Abstract
The pollination biology of Aechmea pectinata (Bromeliaceae) was studied in a submontane rainforest in south‐eastern Brazil. This species has a mainly clumped distribution and its aggregated individuals are likely to be clones. From October to January, during the flowering period, the distal third of its leaves becomes red. The inflorescence produces 1–15 flowers per day over a period of 20–25 d. The flowers are inconspicuous, greenish‐white coloured, tubular shaped with a narrow opening, and the stigma is situated just above the anthers. Anthesis begins at 0400 h and flowers last for about 13 h. The highest nectar volume and sugar concentration occur between 0600 and 1000 h, and decrease throughout the day. Aechmea pectinata is self‐incompatible and therefore pollinator‐dependent. Hummingbirds are its main pollinators (about 90 % of the visits), visiting flowers mainly in the morning. There is a positive correlation between the number of hummingbird visits per inflorescence and the production of nectar, suggesting that the availability of this resource is important in attracting and maintaining visitors. The arrangement of the floral structures favours pollen deposition on the bill of the hummingbirds. Flowers in clumps promote hummingbird territoriality, and a consequence is self‐pollination in a broader sense (geitonogamy) as individuals in assemblages are genetically close. However, trap‐lining and intruding hummingbirds promote cross‐pollination. These observations suggest that successful fruit set of A. pectinata depends on both the spatial distribution of its individuals and the interactions among hummingbirds.
Key words: Aechmea pectinata, hummingbird‐pollination, inconspicuous flowers, nectar production, reproduction, Atlantic forest
INTRODUCTION
Bromeliaceae is the largest family of plants in the Atlantic forest showing a high degree of endemism (Martinelli, 1997), and its species constitute one of the most important nectar sources available to hummingbirds (Snow and Snow, 1986; Araújo et al., 1994; Sazima et al., 1995, 1996). Sick (1984) suggested that the evolution of bromeliads and hummingbirds is parallel, and according to McWilliams (1974), Bawa (1990) and Sazima et al. (2000) hummingbirds have been considered as the major pollinators of these plants.
Hummingbirds are nectar‐feeding specialists (Brown and Bowers, 1985), whose visitation behaviour is influenced by the availability of nectar in the flowers (see Heinrich, 1975; Feinsinger, 1976; Roubik, 1989). In addition, the spatial distribution of this resource promotes different foraging strategies in these birds (Feinsinger, 1978; Snow and Snow, 1986; Locatelli and Machado, 1999; Buzato et al., 2000).
Aechmea pectinata Baker (Bromeliaceae) presents an irregular and discontinuous distribution along the south/south‐eastern Brazilian coast (Reitz, 1983; Wendt, 1997). It occurs in the ‘restinga’ scrub, in the mangrove and on rocky shores, either as a terrestrial, epiphytic or saxicolous plant. It grows mainly in assemblages of 10–15 individuals, which are likely to be clones as asexual reproduction is common in Bromeliaceae (Rauh, 1990; Benzing, 2000), even if isolated individuals also occur (M. B. F. Canela and M. Sazima, pers. obs.). Although cursory reports of the floral features of A. pectinata and its visitors were made by Snow and Snow (1986), Sazima et al. (1995) and Buzato et al. (2000), no detailed information about the floral biology and reproductive system of this species is available.
This study sought to relate data on phenology, floral morphology and biology, as well as breeding system with the composition and dynamics of the pollinators of A. pectinata. The main purpose was two‐fold: (1) to verify if pollinator visitation is correlated with nectar production, and (2) to evaluate if the reproductive success of this bromeliad species is influenced by its spatial distributions, via pollinator behaviour.
MATERIALS AND METHODS
Study site
This study was carried out in the Atlantic Forest at Picinguaba (Parque Estadual da Serra do Mar, Ubatuba, São Paulo State, Brazil) approx. 23°22′S and 44°50′W, at sea level. The climate is wet tropical (‘Af.’; see Köppen, 1948), with an annual rainfall of up to 2600 mm, an average annual temperature of 21 °C and no well‐defined dry‐cold season, even during the so‐called dry months, from May to September (data source: Instituto Agronômico de Campinas, Campinas, Brazil).
Procedure
Fieldwork was performed during two consecutive flowering seasons of A. pectinata, from October 2000 to January 2001 and from December 2001 to February 2002. Epiphytic, terrestrial and saxicolous individuals (n = 91) were sampled in the mangrove, the restinga scrub and on rocky outcrops. Inflorescences were observed in situ to determine the number of open flowers per day, features of anthesis, visitation frequency and foraging behaviour of flower visitors. Floral and vegetative structures related to attraction were also recorded. The internal length of corollas was measured from base to opening (‘effective length’; Wolf et al., 1976).
Nectar sugar concentration (n = 20) was measured with a pocket refractometer and its volume (n = 50) with microlitre syringes (Dafni, 1992). These measures were made throughout anthesis, at 2‐h intervals, on previously bagged flowers of 18 individuals. The accumulated nectar volume (n = 46) and its respective sugar concentration (n = 42) were also measured on bagged flowers of nine individuals, at the end of anthesis. Pollen viability (n = 10 flowers, 10 individuals) was estimated by cytoplasmic staining, using the aceto‐carmine technique (Radford et al., 1974). Stigmatic receptivity (n = 70 flowers, 24 individuals) was tested using the H2O2 (10 V) catalase activity method (Zeisler, 1938).
Visits were observed directly or through binoculars for 36 individuals from 0400 to 1800 h, totalling 112 h in 19 days. Some visits were photographed and videotaped. The identification of hummingbirds was made according to Ruschi (1982) and confirmed by a specialist. The bill length of the hummingbirds is as given in Grantsau (1989).
The breeding system was assessed by hand‐pollination treatments on previously bagged flowers of 17 individuals: manual self‐pollination, cross‐pollination (previously emasculated flowers) and autonomous self‐pollination (bagged buds); flowers under natural conditions of pollination were marked as control (see Table 1). Fruit set for all treatments was determined approx. 2 months after pollination. Pistils of self‐ and cross‐pollinated flowers (n = 5 flowers for each treatment, 14 individuals) were fixed in FAA 12, 24, 36, 48 and 72 h after pollination, and pollen tube growth was analysed by fluorescence microscopy (Martin, 1959). To compare the fruit set per plant resulting from activity of pollinators in clumped individuals vs. isolated ones, data were recorded from 22 randomly chosen individuals of four assemblages and of eight isolated individuals.
Table 1.
Fruit‐set of autonomously self‐pollinated, hand‐pollinated and pollinated under natural conditions Aechmea pectinata flowers
| Treatments | Fruit set (%) |
| Autonomous self‐pollination | 0 (0/171) |
| Manual self‐pollination – same flower | 0 (0/37) |
| Cross‐pollination | 96 (24/25) |
| Natural conditions (control) | 56 (398/716) |
Numbers in parenthesis are the number of fruits/flowers, respectively.
Daily nectar production and hummingbird visitation were evaluated for normality through the Kolmogorov–Smirnov test (BioEstat 2.0). As our data are non‐parametric, the median (M) was calculated and variations were analysed through box‐plot graphs (Systat 8.0), Kruskall–Wallis test (H), Mann–Whitney test (U), Spearman correlation and a regression analysis (BioEstat 2.0).
RESULTS
Flowering phenology and floral features
Aechmea pectinata flowers continuously from late October to mid‐January. During the flowering period, the distal third of the leaves’ length becomes red (Fig. 1A), which makes each individual very showy. This coloration gradually fades away during the fruiting season, which occurs from February to March.
Fig. 1. Individual, pollinators and pollen tubes of Aechmea pectinata in the rainforest, south‐eastern Brazil. A, Individual showing highly visible red coloured leaf tips and a bent inflorescence; note that the inflorescence is very inconspicuous. B, A male Thalurania glaucopis visiting a flower on the upper side of a bent inflorescence. C, Ramphodon naevius, a trap‐liner hummingbird, visiting an erect inflorescence; note the pollen load on its bill tip. D, Signs of incompatibility such as curvature and thickening of the callose deposit on the extremity of the pollen tubes (arrows) on a flower fixed 12 h after manual self‐pollination.
The inflorescence is strobiliform, 10–15 cm long and 6–8 cm wide; it bears 150–250 flowers, and is supported by a 40–60 cm long stalk (Fig. 1A) that makes flowers more accessible to visitors. The inflorescence is usually erect, although bent ones may occur in epiphytic (Fig. 1A) or saxicolous individuals. In each inflorescence, 1–15 flowers open per day over a period of 20–25 days. Flowers are sessile, actinomorphic, with a tubular‐shaped 30 mm long corolla and a narrow opening. The flowers are inconspicuous: bracts are greenish, sepals are greenish white, and petals are yellowish white (Fig. 1C), making it difficult even to distinguish the inflorescences from the foliage (Fig. 1A).
Prior to bud opening the style lengthens so that the stigma slightly exceeds the anthers. Anthesis of the A. pectinata flowers begins at 0400 h, and is characterized by discrete separation and outward curvature of petal tips, resulting in an approx. 2 mm wide opening. In open flowers, the apex of the stigma slightly extends out of the corolla. The stigma is receptive throughout the flower life. Anthers are juxtaposed, included in the corolla, extrorse and open longitudinally. The somewhat sticky pollen is available from the first hour of anthesis and it presents high viability (93 %). Nectar accumulates at the base of the corolla tube. Flowers remain open for approx. 13 h.
Both nectar volume (H = 134·00, P < 0·001) and sugar concentration (H = 80·41, P < 0·001) varied significantly throughout the day. This variation was most evident between the morning and afternoon periods (Fig. 2A and B). At the onset of anthesis (0400 h), flowers have no nectar (M = 0 µl, n = 30, four individuals), but production begins soon after and reaches its peak at 0800 h (M = 21·5 µl), after which it gradually decreases until late afternoon (Fig. 2A). Sugar concentration was also greatest in the early morning, at 0600 h (M = 30 %, n = 20); it decreased to half this value between 1000 and 1200 h, and continued decreasing gradually until late afternoon when it stabilized (Fig. 2B). Nectar volume is positively correlated with sugar concentration (r = 0·85, P < 0·01). The accumulated nectar volume throughout the day (M = 79·5 µl, n = 46) and the sum of its partial values (M = 67·0 µl, n = 43) did not differ significantly (U = 959·50, P > 0·05), indicating that nectar production is continuous and reabsorption does not occur. Sugar concentration in accumulated nectar (M = 26·7 %, n = 42) was also similar (U = 756·00, P > 0·05) to the concentration registered in the first hours of anthesis (M = 25 %, n = 40).
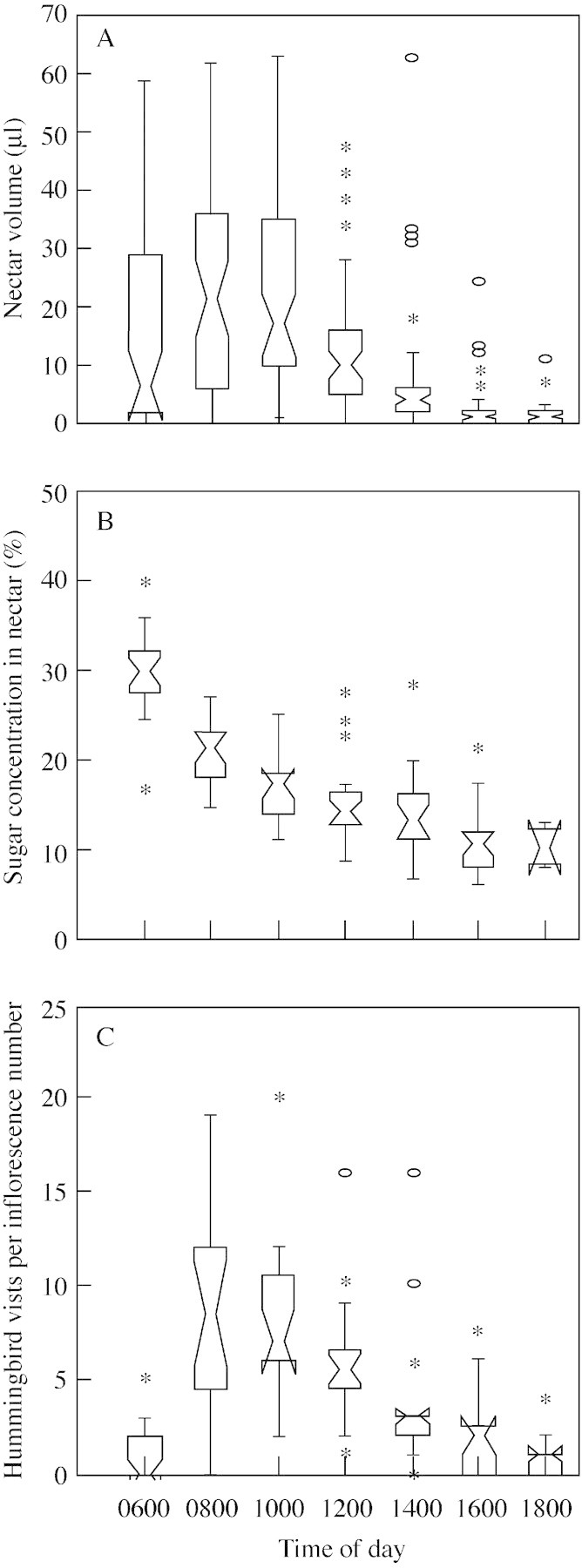
Fig. 2. Nectar features and number of visits in Aechmea pectinata. A, Variation in nectar volume. B, Sugar concentration in nectar. C, Number of hummingbird visits per inflorescence. The boxes represent the inter quartile range and their centres the medians. The interval between the slanted lines around the medians is the 95 % confidence limit. Inferior and superior vertical lines represent the total range of the distribution (25 and 75 %). Extreme values are represented by asterisks, and outliers by open circles. Overlap between boxes indicates no statistical difference.
Floral visitors
Of the 535 visits to A. pectinata flowers, 489 were made by hummingbirds (91 %) and the remaining ones by insects, namely bees and butterflies. The most frequent hummingbird visitor, Thalurania glaucopis Gmelin (Trochilinae) was accountable for approx. 42 % of the visits (n = 489), 83 % of which were made by males (Fig. 1B). Amazilia fimbriata Elliot (Trochilinae) made 36 % of the visits, and Ramphodon naevius Dummont (Phaethornithinae) 20 % (Fig. 1C). Phaethornis ruber Linné and Melanotrochilus fuscus Vieillot (Phae thornithinae) were rarely observed (only 1 % of the visits).
Most of the hummingbird visits (55 %) occurred between 0600 and 1000 h (x̄ = 3·8 visits plant–1 h–1), the others occurring throughout the rest of the day at a diminishing frequency (Fig. 2C). This variation in the number of hummingbird visits throughout the day (H = 72·02, P < 0·001) is correlated with nectar production, for both volume (r2 = 0·97, P < 0·001; Fig. 3A) and sugar concentration (R2 = 0·93, P < 0·005; Fig. 3B).
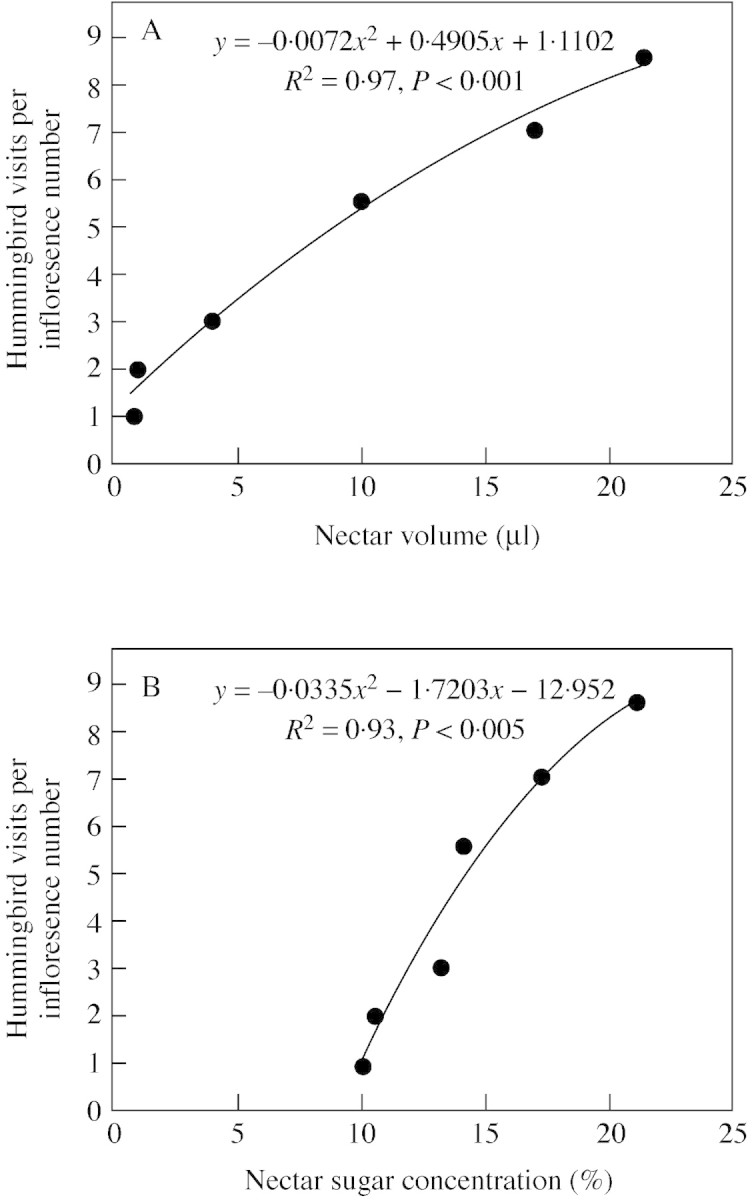
Fig. 3. Positive correlation between the number of hummingbird visits per inflorescence and nectar offered by Aechmea pectinata flowers: A, Nectar volume; B, sugar concentration. The values of nectar production from 0400–0600 h were excluded from this analysis as they correspond to a period of low hummingbird activity.
Although periods of 30–40 min between visits were common, intervals were highly irregular and the longest ones occurred mainly in the afternoon. Visit duration at each flower varied from 1 to 10 s. When taking nectar, hummingbirds contacted anthers, receiving pollen on their bill (Fig. 1C).
Thalurania glaucopis and Amazilia fimbriata were more frequent on clumped individuals of Aechmea pectinata than on isolated ones, being often found on the most conspicuous inflorescences or on those with the greatest number of flowers. They visited every flower on each inflorescence before moving to another one, and sometimes returned to one they had previously visited. Between visits, the hummingbirds commonly remained perched nearby. Thalurania glaucopis (mainly males) and Amazilia fimbriata frequently showed aggressive behaviour and interacted agonistically with intruding hummingbirds, be these of another or the same species; they also excluded and chased bees. Ramphodon naevius (both males and females) visited Aechmea pectinata inflorescences in a trap‐line fashion, sometimes interacting agonistically with other visitors. Individuals of these hummingbird species commonly alternated their roles when visiting A. pectinata, becoming territory intruders.
Reproduction
Aechmea pectinata is hermaphroditic, homogamic and self‐incompatible. In flowers fixed 12 h after manual self‐pollination, some pollen had germinated, tubes were short and showed evidence of incompatibility (see Murray, 1990), such as curvature and thickening of the callose plugs on their tips (Fig. 1D). Flowers fixed 48 h after manual self‐pollination did not show developed pollen tubes. In cross‐pollination experiments, flowers fixed 12 h after pollination showed high quantities of germinated pollen and well‐developed tubes up to two‐thirds of the style length; fertilization occurred after 24 h. Under natural conditions fertilization occurred after a similar period.
None of the autonomously or manually self‐pollinated flowers produced fruits, which contrasts with the fruiting of 96 % of the manually cross‐pollinated flowers and 56 % of the flowers under natural conditions (Table 1).
Of the clumped individuals (n = 22), only 23 % produced fruits, whereas 88 % of the isolated individuals (n = 8) produced fruits. Fruits are highly apparent because they become reddish when ripe. In erect inflorescences most of the flowers set fruit, whereas in bent ones only the flowers accessible to hummingbirds (Fig. 1B) developed fruits.
DISCUSSION
The annual flowering pattern (Newstrom et al., 1994) of A. pectinata at the population level, as well as the individual flowering strategy, characterized as ‘steady‐state’ type by Gentry (1974), are common in ornithophilous species of Bromeliaceae (e.g. Araújo et al., 1994; Sazima et al., 1996; Martinelli, 1997; Buzato et al., 2000). A well‐defined and synchronized blooming period, as observed for A. pectinata, indicates specialization to a given kind of pollinator (Stiles, 1978; Fleming, 1982), and hummingbirds prefer plants that offer regular and constant resources (Wolf et al., 1976; Real and Rathcke, 1991).
Aechmea pectinata presents some floral features related to ornithophily, such as tubular shaped flowers, abundant nectar, absence of odor and long distance between nectar and sexual organs (see Faegri and van der Pijl, 1980; Proctor et al., 1996). Since its flowers are inconspicuous, showing an unusual colour for hummingbird‐pollinated species (Faegri and van der Pijl, 1980), the red‐coloured leaves are responsible for attracting hummingbirds. Coloured leaves and bracts, and not flowers, that attract pollinators are common in other ornithophilous species of Bromelia ceae, as well as in Gesneriaceae and Heliconiaceae (see Stiles, 1981; Araújo et al., 1994; Sazima et al., 1995, 1996, 2000; Martinelli, 1997; Benzing, 2000).
The morphology of A. pectinata flowers favours legitimate access to nectar by visitors with long and stiff mouth parts, namely hummingbirds, and excludes other types of visitors (see Proctor and Yeo, 1972; Faegri and van der Pijl, 1980). The moderately long floral tube allows visits of either short‐billed Trochilinae (11–24 mm bill length) or long‐billed Phaethornithinae (22–46 mm bill length) (see Feinsinger and Colwell, 1978). In addition to their corolla length and shape, the anther arrangement in A. pectinata promotes pollen deposition on the bill of the hummingbird. Pollen deposition on the bill is particularly frequent in Bromeliaceae visitors (see Sazima et al., 1995, 1996; Buzato et al., 2000). This seems to be an efficient way of transferring pollen, as the pollen of most Bromeliaceae species is somewhat sticky (Percival, 1969; see Wanderley and Melhem, 1991; Halbritter, 1992) and hummingbirds usually visit a certain number of flowers before cleaning their bill (Grant and Grant, 1968; Sick, 1984; M. B. F. Canela, pers. obs.).
The somewhat short anthesis period of A. pectinata flowers is a feature common to several bromeliad species (e.g. Araújo et al., 1994; Sluys and Stotz, 1995; Martinelli, 1997; Siqueira, 1998; Sluys et al., 2001; Wendt et al., 2002), which may be related either to homogamy or to the efficiency of pollinators that effect pollination after just a few visits (Ramirez et al., 1990). The same may be true for Bromelia antiacantha Bertoloni (M. B. F. Canela and M. Sazima, pers. obs.) and for Hohenbergia ridleyi (Baker) Mez (Siqueira, 1998).
Nectar volume and sugar concentration in A. pectinata were similar to the data of Snow and Snow (1986), Sazima et al. (1995) and Buzato et al. (2000) for this species, and to those of ornithophilous species in general (Arizmendi and Ornelas, 1990; Sazima et al., 1996; Locatelli and Machado, 1999). In addition, major nectar production early in the day is a consistent tendency in ornithophilous flowers (Feinsinger, 1976), matching the period of hummingbird activity (Benzing, 1980; Sick, 1984). The positive correlation between nectar production and hummingbird visits to A. pectinata suggests that both the quantity and quality of nectar are important factors in attracting and maintaining bird visits (see Percival, 1969; Baker, 1975).
The behaviour of Thalurania glaucopis and Amazilia fimbriata at A. pectinata may be classified as territorial (Feinsinger and Colwell, 1978). Ramphodon naevius is regarded here as a typical high‐reward trap‐liner with no extensive territorial behaviour (cf. Stiles, 1975; Stiles and Freeman, 1993), although it can behave aggressively along its routes and exclude hummingbird intruders which it may come across (see Sazima et al., 1995). Phaethornithinae species are known to occasionally hold temporary flower‐centred territories (Stiles, 1975; Feinsinger and Colwell, 1978), a ‘patrolling’ behaviour that may be regarded as a specific resource defence (cf. Wolf et al., 1976). Although hummingbirds are especially suited to a primary role, some individuals of the visiting species of A. pectinata frequently acted as territory parasites (Feinsinger and Colwell, 1978) and became intruders. The temporary alternation between different roles may be due to shifts in nectar availability of A. pectinata or to the spectrum of hummingbirds at a given site (see Sazima et al., 1996; Locatelli and Machado, 1999; Buzato et al., 2000).
As a self‐incompatible species, A. pectinata is pollinator‐dependent. Although incompatibility is not common in Bromeliaceae, even several genetically self‐compatible species depend on pollinators because of different floral mechanisms (dichogamy, herkogamy) that hinder autonomous self‐pollination (see Gardner, 1986; Araújo et al., 1994; Martinelli, 1997; Siqueira, 1998; Wendt et al., 2001, 2002). In addition to self‐incompatibility, the low fruit set of A. pectinata individuals in large assemblages in comparison with the high fruit set of isolated plants is probably related to the territorial behaviour of the hummingbirds, which is favoured by the clumped distribution of the individuals and the long flowering period. Aggregated individuals concentrate the nectar source, thereby reducing the energy costs of the pollinator while searching for food (Locatelli and Machado, 1999) and promoting territoriality among visiting hummingbirds (Feinsinger, 1978; Snow and Snow, 1986; Buzato et al., 2000). Territoriality prevents the visits of trap‐liner hummingbirds carrying pollen from distant plants and, in the case of A. pectinata, it promotes self‐pollination in a broader sense (geitonogamy) since individuals in assemblages are thought to be genetically close. Although less attractive to pollinators, isolated plants have a great chance of being effectively cross‐pollinated (Janzen, 1971; Stiles, 1975).
The irregular intervals between hummingbird visits to Aechmea pectinata suggest that intruders were frequent, and sometimes not excluded by primary territory holders. Areas of significant aggregated resources attract territory parasites (Wolf and Stiles, 1970), and the hummingbirds that successfully manage to invade such territory have increased possibilities of carrying out cross‐pollination in aggregated individuals. Therefore, intruder individuals of Thalurania glaucopis, Amazilia fimbriata and, mainly, Ramphodon naevius potentially play an important role in the fruit set of Aechmea pectinata.
Due to the high number of individuals and flowers available over a long and continuous period, A. pectinata represents a highly important floral resource for its visitors. The dependency of A. pectinata on a pollinator, in spite of its inconspicuous flowers, is related to such characteristics as leaf coloration, floral morphology and nectar availability, which attract and maintain hummingbird visits. However, successful fruiting is also related to other factors such as the spatial distribution of its individuals and the dynamics of the hummingbird community.
ACKNOWLEDGEMENTS
We thank the Instituto Florestal for logistical support at the Parque Estadual da Serra do Mar; C. F. de Oliveira for help in the field and pleasant company; E. Penna‐Firme and M. Lugero from Camping Caracol for hospitality; I. F. Bressan for technical assistance; I. Sazima for hummingbird identification; C. Westerkamp, J. Semir and T. Wendt for suggestions on early versions of the manuscript; M. A. Aizen and an anonymous reviewer for helpful comments; A. François and A. Bahrami for English revision. Financial support was provided by FAPESP and CNPq. This manuscript is part of the Master’s Degree thesis of M.B.F.C. at the Departamento de Botânica, Pós‐Graduação Biologia Vegetal, Universidade Estadual de Campinas, São Paulo, Brasil.
Supplementary Material
Received: 14 May 2003;; Returned for revision: 17 July 2003. Accepted: 13 August 2003
References
- AraújoAC, Fischer EA, Sazima M.1994. Floração seqüencial e polinização de três espécies de Vriesea (Bromeliaceae) na região da Juréia, sudeste do Brasil. Revista Brasileira de Botânica 17: 113–118. [Google Scholar]
- ArizmendiMC, Ornelas JF.1990. Hummingbirds and their floral resources in a tropical dry forest in Mexico. Biotropica 22: 172–180. [Google Scholar]
- BakerHG.1975. Studies of nectar‐constitution and pollinator‐plant coevolution. In: Gilbert LE, Raven PH, eds. Plant and animal co‐evolution Austin: University of Texas Press, 100–140. [Google Scholar]
- BawaKS.1990. Plant–pollinator interactions in tropical rain forests. Annual Reviews of Ecology and Systematics 21: 399–422. [Google Scholar]
- BenzingDH.1980.The biology of the bromeliads. Eureka CA: Mad River Press. [Google Scholar]
- BenzingDH.2000.Bromeliaceae: profile of an adaptative radiation. Cambridge: Cambridge University Press. [Google Scholar]
- BrownJA, Bowers MA.1985. Community organization in hummingbirds: relationships between morphology and ecology. Auk 102: 251–269. [Google Scholar]
- BuzatoS, Sazima M, Sazima I.2000. Hummingbird‐pollinated floras at three Atlantic Forest sites. Biotropica 32: 824–841. [Google Scholar]
- DafniA.1992.Pollination ecology: a practical approach. Oxford: IRL Press. [Google Scholar]
- FaegriK, van der Pijl L.1980.The principles of pollination ecology. New York: Pergamon Press. [Google Scholar]
- FeinsingerP.1976. Organization of a tropical guild of nectarivorous birds. Ecological Monographs 46: 257–291. [Google Scholar]
- FeinsingerP.1978. Ecological interactions between plants and hummingbirds in a successional tropical community. Ecological Monographs 48: 269–287. [Google Scholar]
- FeinsingerP, Colwell RK.1978. Community organization among neotropical nectar‐feeding birds. American Zoology 18: 779–795. [Google Scholar]
- FlemingTH.1982. Foraging strategies of plant‐visiting bats. In: Kunz TH, ed. Ecology of bats New York: Plenum Press, 287–325. [Google Scholar]
- GardnerCS.1986. Inferences about pollination in Tillandsia (Bromeliaceae). Selbyana 9: 76–87. [Google Scholar]
- GentryAH.1974. Flowering phenology and diversity in tropical Bignoniaceae. Biotropica 6: 64–68. [Google Scholar]
- GrantKA, Grant V.1968.Hummingbirds and their flowers. New York: Columbia University Press. [Google Scholar]
- GrantsauR.1989.Os beija‐flores do Brasil. Rio de Janeiro: Editora Expressão e Cultura. [Google Scholar]
- HalbritterH.1992. Morphologie und systematische Bedeutung des Pollens der Bromeliaceae. Grana 31: 197–212. [Google Scholar]
- HeinrichB.1975. Bee flowers: a hypothesis on flowering variety and blooming times. Evolution 29: 325–334. [DOI] [PubMed] [Google Scholar]
- JanzenDH.1971. Euglossini bees as long‐distance pollinators of tropical plants. Science 17: 203–205. [DOI] [PubMed] [Google Scholar]
- KöppenW.1948.Climatologia: con un estudio de los climas de la tierra. México: Fondo de Cultura Económica. [Google Scholar]
- LocatelliE, Machado IC.1999. Comparative study of the floral biology in the ornithophilous species of Cactaceae: Melocactus zehnteneri and Opuntia palmadora Bradleya 17: 75–85. [Google Scholar]
- McWilliamsEL.1974. Evolutionary Ecology. In: Smith LB, Downs R, eds. Flora Neotropica: Pitcairnioideae (Bromeliaceae), Monograph 14, Part 1 New York: New York Botanical Garden, 40–58. [Google Scholar]
- MartinFN.1959. Staining and observing pollen tubes in the style by means of fluorescence. Stain Technology 34: 125–128. [DOI] [PubMed] [Google Scholar]
- MartinelliG.1997. Biologia reprodutiva de Bromeliaceae na Reserva Ecológica de Macaé de Cima. In: Lima HC, Guedes‐Bruni RR, eds. Serra de Macaé de Cima: Diversidade Florística e Conservação em Mata Atlântica Rio de Janeiro: IP/JBRJ, 213–250. [Google Scholar]
- MurrayBG.1990. Heterostyly and pollen‐tube interactions in Luculia gratissima (Rubiaceae). Annals of Botany 65: 691–698. [Google Scholar]
- NewstromLE, Frankie GW, Baker HG.1994. A new classification for plant phenology based on flowering patterns in lowland tropical rain forest trees at La Selva, Costa Rica. Biotropica 26: 141–159. [Google Scholar]
- PercivalM.1969.Floral biology. London: Pergamon Press. [Google Scholar]
- ProctorM, Yeo P.1972.The pollination of flowers. London: Collins London. [Google Scholar]
- ProctorM, Yeo P, Lack A.1996.The natural history of pollination. London: Harper Collins. [Google Scholar]
- RadfordAE, Dickinson WC, Massey JR, Bell CR.1974.Vascular plant systematics. New York: Harper & Row. [Google Scholar]
- RamirezN, Gil C, Hokche O, Seres A, Brito Y.1990. Biologia floral de una comunidad arbustiva tropical en la Guayana Venezolana. Annals of the Missouri Botanical Garden 77: 383–397. [Google Scholar]
- RauhW.1990.The bromeliad lexicon. 2nd edn. London: Sterling Publishing. [Google Scholar]
- RealLA, Rathcke BJ.1991. Individual variation in nectar production and its effects on fitness in Kalmia latifolia Ecology 72: 149–155. [Google Scholar]
- ReitzR.1983. Bromeliáceas e a malária‐bromélia endêmica. In: Reitz R, ed. Flora Ilustrada Catarinense, Part 1 Itajai: Herbario Barbosa Rodrigues, 433–437. [Google Scholar]
- RoubikDW.1989.Ecology and natural history of tropical bees. Cambridge: Cambridge University Press. [DOI] [PubMed] [Google Scholar]
- RuschiA.1982.Beija‐flores do Estado do Espírito Santo. São Paulo: Editora Rios. [Google Scholar]
- SazimaI, Buzato S, Sazima M.1995. The saw‐billed hermit Ramphodon naevius and its flowers in southeastern Brazil. Journal für Ornithologie 136: 195–206. [Google Scholar]
- SazimaI, Buzato S, Sazima M.1996. An assemblage of hummingbird‐pollinated flowers in a montane forest in southeastern Brazil. Botanica Acta 109: 149–160. [Google Scholar]
- SazimaM, Buzato S, Sazima I.2000. Polinização por beija‐flores em Nidularium e gêneros relacionados. In: Leme EC, ed. Bromélias da Mata Atlântica – Nidularium Rio de Janeiro: Hamburg Donnelley Gráfica Editora Ltda, 188–195. [Google Scholar]
- SickH.1984.Ornitofilia brasileira, uma introdução. Brasília: Ed. Universidade de Brasília. [Google Scholar]
- SiqueiraJAF.1998. Biologia floral of Hohenbergia ridleyi (Baker) Mez. Bromélia 5: 3–13. [Google Scholar]
- SluysMV, Stotz DF.1995. Padrões de visitação a Vriesea neoglutinosa por beija‐flores no Espírito Santo, sudeste do Brasil. Bromélia 2: 27–35. [Google Scholar]
- SluysMV, Cardozo CA, Mangolin R, Rocha CFD.2001. Taxas de visitação de polinizadores a Vriesea procera (Bromeliaceae) na Ilha Grande/RJ, sudeste do Brasil. Bromélia 6: 19–24. [Google Scholar]
- SnowDW, Snow BK.1986. Feeding ecology of hummingbirds in the Serra do Mar, southeastern Brazil. El Hornero 12: 286–296. [Google Scholar]
- StilesFG.1975. Ecology, flowering phenology and hummingbird pollination of some Costa Rican Heliconia species. Ecology 56: 285–310. [Google Scholar]
- StilesFG.1978. Temporal organization of flowering among the hummingbird foodplants of a tropical wet forest. Biotropica 10: 194–210. [Google Scholar]
- StilesFG.1981. Geographical aspects of bird‐flower coevolution, with particular reference to Central America. Annals of the Missouri Botanical Garden 68: 323–351. [Google Scholar]
- StilesFG, Freeman CE.1993. Patterns in floral nectar characteristics of some bird‐visited plant species from Costa Rica. Biotropica 25: 191–205. [Google Scholar]
- WanderleyMGL, Melhem TS.1991. Flora polínica da Reserva do Parque do Ipiranga (São Paulo, Brasil). Hoehnea 18(1): 5–42. [Google Scholar]
- WendtT.1997. A review of the subgenus Pothuava (Baker) Baker of Aechmea Ruiz & Pav. (Bromeliaceae) in Brazil. Botanical Journal of the Linnean Society 125: 245–271. [Google Scholar]
- WendtT, Canela MBF, Faria APG, Rios RI.2001. Reproductive biology and natural hybridization between two endemic species of Pitcairnia (Bromeliaceae). American Journal of Botany 88: 1760–1767. [PubMed] [Google Scholar]
- WendtT, Canela MBF, Klein DE, Rios RI.2002. Selfing facilitates reproductive isolation among three sympatric species of Pitcairnia (Bromeliaceae). Plant Systematics and Evolution 232: 201–212. [Google Scholar]
- WolfLL, Stiles FG.1970. Evolution of pair cooperation in a tropical hummingbird. Evolution 24: 759–773. [DOI] [PubMed] [Google Scholar]
- WolfLL, Stiles FG, Hainsworth FR.1976. Ecological organization of a tropical highland hummingbird community. Journal of Animal Ecology 32: 349–379. [Google Scholar]
- ZeislerM.1938. Über die Abgrenzung der eigentlichen Narbenfläche mit Hilfe von Reaktionen. Beihefte zum Botanischen Zentralblatt 58: 308–318. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



