (Zeisler, 1938) and pollen viability was estimated by cytoplasmic staining, using the aceto‐carmine technique (Radford et al., 1974). Controlled pollinations were performed on eight individual plants in order to establish the breeding system following methods outlined in Radford et al. (1974). Fruit set was recorded on maturing fruits. For pollen tube growth, fixed hand‐pollinated pistils were analysed under fluorescence microscopy (Martin, 1959). Plant vouchers are deposited in the herbarium of the Universidade Estadual de Campinas (UEC 119286, 119287). Bats were photographed on flowers and fruits during visits and species identification was based on photographs. Species recognition at a well‐studied site may be very reliable even at species level (see Sazima and Sazima, 1977; Sahley, 1995; Sazima et al., 1999), and depends on a good knowledge of species occurring in the area. Photographs of bat visits on flowers were used to assess pollen placement on the body parts of bats, and mist‐netted individuals were examined for seed loads in their faeces.
RESULTS AND DISCUSSION
Plant habit, phenology, flower features and floral biology
At the study sites Dyssochroma viridiflorum occurs mainly as a saxicolous (rock‐dwelling) shrub near the seashore or as an epiphyte on various tree species. Saxicolous habit is not mentioned by Knapp et al. (1997), but seems to be a common feature for this species in the lowland rainforest (Hunziker, 1979, 2001). It was found that, at the study sites, D. viridiflorum blooms year‐round, a phenological pattern defined as continual by Newstrom et al. (1994). Such a phenological pattern is unusual in Neotropical bat‐pollinated species and until now it has been recorded for Marcgravia serrae (Marcgraviaceae) in Costa Rica (Tschapka and Helversen, 1999), and for Marcgravia polyantha, Abutilon aff. regnellii (Malvaceae) and Irlbachia alata (Gentianaceae) in Brazil (Machado et al., 1998; Sazima et al., 1999). A given D. viridiflorum individual may bear buds, open flowers, developing fruits and ripe fruits at the same time, thus providing night meals for both nectarivorous and frugivorous bats (see below). One individual may produce one to ten flowers per night, a trait which promotes the trapline mode of foraging and seems widespread amongst bat‐pollinated species (Heithaus et al., 1975; Sazima et al., 1999). Inflorescences are terminal, which agrees with the records of Knapp et al. (1997), and bear one to four flowers at a time. Mostly one and, occasionally, two open flowers per inflorescence per night were found, a common trait in the bat‐pollinated species of Merinthopodium and Trianaea (Solanaceae) (Vogel, 1958, 1969; Voss et al., 1980), as well as in several other bat‐pollinated species (e.g. Irlbachia alata, Machado et al., 1998; Vriesea spp., Bromeliaceae, Sazima et al., 1999). Peduncles of D. viridiflorum are short (30–40 mm), and the flowers point downwards (Fig. 1A and B). Such an orientation in Neotropical bat‐pollinated flowers is uncommon, but it is known also to occur in species of the two other solanaceous genera, Merinthopodium and Trianaea (Voss et al., 1980; Knapp et al., 1997).
Fig. 1. A, Glossophaga soricina about to visit a newly opened flower. Note the downward orientation of Dyssochroma viridiflorum flowers with both stamens and style exerted beyond the corolla opening, and whitish pollen from previous visits on the bat belly. B, The same bat species clinging to an already visited first‐day flower. Note the bat left wing claw grasping the flower, the claw marks on the corolla, and the anthers touching the bat hind‐body. C, Carollia perspicillata biting off a piece of a ripe fruit. Note the bat feet grasping the fruit. D, Sturnira lilium about to bite off a piece of an already chewed fruit. Note yellowish white pulp and brownish, small seeds.
The broadly flaring to campanulate corolla of D. viridiflorum (Fig. 1A and B) has an average effective length of 63·7 mm (s.d. = 5·7, n = 10), and the average largest diameter of the corolla opening is 30 mm (s.d. = 3·5, n = 10). The corolla is greenish externally and yellowish‐green internally, both stamens and style being green and exerted to about the same level, well beyond the corolla opening (Fig. 1A and B) even in flowers in post‐flowering stage. There are no modifications in the position of the sexual organs during flowering. Filaments are hairy at the lower quarter of its length, a device which may prevent the nectar from dropping by holding it through capillary forces, as recorded in other pendulous bat‐flowers (e.g. Merinthopodium; see Helversen, 1993). Anthers are large (10–18 mm), and dehisce longitudinally to expose copious, whitish, and somewhat sticky pollen. The style is glabrous, and the green, clavate stigma is placed in the middle of the anthers (Fig. 1B). Morphological features of the flowers fit well into the description of Hunziker (2001) and Knapp et al. (1997), although the latter authors note that filaments are glabrous along their whole length.
Young buds of D. viridiflorum are protected by the calyx and immersed in watery fluid, the so‐called water calyces which occur in some Solanaceae and related families (Endress, 1994). Buds in pre‐anthesis are swollen and open in a popping‐like way, the corolla lobes moving very quickly, becoming strongly flexed in about 2 min (Fig. 1A and B). The flowers open nearly synchronously around nightfall (1830 h), but sometimes flower opening was delayed until early night, at 2000 h, mostly on cold nights. Flowers last two nights, a feature recorded for a number of other glossophagine‐pollinated species (Helversen, 1993; Machado et al., 1998; Sazima et al., 1999). Nectar is available in the flower upon opening and has a mushroom‐like scent [Hunziker (2001) notes that flowers are ‘perfumed’], scented nectar being recorded for several chiropterophilous plant species (Sazima et al., 1999).
Mean nectar production and concentration of D. viridiflorum is about the same on the first and the second nights, respectively: volume 189·8 ± 117·5 µL (n = 10) and 189·0 ± 128·7 µL (n = 5), concentration 21·7 ± 2·7 % (n = 10) and 18·2 ± 2·01% (n = 5). The nectar concentration range is slightly above the average of the frequency distribution presented by Helversen (1993) for 33 neotropical bat‐pollinated species. During daytime, the available nectar is taken by the hummingbird Eupetomena macroura, which pierces the corolla base. The long corolla of D. viridiflorum prevents this hummingbird from legitimately visiting the flowers, and thus it does not act as a pollinator as recorded for other non‐ephemeral chiropterophilous flowers (Buzato et al., 1994; Sazima et al., 1994).
The stigmatic surface is receptive on flower opening and remains so throughout the second night. Pollen is available on flower opening, easily shed at touch, and most of it is depleted during the first night. Pollen viability is about 93 % throughout the two nights of anthesis. Fruit set from open pollinations (natural conditions) is high, about 70 %, which indicates that bats are able pollen vectors since D. viridiflorum is self‐incompatible, a trait confirmed by hand‐pollination treatments (Table 1). In spite of the pollen tubes’ development along the style (Fig. 2A), rejection of self‐pollen may occur very late within the ovules in some species of Solanaceae (Aguilar and Bernardello, 2001), and this may indeed be the case in D. viridiflorum. Further studies may indicate the presence of an ovarian self‐incompatibility system in this species.
Table 1.
Fruit set from autonomous, manual self‐pollinated and open‐pollinated Dyssochroma viridiflorum flowers
| Treatments | Fruit set (%) |
| Autonomous self‐pollination | 0 (0/17) |
| Manual self‐pollination | 0 (0/12) |
| Open pollination | 72 (18/25) |
Figures in parenthesis are number of fruits/number of flowers.
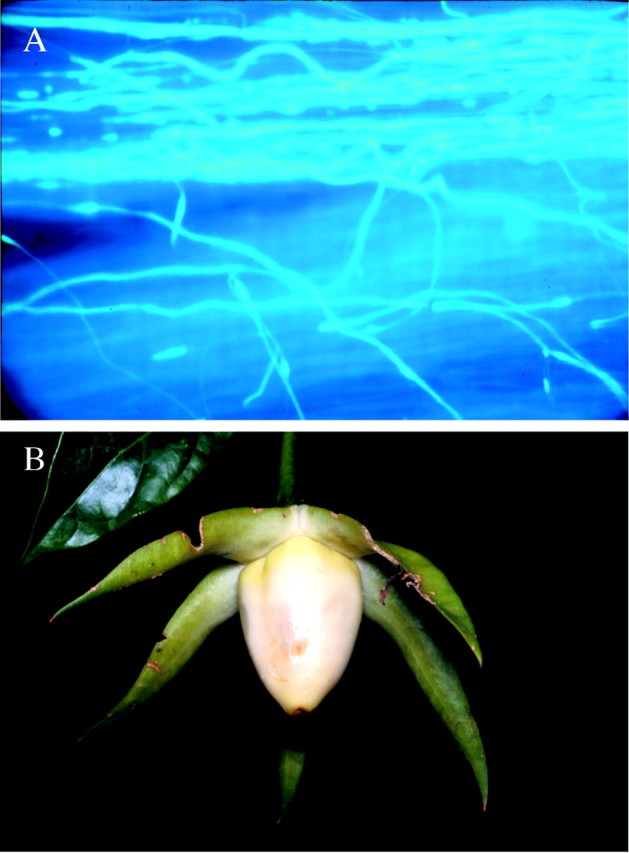
Fig. 2. A, Self‐pollen tubes growing along the style. B, A ripe fruit of D. viridiflorum exposed by the very fleshy, flexed calyx lobes which totally hide the berry until it is ripe.
Fruits ripen in 30–35 d, during which period they are fully covered by the calyx lobes. Ripe fruits are conical, yellowish‐white berries, and completely exposed by the very fleshy flexed calyx lobes (Fig. 2B). Thus, the exposed fruits may be detected by the two bat species by both olfaction (long‐distance) and echo‐location (short‐distance) (see Thies et al., 1998). Fruits are 25–30 mm long, 20–25 mm at greatest diameter, and have a sweet, tomato‐like scent. Each berry produces many brown reniform seeds which are 2 × 4 mm. Morphological features of the fruit and seeds studied are essentially the same as those described by Hunziker (2001), and do not differ substantially from those of the other, probably bat‐dispersed species of Trianaea and Merinthopodium (Knapp, 1997).
Bat visits to flowers and fruits
Three phyllostomid bat species were recorded visiting Dyssochroma viridiflorum, one species visiting the flowers, and two species feeding on the ripe fruits. The flower‐visitor was the ubiquitous long‐tongued bat Glossophaga soricina (Glossophaginae) (Fig. 1A and B), and the fruit‐eaters were the short‐tailed bat Carollia perspicillata (Carolliinae) (Fig. 1C), and the yellow‐shouldered bat Sturnira lilium (Stenodermatinae) (Fig. 1D). Glossophagines are small bats with a manoeuvrable flight well suited to taking nectar from flowers, whereas carolliines and stenodermatines are slightly larger and heavier, but nevertheless still suited to taking fruits from delicate branches or visit flowers for nectar (Sazima 1976; Sazima and Sazima, 1978; Heithaus, 1982; Sazima et al., 1999).
The first bats arrive at the flowering and fruiting plants at nightfall (1820–1830 h). After complete flower opening (1830–2000 h), long‐tongued bats start visiting the flowers. On the first visit, the bat makes a flight pass close to the flower, these passes being repeated over the night and interspersed with visits to the sampled flower (for scented nectar as a clue for bats, see Sazima et al., 1999). This reconnaissance may indicate that the bats are continually assessing the state of a given flower. Alternatively, the bats may be assessing conspecific odour signals, which would indicate that a given flower was recently visited by another bat. Both behaviours would minimise overlap in the use of the same flower’s resource (Lemke, 1984; Buzato et al., 1994).
During the actual visit, the long‐tongued bat approaches the flower from below and clings on to it, while tucking its snout into the corolla (Fig. 1A and B). During the clinging visit the bat’s wing claws grasp the flower and pierce the corolla walls, leaving clearly visible marks (‘Krallenspuren’ of Vogel, 1958, 1969). The claw marks are useful in assessing the number of visits to a given flower (as the bat does not change position while on the flower) and are still used as indirect evidence of bat visits to a given flower species (e.g. Helversen, 1993; for similar marks left by hummingbirds, see Buzato et al., 1994). The clinging visit is brief (about 500 ms), during which time the bat hangs on the flower, its feet bent and the interfemural membrane folded backwards, probably so as not to be spoiled further with pollen (Fig. 1B). The bat leaves the flower by releasing its grasp on the corolla and letting itself drop a little (most likely in order to gain free space) before resuming flight.
The bats’ clinging visits to D. viridiflorum are probably because of the combination of a long corolla and a pendulous, vertical position making it difficult to take nectar while hovering below the flower. At flowers with an even longer corolla, such as those of Hillia illustris (about 62 mm), the long‐tongued bats are able to take nectar while hovering (Sazima et al., 1999). Thus, pictures of bats hovering below flowers of Trianaea spp. (Baker, 1973; Helversen 1993; Walker, 2001, the two latter as Markea) show the approach phase either of a clinging or a hovering visit. It is predicted that other pendulous and long‐flowered, bat‐pollinated species in Juanulloeae are visited in a way similar to that described here for Dyssochroma.
The first visit to a D. viridiflorum fruit is similar to that on a flower, as the bat makes a close flight pass before the first actual contact (for similar findings, see Kalko and Condon, 1998; Thies et al., 1998). As the fruit is scented, it is quite probable that during the pass the bat evaluates the state of a given fruit, including its ripeness and accessibility on the branch (Fleming et al., 1977; Thies et al., 1998). Additionally, the flexed calyx exposes the fruit making it accessible to echo‐location reconnaissance (Kalko and Condon, 1998).
The actual visits differ between the two bat species, as Carollia perspicillata habitually approaches the fruit from below and lands on it directly, clinging to the fruit head‐up with use of its wing and feet claws (Fig. 1C), whereas Sturnira lilium approaches the fruiting branch from above, lands on it and clings on to both the branch and the fruit head‐down (Fig. 1D). These two approaches may reflect the flying ability of the two bats, as Carollia has a manoeuvrable flight comparable to that of Glossophaga (Sazima, 1976; Sazima and Sazima, 1978), whereas Sturnira displays a less manoeuvrable flight, similar to that of other small to medium‐sized fruit‐eating stenodermatines (Heithaus et al., 1975; Sazima and Sazima, 1975).
From these different postures, both bat species habitually chew off a piece of the fruit within a very short visit (1–2 s) and leave the fruit in a way similar to the flower visit described for the long‐tongued bat. Faeces of bats caught with mist nets, as well as faeces sprayed over the vegetation and boulders contained up to 11 seeds from D. viridiflorum per sample, along with seeds from Piper spp. (Piperaceae) and Solanum spp. (Solanaceae).
A given flower on a D. viridiflorum plant was visited at 20–60‐min intervals by one or two individual bats, in about four to six visits over a 4‐h period. On the other hand, a given fruit was visited in pulses composed of a series of repeated landings by one to four individual bats flying around the plant and succeeding each other, interspersed by 5–15‐min intervals, in about 30–40 visits until completely eaten away in about 1 h. Dyssochroma viridiflorum offers few flowers and/or fruits per night and is attended by two bat groups with different foraging strategies. Glossophagine bats are regarded as foraging along a trapline route (Baker, 1973; Fleming, 1982; Lemke, 1984), a pattern well suited for most bat‐pollinated species in the Atlantic rainforest (Sazima et al., 1999). On the other hand, stenodermatine and carolliine bats usually forage by commuting from one food source to another, and may feed in groups (Heithaus et al., 1975; Fleming et al., 1977; Fleming, 1982). As one fruit of D. viridiflorum may be consumed in about 1 h, and as there are no more than two fruits per plant, this plant offers a very limited food supply to fruit‐eating bats. The small seeds of Dyssochroma are easily swallowed along with the pulp by small fruit‐bats such as Carollia and Sturnira and dispersed along their flying paths, in a fate similar to other small‐seeded plants such as Cecropia (Cecropiacae) and Piper (Heithaus et al., 1975; Heithaus, 1982; Fleming, 1982).
Concluding remarks
Most, if not all, species of Markea recorded as bat‐pollinated (Vogel, 1958; Voss et al., 1980; Dobat and Peikert‐Holle, 1985; Walker, 2001) are presently merged within either the genera Merinthopodium or Trianaea (Knapp et al., 1997). Most probably there is not a bat‐pollinated Markea, the species of this genus being pollinated by hummingbirds or insects (Vogel, 1969; Cocucci, 1999). The clade containing Dyssochroma, Merinthopodium and Trianaea (all three genera with bat‐pollinated species), is regarded as one of the most derived within Juanulloeae (Knapp et al., 1997). The sister‐group Juanulloa parviflora and the next clade, composed of J. speciosa and J. ochracea, conform to the hummingbird‐pollination habit (Knapp et al., 1997). This situation seems to strengthen the hypothesis that several Neotropical bat‐pollinated plant groups evolved from bird‐pollinated ancestors, an idea supported by studies by several authors (e.g. Vogel, 1969, 1980; Gottsberger, 1972, 1986; Sazima and Sazima, 1988; Buzato et al., 1994; Sazima et al., 1994).
Judging from literature accounts of bat visits to flowers of Trianaea and Merinthopodium (Vogel, 1958; Baker, 1973; Voss et al., 1980; Walker, 2001) and description of their fruits (Knapp et al., 1997), species in these two genera share phyllostomid bats with Dyssochroma for pollination and seed dispersal (e.g. Dinerstein, 1986). Thus, it is suggested that these three genera probably form a derived, bat‐dependent clade within the Juanulloeae (Knapp et al., 1997). Molecular studies may strengthen or weaken this hypothesis.
ACKNOWLEDGEMENTS
We thank Volker Bittrich, João Renato Stehmann, Marco Tschapka and an anonymous reviewer for kindly reading the manuscript (J.R.S. also for confirming the identity of the population studied); the Instituto Florestal for permission to work at the Núcleo Picinguaba of the Parque Estadual da Serra do Mar; and the CNPq for essential financial support. This paper is dedicated to Ricardo and Cristina Sazima in appreciation for the nights of their childhood that they shared with us (not always willingly on their part, it has to be said) studying bats and flowers.
Supplementary Material
Received: 30 May 2003;; Returned for revision: 17 July 2003. Accepted: 30 July 2003; Published electronically: 19 September 2003
References
- AguilarR, Bernardello G.2001. The breeding system of Lycium cestroides: a Solanaceae with ovarian self‐incompatibility. Sexual Plant Reproduction 13: 273–277. [Google Scholar]
- BakerHG.1973. Evolutionary relationships between flowering plants and animals in American and African tropical forests. In: Meggers BJ, Ayensu ES, Duckworth WD, eds. Tropical forest ecosystems in Africa and South America: a comparative review. Washington, DC: Smithsonian Institution, 145–159. [Google Scholar]
- BuzatoS, Sazima M, Sazima I.1994. Pollination of three species of Abutilon (Malvaceae) intermediate between bat and hummingbird flower syndromes. Flora 189: 327–334. [Google Scholar]
- BuzatoS, Sazima M, Sazima I.2000. Hummingbird‐pollinated floras at three Atlantic forest sites. Biotropica 32: 824–841. [Google Scholar]
- CocucciA.1999. Evolutionary radiation in neotropical Solanaceae. In: Nee M, Symon DE, Lester RN, Jessop JP, eds. Solanaceae, Vol. IV Kew, Royal Botanic Gardens, 9–22. [Google Scholar]
- DinersteinE.1986. Reproductive ecology of fruit bats and the seasonality of fruit production in a Costa Rican cloud forest. Biotropica 18: 307–318. [Google Scholar]
- DobatK, Peikert‐Holle T.1985.Blüten und Fledermäuse, Bestäubung durch Fledermäuse und Flughunde (Chiropterophilie). Frankfurt: Waldemar Kramer. [Google Scholar]
- EitenG.1970. A vegetação do Estado de São Paulo. Boletim do Instituto de Botânica (São Paulo) 7: 1–147. [Google Scholar]
- EndressPK.1994.Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press. [Google Scholar]
- FaegriK, van der Pijl L.1980.The principles of pollination ecology. Oxford: Pergamon Press. [Google Scholar]
- FischerEA, Araújo AC.1995. Spatial organization of a bromeliad community in the Atlantic rainforest, south‐eastern Brazil. Journal of Tropical Ecology 11: 559–567. [Google Scholar]
- FlemingTH.1982. Foraging strategies in plant‐visiting bats. In: Kunz TH, ed. Ecology of bats. New York: Plenum Press, 287–325. [Google Scholar]
- FlemingTH.1988.The short‐tailed fruit bat. Chicago: University of Chicago Press. [Google Scholar]
- FlemingTH, Heithaus ER, Sawyer WB.1977. An experimental analysis of the food location behavior of frugivorous bats. Ecology 58: 619–627. [Google Scholar]
- GottsbergerG.1972. Blütenbiologische Beobachtungen an brasilian ischen Malvaceen. Österreichische Botanische Zeitschrift 120: 439–509. [Google Scholar]
- GottsbergerG.1986. Some pollination strategies in Neotropical savannas and forests. Plant Systematics and Evolution 152: 29–45. [Google Scholar]
- HeithausER.1982. Coevolution between bats and plants. In: Kunz TH, ed. Ecology of bats New York: Plenum Press, 327–367. [Google Scholar]
- HeithausER, Fleming TH, Opler PA.1975. Foraging patterns and resource utilization in seven species of bats in a seasonal tropical forest. Ecology 56: 841–854. [Google Scholar]
- HelversenvonO.1993. Adaptations of flowers to the pollination by glossophagine bats. In: Barthlott W, Naumann CM, Schmidt‐Loske K, Schuchmann K‐L, eds. Plant–animal interactions in tropical environments Bonn: Museum Alexander König, 41–59. [Google Scholar]
- HunzikerAT.1979. South American Solanaceae. In: Hawkes JG, Lester RN, Skelding AD, eds. The biology and taxonomy of the Solanaceae London: Academic Press, 49–85. [Google Scholar]
- HunzikerAT.2001.Genera Solanacearum: the genera of Solanaceae illustrated, arranged according to a new system. Ruggell: ARG Gantner Verlag K.‐G. [Google Scholar]
- KalkoEKV, Condon MA.1998. Echolocation, olfaction and fruit display: how bats find fruit of flagellichorous cucurbits. Functional Ecology 12: 364–372. [Google Scholar]
- KearnsCA, Inouye DW.1993.Techniques for pollination biologists. Niwot‐Colorado: University Press of Colorado. [Google Scholar]
- KnappS, Persson V, Blackmore S.1997. A phylogenetic conspectus of the tribe Juanulloeae (Solanaceae). Annals of the Missouri Botanical Garden 84: 67–89. [Google Scholar]
- KornerupA, Wanscher JH.1963.Taschenlexikon der Farben. Göttingen: Muster‐Schmidt. [Google Scholar]
- LemkeTO.1984. Foraging ecology of the long‐nosed bat, Glossophaga soricina, with respect to resource availability. Ecology 65: 538–548. [Google Scholar]
- MachadoICS, Sazima I, Sazima M.1998. Bat pollination of the terrestrial herb Irlbachia alata (Gentianaceae) in northeastern Brazil. Plant Systematics and Evolution 209: 231–237. [Google Scholar]
- MartinFN.1959. Staining and observing pollen tubes in the style by means of fluorescence. Stain Technology 34: 125–128. [DOI] [PubMed] [Google Scholar]
- MartinoAMG, Aranguren JO, Arends A.2002. Feedings habits of Leptonycteris curasoe in northern Venezuela. Southwestern Naturalist 47: 78–85. [Google Scholar]
- NewstromLE, Frankie GW, Baker HG.1994. A new classification for plant phenology based on flowering patterns in lowland tropical rain forest trees at La Selva, Costa Rica. Biotropica 26: 141–159. [Google Scholar]
- OlmsteadRG, Sweere JA, Spangler RE, Bohs L, Palmer JD.1999. Phylogeny and provisional classification of the Solanaceae based on chloroplast DNA. In: Nee M, Symon DE, Lester RN, Jessop JP, eds. Solanaceae, Vol. IV Kew, Royal Botanic Gardens, 111–137. [Google Scholar]
- PetitS.1997. The diet and reproductive schedules of Leptonycteris curasoe curasoe and Glossophaga longirostris elongata (Chiroptera: Glossophaginae) on Curaçao. Biotropica 29: 214–223. [Google Scholar]
- RadfordAE, Dickinson WC, Massey JR, Bell CR.1974.Vascular plant systematics. New York: Harper & Tow Publishing. [Google Scholar]
- SahleyG.1995. Peru’s bat–cactus connection. Bats 13: 6–11. [Google Scholar]
- SazimaI.1976. Observations on the feeding habits of phyllostomatid bats (Carollia, Anoura and Vampyrops) in south‐eastern Brazil. Journal of Mammalogy 57: 381–382. [Google Scholar]
- SazimaI, Sazima M.1977. Solitary and group foraging: two flower‐visiting patterns of the lesser spear‐nosed bat, Phyllostomus discolor Biotropica 9: 213–215. [Google Scholar]
- SazimaM, Sazima I.1975. Quiropterofilia em Lafoensia pacari St. Hil. (Lythraceae), na Serra do Cipó, Minas Gerais. Ciência e Cultura 27: 405–416 [Google Scholar]
- SazimaM, Sazima I.1978. Bat pollination of the passion flower, Passiflora mucronata, in southeastern Brazil. Biotropica 10: 100–109. [Google Scholar]
- SazimaM, Sazima I.1988.Helicteres ovata (Sterculiaceae), pollinated by bats in southeastern Brazil. Botanica Acta 101: 269–271. [Google Scholar]
- SazimaM, Buzato S, Sazima I.1999. Bat‐pollinated flower assemblages and bat visitors at two Atlantic forest sites in Brazil. Annals of Botany 83: 705–712 [Google Scholar]
- SazimaM, Sazima I, Buzato S.1994. Nectar by day and night: Siphocampylus sulfureus (Lobeliaceae) pollinated by hummingbirds and bats. Plant Systematics and Evolution 191: 236–247. [Google Scholar]
- ThiesW, Kalko EKV, Schnitzler H‐U.1998. The roles of echolocation and olfaction in two Neotropical fruit‐eating bats, Carollia perspicillata and C. castanea, feeding on Piper Behavioural Ecology and Sociobiology 42: 397–409. [Google Scholar]
- TschapkaM, Helversen von O.1999. Pollinators of syntopic Marcgravia species in Costa Rican lowland rain forest: bats and opossums. Plant Biology 1: 382–388. [Google Scholar]
- VogelS.1958. Fledermausblumen in Südamerika. Österreichische Botanische Zeitschrift 104: 491–530. [Google Scholar]
- VogelS.1969. Chiropterophilie in der neotropischen Flora. II. Flora 158: 185–222. [Google Scholar]
- VogelS.1980. Florengeschichte im Spiegel blütenökologischer Erkennt nisse. Vorträge rheinisch‐westfälische Akademie der Wissenschaften 291: 7–48. [Google Scholar]
- VossR, Turner M, Inouye R, Fisher M, Cort R.1980. Floral biology of Markea neurantha Hemsley (Solanaceae), a bat‐pollinated epiphyte. American Midland Naturalist 103: 262–268. [Google Scholar]
- WalkerSM.2001. Conservation progress in Latin America. Bats 19: 1–2. [Google Scholar]
- WolfLL, Stiles FG, Hainsworth FR.1976. Ecological organization of a tropical highland hummingbird community. Journal of Animal Ecology 32: 349–379. [Google Scholar]
- ZeislerM.1938. Über die Abgrenzung der eigentlichen Narbenfläche mit Hilfe von Reaktionen. Beihefte zum Botanischen Zentralblatt 58: 308–318. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



