Abstract
Prostaglandin (PG) E2, a major product of cyclooxygenase (COX)-2, acts as an immunomodulator at the maternal-fetal interface during pregnancy. It exerts biologic function through interaction with E-prostanoid (EP) receptors localized to the placenta. The activation of the COX-2/PGE2/EP signal pathway can alter the expression of the ATP-binding cassette (ABC) transporters, multidrug resistance protein 1 [P-glycoprotein (Pgp); gene: ABCB1], and breast cancer resistance protein (BCRP; gene: ABCG2), which function to extrude drugs and xenobiotics from cells. In the placenta, PGE2-mediated changes in ABC transporter expression could impact fetal drug exposure. Furthermore, understanding the signaling cascades involved could lead to strategies for the control of Pgp and BCRP expression levels. We sought to determine the impact of PGE2 signaling mechanisms on Pgp and BCRP in human placental cells. The treatment of placental cells with PGE2 up-regulated BCRP expression and resulted in decreased cellular accumulation of the fluorescent substrate Hoechst 33342. Inhibiting the EP1 and EP3 receptors with specific antagonists attenuated the increase in BCRP. EP receptor signaling results in activation of transcription factors, which can affect BCRP expression. Although PGE2 decreased nuclear factor κ-light chain-enhancer of activated B activation and increased activator protein 1, chemical inhibition of these inflammatory transcription factors did not blunt BCRP up-regulation by PGE2. Though PGE2 decreased Pgp mRNA, Pgp expression and function were not significantly altered. Overall, these findings suggest a possible role for PGE2 in the up-regulation of placental BCRP expression via EP1 and EP3 receptor signaling cascades.
Introduction
Inflammation has an important role in the pathophysiology of several common and serious pregnancy disorders that are each associated with adverse outcomes and compromised placental function. The inflammatory responses observed during pregnancy are complex and the result is often dependent on the timing, severity, and type of stimulus. An inflammatory response can be triggered by a wide variety of pathologic stimuli, including hypoxia, infection, tissue damage, trauma, and cellular stress (Guo et al., 2010; Bloise et al., 2013). It is increasingly clear that different stimuli can induce alternative patterns of inflammatory molecules, thereby invoking different regulatory patterns of gene expression (Ho and Piquette-Miller, 2006).
Studies of placental cells and tissue in vitro document their responsiveness to inflammatory stimuli with increased production of cytokines, chemokines, and prostaglandins (PGs) (Keelan et al., 2003). PGs are potent mediators of immune responses (Betz and Fox, 1991; Snijdewint et al., 1993; Roper and Phipps, 1994) and inflammation (Gilroy et al., 1999; Trebino et al., 2003). They play a role in many aspects of pregnancy including maternal-fetal tolerance, parturition, and innate immunity. PGE2 is the predominant PG produced by the placenta (Myatt, 1990). As a result, levels at the placental interface are likely higher than in the peripheral circulation. PGE2 is derived from arachidonic acid via the activities of cyclooxygenase (COX)-2. The effects of PGE2 are facilitated by specific-membrane bound G protein–coupled E-prostanoid (EP) receptors (EP1–EP4), which each have distinct signal transduction profiles and often differing cellular actions (Hata and Breyer, 2004).
The activation of COX-2 affects the ATP-binding cassette (ABC) transporters, multidrug resistance protein (MDR) 1 [P-glycoprotein (Pgp); gene: ABCB1], multidrug resistance–associated protein (MRP) 1 (gene: ABCC1), and breast cancer resistance protein (BCRP; gene: ABCG2) (Surowiak et al., 2008; Liu et al., 2010), which play important roles in extruding drugs and xenobiotics out of cells. COX-2 up-regulates the expression and activity of Pgp and BCRP in several cell types (Fantappiè et al., 2002; Patel et al., 2002). Furthermore, specific COX-2 inhibitors, such as celecoxib and NS398 (N-[2-(cyclohexyloxy)-4-nitrophenyl]methanesulfonamide), block the COX-2–mediated increase in Pgp and BCRP, suggesting that PGE2 may be implicated in this response (Arico et al., 2002; Patel et al., 2002; Zatelli et al., 2007; Arunasree et al., 2008; Ko et al., 2008; Zrieki et al., 2008; Roy et al., 2010).
Pgp and BCRP are the most abundant ABC efflux transporters on the apical brush-border membrane of the placenta. These proteins pump compounds back into the maternal circulation and are generally believed to have a protective role (Ceckova-Novotna et al., 2006; Mao, 2008). However, it is easy to see how problems could arise if they were to prevent drug delivery to the fetus where the medication was intended to have a therapeutic effect. In either instance, changes in Pgp and/or BCRP expression in the placenta could affect fetal drug exposure.
The functional expression of Pgp and BCRP can be up- or down-regulated by exposing placental cells to various inflammatory agents. For example, acute inflammation, such as that mediated by microbial products such as lipopolysaccharide, are known to down-regulate the expression of several placental transporters, including Pgp (Chen et al., 2005; Wang et al., 2005; Petrovic et al., 2007, 2008; Bloise et al., 2013). On the other hand, chronic, sublethal exposure to lipopolysaccharide had no effect on overall placental Pgp activity (Bloise et al., 2013). In other examples of chronic inflammation, such as that observed in subjects with rheumatoid arthritis, Pgp expression is increased (Dumoulin et al., 1997; Llorente et al., 2000). We previously documented up-regulation of placenta Pgp and BCRP expression in the presence of histologic chorioamnionitis (Mason et al., 2011). Taken together, the findings suggest there are many interactive and reactive mediators of inflammation that can alter these transporters in the placenta. To date, studies have primarily focused on the effects of proinflammatory cytokines (Ho and Piquette-Miller, 2006; Evseenko et al., 2007). PGs are released by the actions of proinflammatory cytokines. It has been shown that interleukin (IL)-1β, IL-6, tumor necrosis factor-α, and IL-8 directly stimulate PGE2 production by cells obtained from term placenta (Furuta et al., 2000). Surprisingly, there is no information on the effects of PGE2 on ABC efflux transporters in the placenta.
In this investigation, we tested the hypothesis that PGE2 increases Pgp and BCRP expression and activity in placental cells via distinct EP receptor signaling pathways. Identification of these signaling pathway(s) could provide important information about mechanisms of inflammation on transporter regulation and facilitate the development of strategies to manipulate Pgp and BCRP expression levels via EP receptor inhibition.
Materials and Methods
The PG receptor antagonists SC-19220 (8-chloro-dibenz[b,f][1,4]oxazepine-10(11H)-carboxy-(2-acetyl)hydrazide), AH-6809 (6-isopropoxy-9-oxoxanthene-2-carboxylic acid), and L-161982 (N-[[4′-[[3-butyl-1,5-dihydro-5-oxo-1-[2-(trifluoromethyl)phenyl]-4H-1,2,4-triazol-4-yl[methyl][1,1′-biphenyl]-2-yl[sulfonyl[-3-methyl-2-thiophenecarboxamide) were purchased from Cayman Chemical (Ann Arbor, MI). PGE2, L-798106 [(2E)-N-[(5-bromo-2-methoxyphenyl)sulfonyl]-3-[2-(2-naphthalenylmethyl)phenyl]-2-propenamide], verapamil, fumitremorgin C (FTC), MK-571 (3-[[[3-[(1E)-2-(7-chloro-2-quinolinyl)ethenyl]phenyl][[3-(dimethylamino)-3-oxopropyl]thio]methyl]thio]propanoic acid), sulprostone, anti-MDR1 monoclonal antibody (clone F4), and Percoll were purchased from Sigma-Aldrich (St. Louis, MO). BAY-11-7082 (3-[(4-methylphenyl)sulfonyl]-(2E)-propenenitrile) and SR 11302 [(E,E,Z,E)-3-methyl-7-(4-methylphenyl)-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraenoic acid] were from Cayman Chemical and Tocris Bioscience (R&D Systems, Minneapolis, MN), respectively. The antibody against BCRP (clone BXP21) was purchased from EMD Millipore (Billerica, MA). All receptor antagonists were dissolved in DMSO as 10 mM stock solutions and stored at −20°C; PGE2 (1 mg/ml) was dissolved in ethanol. The concentration of DMSO and EtOH after the dilution of compounds to their final concentrations was 0.1 and 0.5%, respectively. Lipofectamine 2000, p-nuclear factor κ-light chain-enhancer of activated B (NF-κB)–Luc plasmid, and p activator protein 1 (AP-1)–Luc plasmid were purchased from Life Technologies (Carlsbad, CA). The Dual-Luciferase Reporter Assay System was purchased from Promega (Madison, WI).
Cell Culture.
Jar cells were obtained from American Type Cell Culture (Manassas, VA) and cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 50 U/ml penicillin, and 50 μg/ml streptomycin. Cells were maintained in a humidified atmosphere of 95% air and 5% CO2 at 37°C.
Human placentas were obtained under a protocol approved by the Institutional Review Board at the University of Kansas Medical Center. Cytotrophoblasts were isolated from healthy placenta delivered at term by cesarean section as previously described (Arroyo et al., 2004) with minor modifications. Briefly, 50 g of villous tissue was digested in Hanks’ balanced salt solution media (Sigma-Aldrich) containing 40 mg of DNAse (Roche Diagnostics, Indianapolis, IN), 100 mg of trypsin (Sigma-Aldrich), and 500 units of dispase (BD Biosciences, Bedford, MA). Digestion was performed at 37°C for 60 minutes in an orbital shaking incubator at 350g. Digest media were strained to remove tissue fragments and then centrifuged for 5 minutes at 500g. The cell pellet was resuspended in Dulbecco’s modified Eagle’s medium containing 10% fetal calf serum (FCS; Sigma-Aldrich). Cell suspensions were layered on a discontinuous Percoll gradient (5–70%) and spun for 20 minutes at 1200g. Cells between the 40 and 50% Percoll bands were collected and plated at a density of 6 × 106 cells on 60-mm2 petri dishes. The cells were grown in M199 media, supplemented with 10% FCS, epidermal growth factor (10 ng/ml), insulin (5 ng/ml), transferrin (10 ng/ml), sodium selenite (0.2 nM), and penicillin/streptomycin (100 U/ml) in a 95% air/5% CO2 humidified atmosphere at 37°C. After 24 hours in culture, the cells were washed with phosphate-buffered saline (PBS), and renewed with media containing 10% FCS and nystatin.
The purity of the isolated cells was determined by immunofluorescence staining using anti-cytokeratin 7 and anti-vimentin antibodies. A proportion of ≥90% cytokeratin 7–positive cells (cytotrophoblasts) was considered to be of sufficient purity. Primary trophoblasts from eight different placentas were used for the regulatory experiments, and the data generated were pooled and analyzed collectively.
Assessment of Cytotrophoblast Cell Biochemical Differentiation.
The β-subunit of human chorionic gonadotropin (hCG) is produced by terminally differentiated syncytiotrophoblast and was measured as an indicator of cytotrophoblast differentiation in culture after their isolation from term placenta. Culture medium was collected at 24, 48, 72, 96, and 120 hours of cytotrophoblast cell culture, and was stored in aliquots at −80°C. β-hCG was measured by enzyme-linked immunosorbent assay following the manufacturer’s instructions (DRG International, Springfield, NJ).
PGE2 Measurements.
Cells were cultured in their respective phenol red free, charcoal-stripped FBS media for 24 hours. Cytotrophoblasts were cultured for 4 days, at which point media were replaced and collected after 24 hours. The PGE2 concentration in the media was then determined using a PGE2 enzyme-linked immunosorbent assay kit (Thermo Fisher Scientific, Rockford, IL) following the manufacturer’s instructions. The sensitivity of the kit was 15.6 pg/ml, and the intra- and inter-assay coefficients of variation were less than 10%. PGE2 concentrations were calculated using a four-parameter logistic curve-fitting program in GraphPad Prism 6 (GraphPad Software, La Jolla, CA).
Treatment.
To examine the effect of PGE2 on Pgp and BCRP transporter expression, cells were cultured in their respective phenol red free medium supplemented with 10% charcoal-/dextran-stripped FBS for 24 hours. Primary trophoblasts were cultured for 4 to 5 days to allow for the development of syncytialization. The medium was then replaced with fresh medium containing 5 µg/ml [14.8 µM] PGE2 and cell culture continued. For pharmacological interventions of PGE2 receptor–specific isoforms, Jar cells were incubated with 10 µM PG receptor antagonists (SC-19220, EP1; AH-6809, EP1 and EP2; L-798106, EP3; and L-161982, EP4) for 1 hour prior to the addition of PGE2 (5 µg/ml). The effects of PGE2 and EP receptor antagonists on mRNA expression were tested at 24 hours. Multiple time points (6–48 hours) were tested in preliminary studies. At 24 hours, we observed clear and consistent changes in transporter mRNA and protein with PGE2 treatment. In consecutive studies, cells were exposed to an EP3 receptor agonist (sulprostone, 10 µM) or an EP1 receptor agonist (17-phenyltrinor PGE2) for 24 hours prior to RNA isolation. Finally, Jar cells were treated with BAY-110782 (10 µM) and SR 11302 (10 µM) for 1 hour prior to the addition of PGE2. Pgp and BCRP mRNA expression were determined at 24h.
RNA Isolation and Purification.
Total cellular RNA was isolated from the cells and purified using the RNeasy Mini Kit according to manufacturer’s instructions (Qiagen, Valencia, CA). Total RNA concentration was determined by UV spectroscopy at 260 nm and RNA integrity and purity were confirmed by a 260/280 ratio (>1.8) and separation on a 1% agarose gel followed by visualization with ethidium bromide.
Quantitative Real-Time Polymerase Chain Reaction.
Primer sequences for both Pgp and BCRP amplifications were previously described (Mason et al., 2011). 18s rRNA was used to normalize mRNA expression levels. Each sample was assayed in duplicate. All primer sets were tested to ensure efficiency of amplification over a wide range of template concentrations. SYBR Green (Bio-Rad Laboratories, Hercules, CA) was used for amplicon detection. A melt curve was used after amplification to ensure that all samples exhibited a single amplicon. The average Ct value (cycle threshold for target or endogenous reference gene amplification) was estimated using the software associated with the iCycler real-time polymerase chain reaction detection system (Bio-Rad Laboratories). Relative changes in mRNA expression of the target genes were analyzed using the ΔΔCt method (2−ΔΔCt) (Livak and Schmittgen, 2001). The average ΔCt was calculated by subtracting the average Ct value of the endogenous reference gene (18s rRNA) from the average Ct value of the target gene for the treatment and control groups. Fold changes in mRNA expression of target genes from the treatment groups were expressed relative to that of the vehicle control group.
Immunoblot Analysis (Western Blotting).
Whole cell lysates were prepared by washing the cells twice with ice-cold PBS and then adding lysis buffer (50 mmol/l Tris-Cl, pH 7.5, 150 mmol/l NaCl, 1 mmol/l EDTA, 1% Igepal Ca 630, 0.1% SDS, 0.5% sodium deoxycholate, protease inhibitor, phosphatase inhibitor cocktail I, phosphatase inhibitor cocktail II) to each well. The cells were collected in lysis buffer using a cell scraper, and the sample was placed on ice and sonicated briefly. The protein content was measured using the Bio-Rad protein assay kit (Bio-Rad Laboratories).
Protein samples were heated in SDS Laemmli loading buffer [1:1 (v/v)] at 95°C for 5 minutes, and total protein (40 μg) was loaded onto a 7.5% SDS-polyacrylamide gel. Electrophoresis was conducted at 100 V. The proteins were transferred overnight at 35 V onto polyvinylidene membrane. Nonspecific binding sites were blocked for 1h with 5% nonfat dry milk in PBS-Tween 0.1% (PBS-T). The polyvinylidene membrane was incubated with Pgp antibody (1:500), BCRP antibody (1:1000), and β-actin antibody (1:20,000) at 4°C overnight, washed three times with PBS-T, and then incubated with a horseradish peroxidase–linked secondary antibody for 1 hour at room temperature. The cells were then washed three times with PBS-T. Detection of the protein−antibody complexes was accomplished via the use of enhanced chemiluminescence reagents and chemiluminescent detection.
Functional Activity.
Efflux transporter-mediated activity was measured by the intracellular accumulation of fluorescent substrates in the presence or absence of specific inhibitors. Drug efflux activity of cells in monolayer culture was measured by inhibition of the cellular uptake of fluorescent Pgp substrates, calcein acetoxymethylester (calcein AM) and Flutax-2 (Oregon green-488 paclitaxel), and BCRP substrate, Hoechst 33342. Calcein AM is a lipophilic, nonfluorescent dye that diffuses into cells where it is cleaved by cytosolic esterase to a green fluorescent dye. Because calcein AM is also a substrate for MRP1, Flutax-2 was used to confirm Pgp activity. Flutax-2 is a commercially available fluorescent derivative of paclitaxel with a high diffusion coefficient to cells (Jang et al., 2001; Díaz et al., 2003). Hoechst 33342 is a dye that fluoresces only when bound to DNA. Thus, the amount of transporter activity is inversely proportional to the accumulation of intracellular fluorescence. The selective Pgp and BCRP inhibitors used were verapamil (100 μM) and FTC (5 μM), respectively.
For functional studies, Jar cells were plated on 24-well plates at 1 × 105 cells/well and incubated for 1 day in phenol red free medium containing 10% charcoal/dextran-stripped FBS before study. Primary trophoblasts were seeded at a density of 5 × 105 cell/well and cultured for 4 days in growth medium containing 10% charcoal-/dextran-stripped FBS prior to treatment. Cells were incubated for 24 hours at 37°C with or without PGE2 (5 μg/ml). The selective blockers were added for 60 minutes at 37°C prior to treatment with fluorescent substrates. Fluorescent substrates were then added to final concentrations of 0.4 μM (calcein AM), 0.5 μM (Flutax-2), and 5 μg/ml (Hoechst 33442). After a 60-minute incubation period, the cells were washed thrice with cold PBS and lysed in 10 mM Tris-HCl-1% Triton X-100 (pH 7.4). The accumulation of fluorescent substrates was measured on a SpectraMax M5 Microplate reader (Molecular Devices, Sunnyvale, CA) at 485/535 nm for calcein AM and Flutax-2, and 355/460 nm for Hoechst 33442. Relative fluorescent units were normalized by protein concentration and expressed as percentage compared with control.
Luciferase Assay.
pNF-κB–Luc plasmid or pAP-1–Luc plasmid was cotransfected with Renilla luciferase plasmid into Jar cells using Lipofectamine 2000 according to the manufacturer’s instructions (Life Technologies). The cells were transfected for 24 hours, washed, and then treated with PGE2 (5 µg/ml) for another 24 hours. Luciferase activity was assayed using the Dual-Luciferase Reporter Assay System (Promega) according the recommended protocol. The luciferase activity in the cell lysates was measured by luminescence using the TD-20/20 luminometer (Promega) and normalized to total protein. Each treatment group was run in triplicate.
Statistical Analyses.
All studies were repeated at least three times. Descriptive statistics were performed for each data set. Graphs were plotted, data were transformed, and statistical analysis was carried out using GraphPad Prism 6.0 (GraphPad Software). β-hCG concentrations were log transformed and analyzed using one-way analysis of variance (ANOVA) followed by Dunnett’s post test. For expression studies, either the unpaired t test or one-way ANOVA followed by the Tukey’s post test was performed as appropriate. Differences in the cellular accumulation of fluorescent substrates were analyzed using a one-way ANOVA followed by Dunnett’s post test. P < 0.05 was considered to be significant.
Results
Differentiation of Cytotrophoblasts in Culture.
We confirmed prior reports of cytotrophoblast morphologic and biochemical differentiation in culture. The morphologic changes were accompanied by biochemical differentiation as indicated by increased hCG secretion. Cytotrophoblast β-hCG secretion increased significantly by 72 hours and peaked at 96 hours in culture (Supplemental Fig. 1).
PGE2 Production by Jar Cells and Primary Trophoblasts.
To address the possible effect of endogenous PGE2 produced in cell culture, we evaluated PGE2 concentrations in culture media from untreated cells. In a 24-hour culture, Jar cells (5 × 105 cells) produced a mean PGE2 concentration of 39.7 ± 2.8 pg/ml (n = 4). The basal level of PGE2 produced in 24 hours by differentiated trophoblasts (6 × 106 cells; cultured for 4 days) was 153.4 ± 23.4 pg/ml (n = 5).
PGE2 Differentially Affects BCRP Expression in Human Placental Cells.
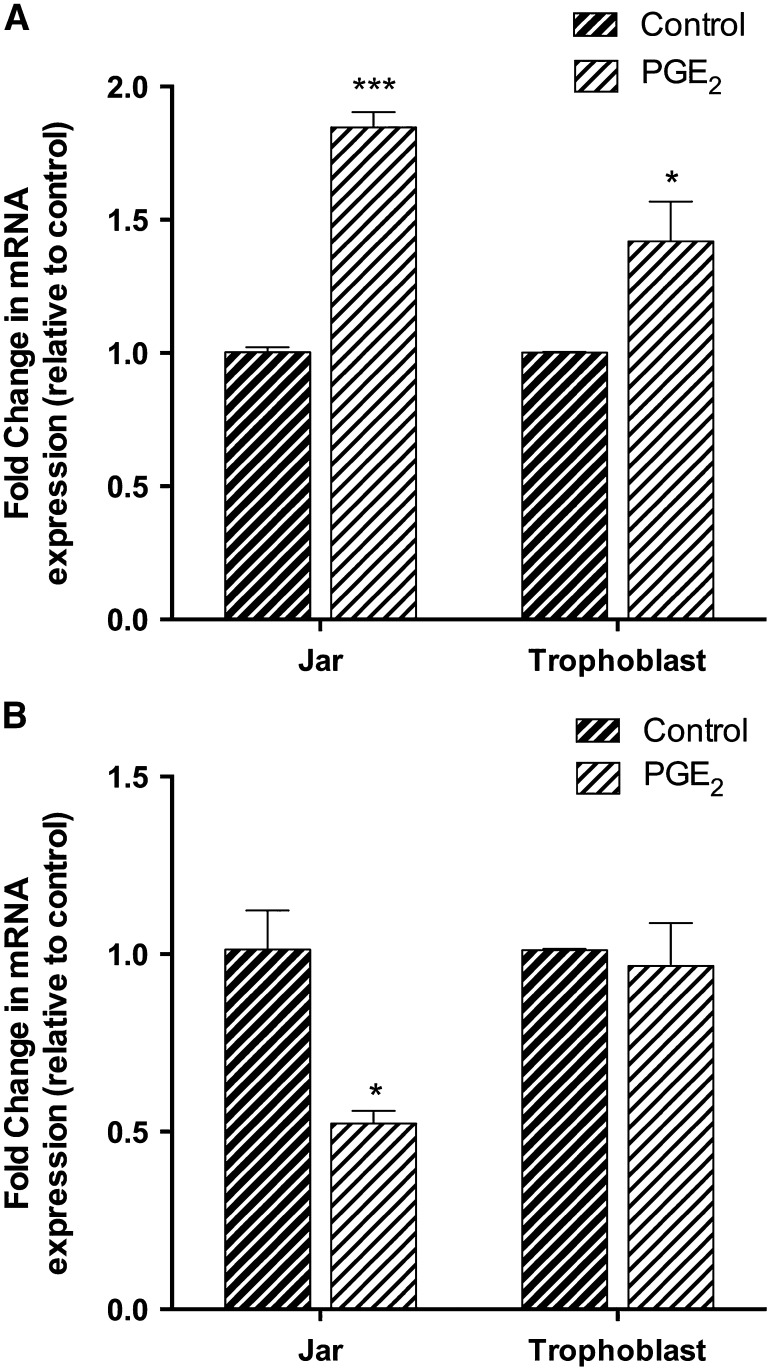
PGE2 increased BCRP mRNA expression nearly 1.9-fold in Jar cells (P < 0.001) and 1.5-fold in human primary trophoblasts (P < 0.05) after 24-hour treatment (Fig. 1A). PGE2 decreased Pgp mRNA in Jar cells by approximately 50% (P < 0.05) compared with the control, but there was no change of expression in human trophoblasts (Fig. 1B).
Fig. 1.
Relative changes in BCRP (A) and Pgp (B) mRNA expression were determined by real-time polymerase chain reaction in human placental choriocarcinoma Jar cells (n = 3) and human primary trophoblast cells (n = 8) stimulated with PGE2 (5 μg/ml) for 24 hours. Samples were normalized to the endogenous reference gene, 18s rRNA, and expressed relative to the vehicle control. The mean fold change was calculated in each sample by the 2ΔΔCt method. Treatment with PGE2 increased BCRP mRNA expression. Data are presented as the mean ± S.E.M. *P < 0.05; ***P < 0.001
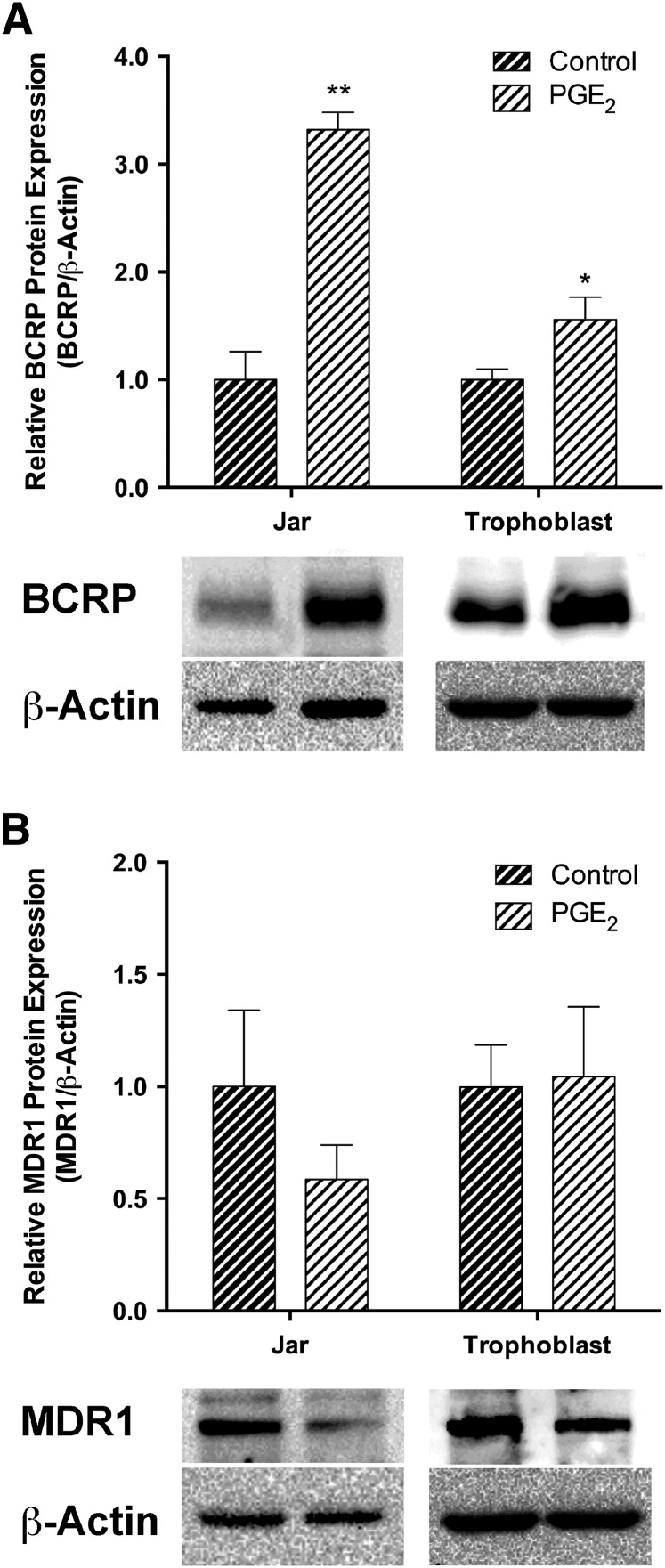
Compared with the untreated control, 24 hours of PGE2 produced a 3.3-fold (P < 0.01) increase in Jar cell BCRP protein and a 1.6-fold (P < 0.05) increase in human primary trophoblasts (Fig. 2A). Pgp protein levels were not statistically altered among cells exposed to PGE2 and vehicle (P = 0.29) (Fig. 2B).
Fig. 2.
The effect of PGE2 on BCRP (A) and Pgp (B) protein expression was determined by Western blot in human placental choriocarcinoma Jar cells (n = 3) and human primary trophoblast cells (n = 8). Immunoblots shown are the representative results obtained in typical experiments. Relative protein levels were normalized to β-actin. Band intensity (intensity/mm2) was expressed as a fold change over the average control for 24-hour PGE2 treatments. Data are presented as the mean ± S.E.M. *P < 0.05; **P < 0.01.
PGE2 Alters BCRP, But Not Pgp Activity.
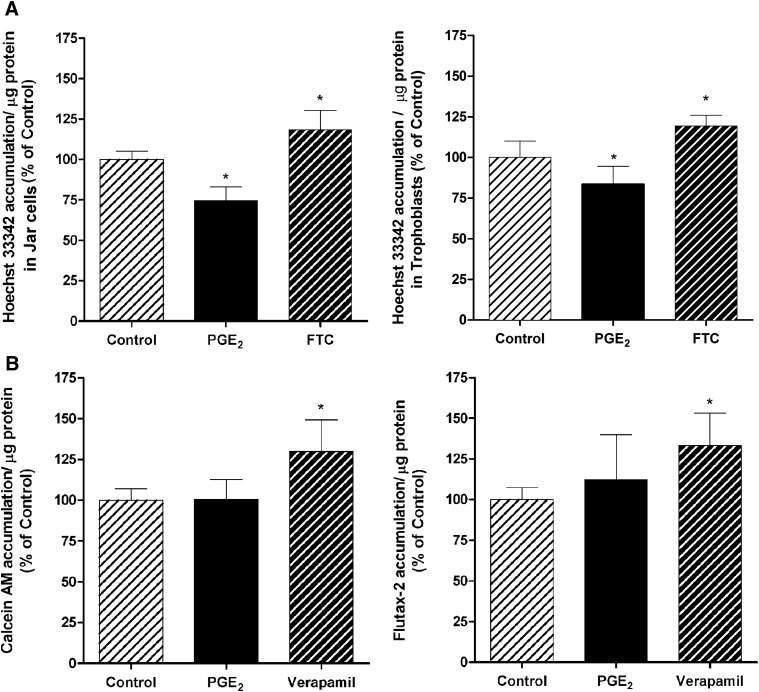
BCRP functional activity was clearly present in Jar cells and primary trophoblasts, which showed about a 13 and 19% increase, respectively, in Hoechst 33342 accumulation after 60-minute incubation in the presence of the BCRP selective blocker FTC (P < 0.05) (Fig. 3A). In agreement with the increased BCRP mRNA and protein levels, PGE2 treatment (5 μg/ml; 24 hours) decreased the accumulation of Hoechst 33342 over 20% compared with the control in Jar cells and around 16% in primary trophoblasts (P < 0.05; Fig. 3A). Accumulation of the Pgp substrate, calcein AM, was about 30% higher in the presence of verapamil (P < 0.05; Fig. 3B), but there was no difference when cells were treated first with PGE2. Cells treated with MK 571 also increased cellular accumulation of calcein AM indicating the presence of MRP1 in Jar cells. However, PGE2 had no effect on the MRP1 mRNA level as determined by quantitative real-time polymerase chain reaction (Supplementary Fig. 2). The treatment of Jar cells with verapamil also increased the cellular accumulation of Flutax-2 by 33% (P < 0.05). PGE2 increased Flutax 12%, but this increase did not achieve statistical significance (Fig. 3B).
Fig. 3.
The functional expression of BCRP in Jar cells and primary trophoblasts (A) and Pgp in Jar cells (B) was evaluated after treatment with PGE2 (5 μg/ml). Drug efflux activity was determined after 24-hour PGE2 exposure, and selective chemical inhibition of the intracellular accumulation of fluorescent BCRP substrate Hoechst 33342 and Pgp substrates calcein AM and Flutax-2. The selective BCRP and Pgp inhibitors used were FTC (5 µM) and verapamil (100 µM), respectively. Data are presented as the mean ± S.E.M. *P < 0.05.
PGE2 Regulates BCRP Expression via EP1 and EP3 Receptors.
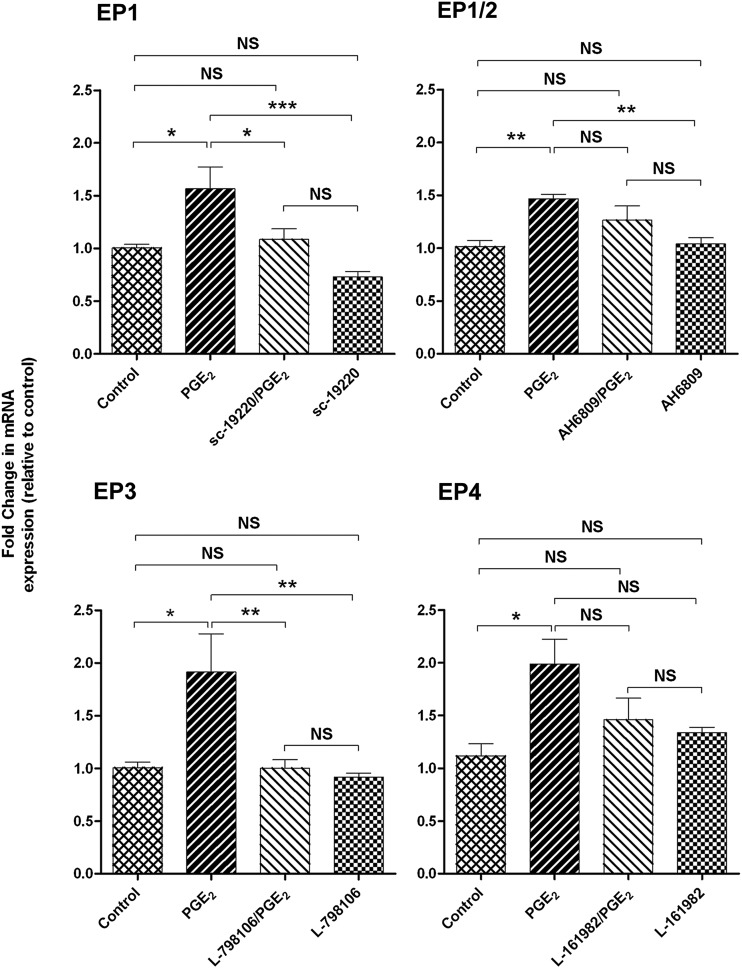
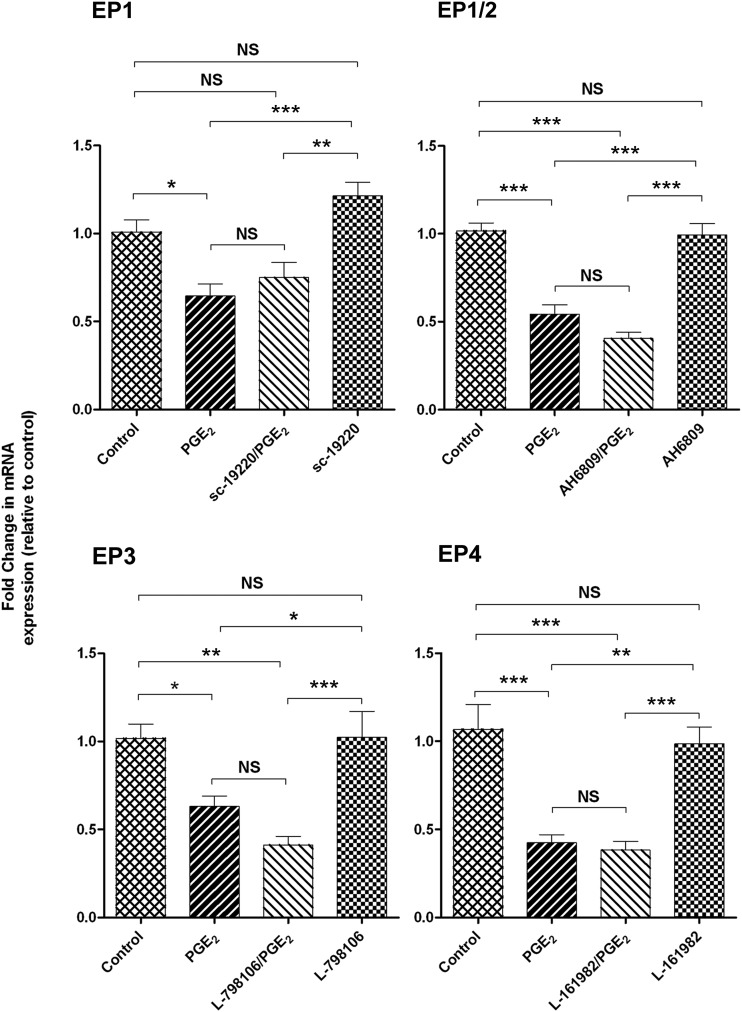
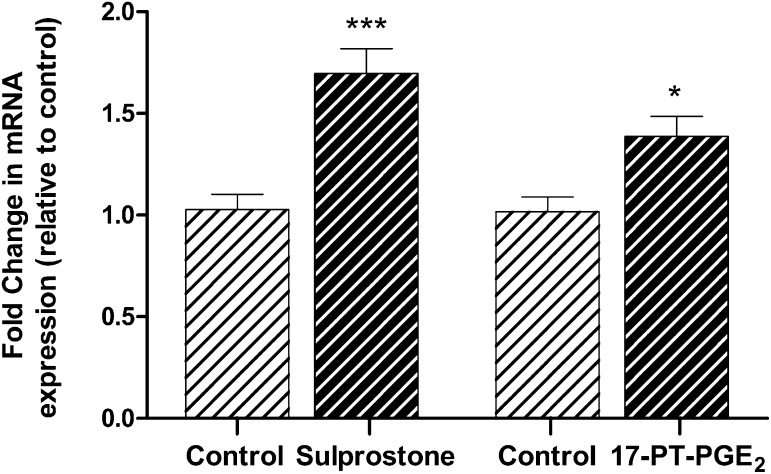
To determine the EP receptors involved in PGE2-mediated up-regulation of BCRP expression, cells were pretreated with an EP receptor antagonist before exposure to PGE2 (5 µg/ml) for 24 hours. Both the selective EP1 antagonist SC-19220 (P < 0.05) and the EP3 antagonist L-798196 (P < 0.01) inhibited the induction of BCRP mRNA by PGE2. Treatment of cells with EP4-selective antagonist L-161982 and EP1/EP2 antagonist AH 6809 slightly reduced BCRP mRNA levels (Fig. 4). All EP receptor antagonists tested failed to fully restore Pgp mRNA levels in Jar cells exposed to PGE2 (Fig. 5). There was no effect on Pgp or BCRP expression when cells were treated with antagonist alone. It should be noted that neither PGE2 nor the EP receptor antagonists altered EP1 and EP3 receptor levels (data not shown), indicating that the effect of these agents on transporter expression is not attributable to changes in the cellular expression of EP1 and EP3, but rather the signaling pathway involved. Sulprostone (EP3 agonist; 10 μM) increased BCRP mRNA expression in Jar cells 1.7-fold (P < 0.001), whereas 17-phenyltrinor PGE2 (EP1 agonist; 10 μM) increased BCRP expression 1.4-fold (P < 0.05; Fig. 6).
Fig. 4.
The effects of PGE2 receptor (EP) antagonism on BCRP mRNA expression in placental Jar cells. The mean fold change in transporter mRNA expression was calculated in each sample by the 2ΔΔCt method. Ct values of the target gene were normalized to the endogenous reference gene, 18s rRNA, and expressed relative to the vehicle control. Data are presented as the mean ± S.E.M. *P < 0.05; **P < 0.01; ***P < 0.001; NS, no significant difference.
Fig. 5.
The effects of PGE2 receptor (EP) antagonism on Pgp mRNA expression in placental Jar cells. The mean fold change in transporter mRNA expression was calculated in each sample by the 2ΔΔCt method. Ct values of the target gene were normalized to the endogenous reference gene, 18s rRNA, and expressed relative to the vehicle control. Data are presented as the mean ± S.E.M. *P < 0.05; **P < 0.01; ***P < 0.001. NS, no significant difference.
Fig. 6.
The EP1 receptor agonist 17-phenyl trinor PGE2 and the EP3 receptor agonist sulprostone increase BCRP mRNA expression. The mean fold change in transporter mRNA expression was calculated in each sample by the 2ΔΔCt method. Ct values of the target gene were normalized to the endogenous reference gene, 18s rRNA, and expressed relative to the vehicle control. Results are expressed as the mean ± S.E.M. *P < 0.05; ***P < 0.001 compared with the vehicle control.
PGE2 Stimulates AP-1 and Represses NF-κB Activation.
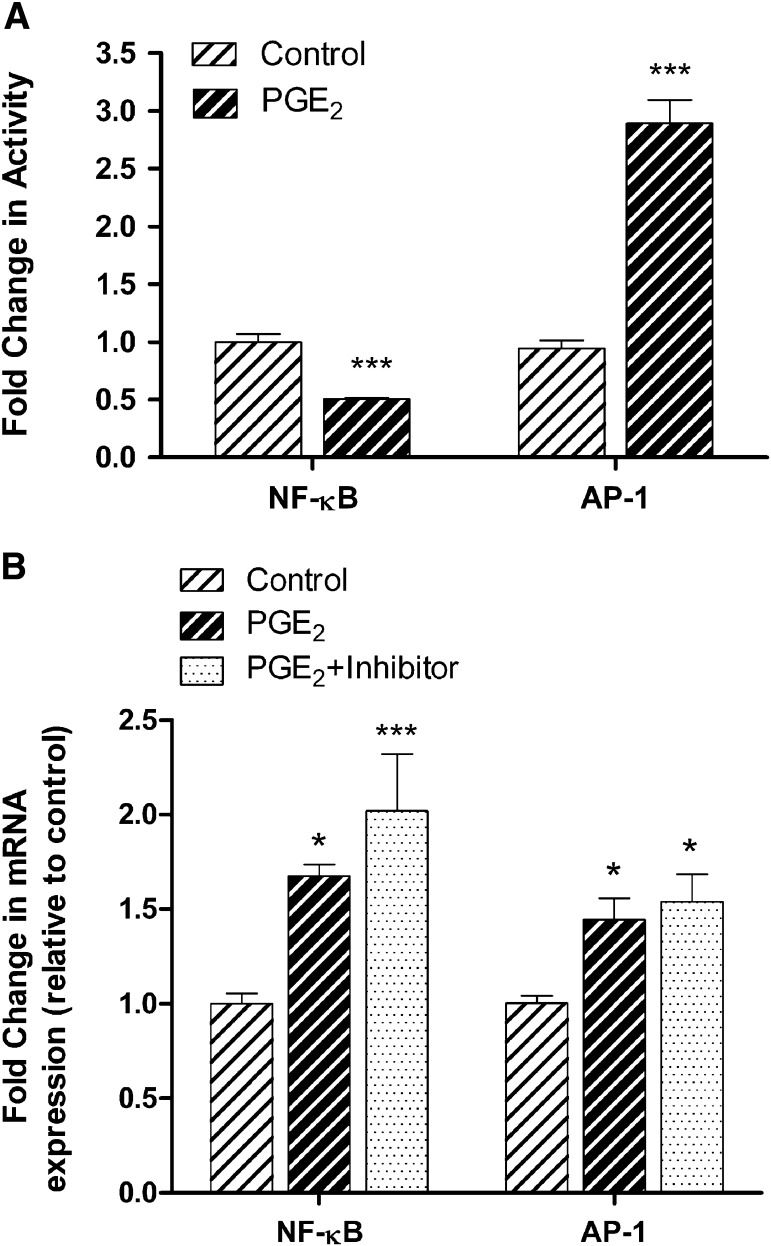
To examine whether NF-κB and AP-1 mediate PGE2-induced BCRP expression in Jar cells, we first transfected the cells with NF-κB cis-reporter plasmid pNF-κB–Luc or AP-1 cis-reporter plasmid pAP-1–Luc. Once complete, PGE2 treatment repressed NF-κB activation as reflected in a nearly 2.0-fold decrease in luciferase activity at 24 hours (P < 0.001). AP-1 luciferase activity was increased 2.9-fold in the presence of PGE2 at 24 hours (P < 0.001, Fig. 7A).
Fig. 7.
The effect of PGE2 on the activation of transcription factors NF-κB and AP-1, and their impact on BCRP transcriptional expression. (A) The effect of PGE2 on NF-κB– and AP-1–dependent transcriptional activity was measured by NF-κB– and AP-1–dependent promoter luciferase reporter gene assay. Cells were cotransfected with pNF-κB–Luc or pAP-1–Luc for 24 hours, and then exposed to PGE2 (5 μg/ml). The luciferase activity was measured after 24-hour administration of PGE2. Data for the NF-κB– and AP-1–dependent promoter activity were normalized to luciferase activity from cotransfection of Renilla luciferase plasmid. (B) Jar cells were treated with the NF-κB inhibitor BAY-11-7082 (10 μM) or the AP-1 inhibitor SR 11302 (10 μM) for 1 hour prior to stimulation with PGE2 for an additional 24 hours. BCRP mRNA expression was determined by real-time polymerase chain reaction. Neither BAY-11-7082 nor SR 11302 affected the PGE2-mediated up-regulation of BCRP. Values are the means ± S.E.M. *P < 0.05; ***P < 0.001 compared with vehicle control.
In subsequent experiments, inhibition of NF-κB and AP-1 with BAY-11-7082 (10 μM) and SR 11302 (10 μM), respectively, had little effect on PGE2-induced BCRP up-regulation (Fig. 7B). The addition of BAY 11-7082 or SR 11302 alone had no effect on BCRP mRNA levels (data not shown).
Discussion
Inflammatory responses during pregnancy can trigger altered expression of placenta transporters, but the factors responsible for these changes remain largely unknown. We find that PGE2, an immunomodulatory lipid associated with the terminal phases of cervical softening and uterine contraction, alters the functional expression of BCRP in both immortalized and primary placental cells. PGE2 increased BCRP mRNA and protein expression (Figs. 1 and 2, respectively), albeit to a lesser extent in human placental trophoblasts. The results correlate with increased BCRP activity in Jar cells and primary trophoblasts, which was identified by a decrease in the cellular accumulation of its substrate, Hoechst 33342 (Fig. 3). These findings are supported by prior studies demonstrating that the administration of a COX-2 inhibitor, celecoxib, which would inhibit PGE2 production, reverses BCRP-related drug resistance via the down-regulation of BCRP mRNA (Ko et al., 2008; Kalalinia et al., 2011).
Activation of COX-2 has also been shown to increase Pgp (Patel et al., 2002; Liu et al., 2010). Thus, we were surprised that PGE2 decreased mRNA levels of Pgp in Jar cells (Fig. 1). Although there was a trend toward decreased Pgp protein expression, the differences failed to achieve statistical significance (P = 0.29) (Fig. 2B). As previously cited, differences at the RNA level do not always closely correlate with ABC protein expression in trophoblast cultures (Evseenko et al., 2006). Furthermore, we found virtually no effect of PGE2 on calcein AM accumulation in Jar cells. Calcein AM is also an MRP1 substrate, which too, is functionally expressed in Jar cells (Evseenko et al., 2007). Although PGE2 had no effect on MRP1 transcripts (Supplemental Fig. 2B), MRP1 inhibition with MK-571 increased the cellular accumulation of calcein AM (Supplemental Fig. 2A). Paclitaxel may be a better substrate for Pgp, even though it can also be transported by MRP2 (Lagas et al., 2006). MRP2 is poorly expressed in Jar cells (Evseenko et al., 2006), and the cellular accumulation of Flutax-2 (i.e., fluorescently labeled paclitaxel) is perhaps more representative of Pgp function. PGE2 modestly increased Flutax-2 accumulation in Jar cells (Fig. 3B). The fact that changes in Pgp gene expression were not followed by similar functional expression indicates that there can be changes in placental Pgp gene expression independent of its activity. A disconnect between Pgp gene expression and activity was previously demonstrated in other placenta models (Petropoulos et al., 2010; Bloise et al., 2013).
There were obvious differences in the extent that PGE2 altered BCRP and Pgp expression in Jar and primary trophoblast cells. This could be attributed to several different factors including those related to immortalization, culture conditions, metabolic status, transporter expression, and interindividual variability (as in the case of primary cultures). ABC transporter expression varies among the various placental cell lines (Evseenko et al., 2006). The choriocarcinoma Jar cell line has been used to study trophoblast biology and transport in vitro (Atkinson et al., 2003; Evseenko et al., 2006; Serrano et al., 2007; Coles et al., 2009). We chose the Jar cell line for this study because it expresses both Pgp and BCRP at levels detectable for quantitation and shows evidence of functional expression of these transporters.
The most prominent effect of PGE2 on transporter expression was observed in the Jar cells. To more closely evaluate the mechanism and identify clear differences, we performed the subsequent studies using these cells. There are several reasons the effect of PGE2 on transporter expression was more prominent in Jar cells. Differentiation (syncytialization) may explain some or all the observed differences between the two cell models. Jar cells proliferate in culture but do not spontaneously fuse, whereas trophoblast cells form differentiated syncytium by days 4 to 5. β-hCG is produced by terminally differentiated syncytiotrophoblasts and is used to assess cytotrophoblast biochemical differentiation in culture. We found that β-hCG levels dramatically increase around 72 hours in culture (Supplemental Fig. 1). BCRP and Pgp expression appear to change as trophoblasts mature in culture. BCRP levels in primary trophoblasts increased at 120 hours of incubation relative to those at 24 hours, whereas Pgp decreased (Evseenko et al., 2006). Even so, our results were similar for trophoblasts cultured for 1 day (data not shown) or 4 to 5 days prior to PGE2 treatment.
Intrinsic differences in the functional expression of the PGE2 machinery (i.e., COX-2, arachidonic acid, EP receptors, etc.) and PGE2 production could also explain observed differences between the cells. For instance, previous reports failed to detect the production of PGE2 by JEG-3 choriocarcinoma cells. In contrast with primary trophoblasts, it has been suggested that the limited arachidonate releasing capacity of JEG-3 cells might be responsible for the low PGE2 production (Premyslova et al., 2006). In theory, this could mean that choriocarcinoma cells, such as Jar, are more sensitive to exogenous PGE2 stimulation compared with the primary trophoblasts. In the present study, we determined that primary trophoblasts seeded at a density of 6 × 106 cells and differentiated for 4 days produced around 153.4 ± 23.4 pg/ml of PGE2 in 24 hours. Jar cells were also capable of producing PGE2. The mean basal concentration of PGE2 produced in Jar cells seeded at a density of 5 × 105 cells was 39.7 ± 2.8 pg/ml. We cannot rule out the possibility that endogenous PGE2 may have some effect on transporter expression.
A primary function of BCRP and Pgp is to transport compounds out of placental cells and prevent access to the fetal circulation. In tissue barriers, BCRP and Pgp often act in concert (de Vries et al., 2007), implying that the loss of either transporter alone does not result in an appreciable increase in penetration of dual substrates. For example, in Pgpa/Pgpb knockout mice (where bcrp1 is present), bcrp1 alone is sufficient to limit brain uptake of xenobiotics (Agarwal et al., 2011). The same can be said of mdr1a/mdr1b in bcrp1 knockout mice. Thus, the greatest impact on transplacental passage of dual substrates may be seen when both transporters are similarly altered or when one of these transporters is predominately expressed in the cell.
BCRP mRNA levels are highest in placenta compared with other tissues, and BCRP is approximately 10 times greater than Pgp in human term placenta (Ceckova et al., 2006). Many drugs commonly administered to pregnant women such as glyburide (Zhou et al., 2008), nitrofurantoin (Merino et al., 2005), and cimetidine (Pavek et al., 2005) are BCRP substrates, and its induction may affect how these drugs are distributed and cleared during pregnancy. The importance of BCRP in placenta is further recognized by its presence on the fetal blood vessel endothelial cells (Ceckova et al., 2006; Yeboah et al., 2006; Mason et al., 2011), where it could efflux potentially harmful exogenous and endogenous substrates into the fetal circulation. In this case, up-regulation of BCRP in fetal blood vessel endothelial cells could have an impact on transport toward the fetus. Interestingly, Yeboah et al. (2006) found that neither the umbilical cord vein nor arteries express BCRP. They speculated that BCRP expression in the fetal blood vessel endothelial cells may be regulated by the local action of factors derived from the placenta itself. PGE2 is produced by the placenta and our data link PGE2 to up-regulation of BCRP, advocating it as a potentially important factor in terms of placental transport.
The heterogeneity of PGE2 immunoregulatory effects may depend on distinctive signaling mechanisms mediated by different PGE2 receptors. All four membrane receptor subtypes (EP1–EP4) have been localized in human placental villous tissue (Grigsby et al., 2006). It is believed that their presence in human placenta may indicate autocrine and paracrine roles for PGE2 in the signaling pathways associated with parturition (Grigsby et al., 2006). We suspect that specific EP receptors are involved in downstream regulation of BCRP and Pgp. We observed that antagonism of the EP1 and EP3 receptors counteracts the increase in BCRP mRNA induced by PGE2 (Fig. 4), and that the EP1 and EP3 agonists alone increased BCRP mRNA expression (Fig. 6). Thus, EP1/EP3 receptors may facilitate PGE2 signals that modulate placental BCRP expression. Further evaluation may reveal that these receptors are potential pharmacological targets, whereby limiting the responsiveness to PGE2 during pathologic pregnancy could potentially mitigate BCRP overexpression while maintaining its relevant transport function. This could allow adequate delivery of drugs intended for the fetus without compromising the protective function of BCRP in the placenta.
The greatest impact on BCRP mRNA expression occurred with the EP3 receptor. This receptor is unique among the EP subtypes in that it seems to be the only prostanoid receptor present in the syncytiotrophoblast layer of placenta from women at various stages of labor (Grigsby et al., 2006). Its expression corresponds to the apical membrane localization of BCRP in syncytiotrophoblasts, supporting its potential involvement in BCRP regulation. The EP3 receptor has multiple isoforms generated by alternative splicing. These variants differ in tissue expression, constitutive activity, and regulation of signaling molecules (Adam et al., 1994; Negishi et al., 1995; Schmid et al., 1995). Their expression profile in the human placenta is unknown, but suggests the potential for isoform-specific regulation of BCRP transcriptional activation. Targeting placenta-specific isoforms of EP3 to regulate BCRP activity could avoid the ever-present possibility of affecting BCRP levels in other tissues.
EP receptor signaling pathways ultimately lead to activation and translocation of transcription factors involved in the regulation of transporter expression. Within the promoter region of BCRP and Pgp are the putative binding sites for both NF-κB and AP-1. These proinflammatory transcription factors could play a role in the regulation of PGE2 on transporter expression. It was previously shown that COX-2/PGE2/EP receptor signaling produces dose-dependent changes in the activity of NF-κB (Hu et al., 2008). Others have observed that NF-κB inhibits the transcription of Pgp/mdr1a/mdr1b (Ogretmen and Safa, 1999) and induces the transcription of BCRP/Bcrp1 (Pradhan et al., 2010). We found that PGE2 decreased NF-κB and increased AP-1 activity (Fig. 7A) in Jar cells, supporting AP-1 activation as a candidate transcriptional pathway for BCRP up-regulation. It is possible that NF-κB and AP-1 may modulate each other and that activation of AP-1 negatively affects NF-κB activity (Fujioka et al., 2004). Although this could explain the effect of PGE2 on NF-κB activity, inhibition of these transcription factors in PGE2-treated Jar cells did not interfere with BCRP induction (Fig. 7B). This indicates that BCRP mRNA may not be directly regulated by NF-κB or AP-1 when stimulated with PGE2. Clearly, a broader investigation into the transcription factor profile activated by PGE2 stimulation would provide insight into those that contribute to the induction of BCRP mRNA.
Both BCRP and Pgp are important elements of barrier function and distinct signaling pathways may regulate their functional expression. This study highlights a potential role of PGE2 in regulating BCRP expression in human placenta cells. Given that placental PGE2 levels rise in response to infection, we postulate that BCRP up-regulation is a placental mechanism to dynamically respond to protect the developing fetus from inflammatory insults, and that PGE2 is a trigger for this cellular response. We cannot rule out the possibility that other PGs, such as PGF2α, may also have an effect on Pgp and BCRP expression. It would be interesting then in future investigations to compare the impact of different PGs on transporter activity.
Supplementary Material
Abbreviations
- ABC
ATP-binding cassette
- AH-6809
6-isopropoxy-9-oxoxanthene-2-carboxylic acid
- ANOVA
analysis of variance
- AP-1
p activator protein 1
- BAY-11-7082
3-[(4-methylphenyl)sulfonyl]-(2E)-propenenitrile
- BCRP
breast cancer resistance protein
- calcein AM
calcein acetoxymethylester
- COX
cyclooxygenase
- Ct
cycle threshold
- EP
E-prostanoid
- FBS
fetal bovine serum
- FCS
fetal calf serum
- FTC
fumitremorgin C
- hCG
human chorionic gonadotropin
- IL
interleukin
- L-161982
N-[[4′-[[3-butyl-1,5-dihydro-5-oxo-1-[2-(trifluoromethyl)phenyl]-4H-1,2,4-triazol-4-yl[methyl][1,1′-biphenyl]-2-yl[sulfonyl[-3-methyl-2-thiophenecarboxamide
- L-798106
(2E)-N-[(5-bromo-2-methoxyphenyl)sulfonyl]-3-[2-(2-naphthalenylmethyl)phenyl]-2-propenamide
- MDR
multidrug resistance protein
- MK-571
3-[[[3-[(1E)-2-(7-chloro-2-quinolinyl)ethenyl]phenyl][[3-(dimethylamino)-3-oxopropyl]thio]methyl]thio]propanoic acid)
- MRP
multidrug resistance–associated protein
- NF-κB
nuclear factor κ-light chain-enhancer of activated B
- NS398
N-[2-(cyclohexyloxy)-4-nitrophenyl]methanesulfonamide
- PBS
phosphate-buffered saline
- PBS-T
PBS-Tween 0.1%
- Pgp
P-glycoprotein
- PG
prostaglandin
- SC-19220
8-chloro-dibenz[b,f][1,4]oxazepine-10(11H)-carboxy-(2-acetyl)hydrazide
- SR 11302
(E,E,Z,E)-3-methyl-7-(4-methylphenyl)-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraenoic acid
Authorship Contributions
Participated in research design: Mason, Dong, Zhou, Weiner.
Conducted experiments: Mason, He.
Contributed new reagents or analytic tools: Lee.
Performed data analysis: Mason.
Wrote or contributed to the writing of the manuscript: Mason, Weiner.
Footnotes
This research is cofunded by the National Institutes of Health Office of Research on Women’s Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of Allergy and Infectious Diseases, and the National Institute of Mental Health [Grants 5K12-HD052027-06, K99-HD068454, and R00-HD068454].
This work was previously presented: Mason CW, Dong Y, Weiner CP (2011) Prostaglandin E2 Alters Drug Efflux Transporters in Human Placental Cells. AAPS Annual Meeting and Exposition; 2011 Oct 23–27; Washington, D.C.
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Adam M, Boie Y, Rushmore TH, Müller G, Bastien L, McKee KT, Metters KM, Abramovitz M. (1994) Cloning and expression of three isoforms of the human EP3 prostanoid receptor. FEBS Lett 338:170–174. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Hartz AM, Elmquist WF, Bauer B. (2011) Breast cancer resistance protein and P-glycoprotein in brain cancer: two gatekeepers team up. Curr Pharm Des 17:2793–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arico S, Pattingre S, Bauvy C, Gane P, Barbat A, Codogno P, Ogier-Denis E. (2002) Celecoxib induces apoptosis by inhibiting 3-phosphoinositide-dependent protein kinase-1 activity in the human colon cancer HT-29 cell line. J Biol Chem 277:27613–27621. [DOI] [PubMed] [Google Scholar]
- Arroyo J, Torry RJ, Torry DS. (2004) Deferential regulation of placenta growth factor (PlGF)-mediated signal transduction in human primary term trophoblast and endothelial cells. Placenta 25:379–386. [DOI] [PubMed] [Google Scholar]
- Arunasree KM, Roy KR, Anilkumar K, Aparna A, Reddy GV, Reddanna P. (2008) Imatinib-resistant K562 cells are more sensitive to celecoxib, a selective COX-2 inhibitor: role of COX-2 and MDR-1. Leuk Res 32:855–864. [DOI] [PubMed] [Google Scholar]
- Atkinson DE, Greenwood SL, Sibley CP, Glazier JD, Fairbairn LJ. (2003) Role of MDR1 and MRP1 in trophoblast cells, elucidated using retroviral gene transfer. Am J Physiol Cell Physiol 285:C584–C591. [DOI] [PubMed] [Google Scholar]
- Betz M, Fox BS. (1991) Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunol 146:108–113. [PubMed] [Google Scholar]
- Bloise E, Bhuiyan M, Audette MC, Petropoulos S, Javam M, Gibb W, Matthews SG. (2013) Prenatal endotoxemia and placental drug transport in the mouse: placental size-specific effects. PLoS ONE 8:e65728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceckova M, Libra A, Pavek P, Nachtigal P, Brabec M, Fuchs R, Staud F. (2006) Expression and functional activity of breast cancer resistance protein (BCRP, ABCG2) transporter in the human choriocarcinoma cell line BeWo. Clin Exp Pharmacol Physiol 33:58–65. [DOI] [PubMed] [Google Scholar]
- Ceckova-Novotna M, Pavek P, Staud F. (2006) P-glycoprotein in the placenta: expression, localization, regulation and function. Reprod Toxicol 22:400–410. [DOI] [PubMed] [Google Scholar]
- Chen YH, Wang JP, Wang H, Sun MF, Wei LZ, Wei W, Xu DX. (2005) Lipopolysaccharide treatment downregulates the expression of the pregnane X receptor, cyp3a11 and mdr1a genes in mouse placenta. Toxicology 211:242–252. [DOI] [PubMed] [Google Scholar]
- Coles LD, Lee IJ, Voulalas PJ, Eddington ND. (2009) Estradiol and progesterone-mediated regulation of P-gp in P-gp overexpressing cells (NCI-ADR-RES) and placental cells (JAR). Mol Pharm 6:1816–1825. [DOI] [PubMed] [Google Scholar]
- de Vries NA, Zhao J, Kroon E, Buckle T, Beijnen JH, van Tellingen O. (2007) P-glycoprotein and breast cancer resistance protein: two dominant transporters working together in limiting the brain penetration of topotecan. Clin Cancer Res 13:6440–6449. [DOI] [PubMed] [Google Scholar]
- Díaz JF, Barasoain I, Andreu JM. (2003) Fast kinetics of Taxol binding to microtubules. Effects of solution variables and microtubule-associated proteins. J Biol Chem 278:8407–8419. [DOI] [PubMed] [Google Scholar]
- Dumoulin FL, Reichel C, Sauerbruch T, Spengler U. (1997) Semiquantitation of intrahepatic MDR3 mRNA levels by reverse transcription/competitive polymerase chain reaction. J Hepatol 26:852–856. [DOI] [PubMed] [Google Scholar]
- Evseenko DA, Paxton JW, Keelan JA. (2006) ABC drug transporter expression and functional activity in trophoblast-like cell lines and differentiating primary trophoblast. Am J Physiol Regul Integr Comp Physiol 290:R1357–R1365. [DOI] [PubMed] [Google Scholar]
- Evseenko DA, Paxton JW, Keelan JA. (2007) Independent regulation of apical and basolateral drug transporter expression and function in placental trophoblasts by cytokines, steroids, and growth factors. Drug Metab Dispos 35:595–601. [DOI] [PubMed] [Google Scholar]
- Fantappiè O, Masini E, Sardi I, Raimondi L, Bani D, Solazzo M, Vannacci A, Mazzanti R. (2002) The MDR phenotype is associated with the expression of COX-2 and iNOS in a human hepatocellular carcinoma cell line. Hepatology 35:843–852. [DOI] [PubMed] [Google Scholar]
- Fujioka S, Niu J, Schmidt C, Sclabas GM, Peng B, Uwagawa T, Li Z, Evans DB, Abbruzzese JL, Chiao PJ. (2004) NF-kappaB and AP-1 connection: mechanism of NF-kappaB-dependent regulation of AP-1 activity. Mol Cell Biol 24:7806–7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta I, Yamada H, Sagawa T, Fujimoto S. (2000) Effects of inflammatory cytokines on prostaglandin E(2) production from human amnion cells cultured in serum-free condition. Gynecol Obstet Invest 49:93–97. [DOI] [PubMed] [Google Scholar]
- Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. (1999) Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med 5:698–701. [DOI] [PubMed] [Google Scholar]
- Grigsby PL, Sooranna SR, Brockman DE, Johnson MR, Myatt L. (2006) Localization and expression of prostaglandin E2 receptors in human placenta and corresponding fetal membranes with labor. Am J Obstet Gynecol 195:260–269. [DOI] [PubMed] [Google Scholar]
- Guo R, Hou W, Dong Y, Yu Z, Stites J, Weiner CP. (2010) Brain injury caused by chronic fetal hypoxemia is mediated by inflammatory cascade activation. Reprod Sci 17:540–548. [DOI] [PubMed] [Google Scholar]
- Hata AN, Breyer RM. (2004) Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther 103:147–166. [DOI] [PubMed] [Google Scholar]
- Ho EA, Piquette-Miller M. (2006) Regulation of multidrug resistance by pro-inflammatory cytokines. Curr Cancer Drug Targets 6:295–311. [DOI] [PubMed] [Google Scholar]
- Hu SS, Bradshaw HB, Chen JS, Tan B, Walker JM. (2008) Prostaglandin E2 glycerol ester, an endogenous COX-2 metabolite of 2-arachidonoylglycerol, induces hyperalgesia and modulates NFkappaB activity. Br J Pharmacol 153:1538–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SH, Wientjes MG, Au JL. (2001) Determinants of paclitaxel uptake, accumulation and retention in solid tumors. Invest New Drugs 19:113–123. [DOI] [PubMed] [Google Scholar]
- Kalalinia F, Elahian F, Behravan J. (2011) Potential role of cyclooxygenase-2 on the regulation of the drug efflux transporter ABCG2 in breast cancer cell lines. J Cancer Res Clin Oncol 137:321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. (2003) Cytokines, prostaglandins and parturition–a review. Placenta 24 (Suppl A):S33–S46. [DOI] [PubMed] [Google Scholar]
- Ko SH, Choi GJ, Lee JH, Han YA, Lim SJ, Kim SH. (2008) Differential effects of selective cyclooxygenase-2 inhibitors in inhibiting proliferation and induction of apoptosis in oral squamous cell carcinoma. Oncol Rep 19:425–433. [PubMed] [Google Scholar]
- Lagas JS, Vlaming ML, van Tellingen O, Wagenaar E, Jansen RS, Rosing H, Beijnen JH, Schinkel AH. (2006) Multidrug resistance protein 2 is an important determinant of paclitaxel pharmacokinetics. Clin Cancer Res 12:6125–6132. [DOI] [PubMed] [Google Scholar]
- Liu B, Qu L, Tao H. (2010) Cyclo-oxygenase 2 up-regulates the effect of multidrug resistance. Cell Biol Int 34:21–25. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. [DOI] [PubMed] [Google Scholar]
- Llorente L, Richaud-Patin Y, Diaz-Borjon A, Alvarado de la Barrera C, Jakez-Ocampo J, de la Fuente H, Gonzalez-Amaro R, Diaz-Jouanen E. (2000) Multidrug resistance-1 (MDR-1) in rheumatic autoimmune disorders. Part I: Increased P-glycoprotein activity in lymphocytes from rheumatoid arthritis patients might influence disease outcome. Joint Bone Spine 67:30–39. [PubMed] [Google Scholar]
- Mao Q. (2008) BCRP/ABCG2 in the placenta: expression, function and regulation. Pharm Res 25:1244–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason CW, Buhimschi IA, Buhimschi CS, Dong Y, Weiner CP, Swaan PW. (2011) ATP-binding cassette transporter expression in human placenta as a function of pregnancy condition. Drug Metab Dispos 39:1000–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino G, Jonker JW, Wagenaar E, van Herwaarden AE, Schinkel AH. (2005) The breast cancer resistance protein (BCRP/ABCG2) affects pharmacokinetics, hepatobiliary excretion, and milk secretion of the antibiotic nitrofurantoin. Mol Pharmacol 67:1758–1764. [DOI] [PubMed] [Google Scholar]
- Myatt L. (1990) Placental biosynthesis, metabolism, and transport of eicosanoids, in Eicosanoids in Reproduction (Mitchell MD, ed) pp 169–198, CRC Press Inc., Boca Raton, FL. [Google Scholar]
- Negishi M, Sugimoto Y, Ichikawa A. (1995) Molecular mechanisms of diverse actions of prostanoid receptors. Biochim Biophys Acta 1259:109–119. [DOI] [PubMed] [Google Scholar]
- Ogretmen B, Safa AR. (1999) Negative regulation of MDR1 promoter activity in MCF-7, but not in multidrug resistant MCF-7/Adr, cells by cross-coupled NF-kappa B/p65 and c-Fos transcription factors and their interaction with the CAAT region. Biochemistry 38:2189–2199. [DOI] [PubMed] [Google Scholar]
- Patel VA, Dunn MJ, Sorokin A. (2002) Regulation of MDR-1 (P-glycoprotein) by cyclooxygenase-2. J Biol Chem 277:38915–38920. [DOI] [PubMed] [Google Scholar]
- Pavek P, Merino G, Wagenaar E, Bolscher E, Novotna M, Jonker JW, Schinkel AH. (2005) Human breast cancer resistance protein: interactions with steroid drugs, hormones, the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine, and transport of cimetidine. J Pharmacol Exp Ther 312:144–152. [DOI] [PubMed] [Google Scholar]
- Petropoulos S, Gibb W, Matthews SG. (2010) Effect of glucocorticoids on regulation of placental multidrug resistance phosphoglycoprotein (P-gp) in the mouse. Placenta 31:803–810. [DOI] [PubMed] [Google Scholar]
- Petrovic V, Teng S, Piquette-Miller M. (2007) Regulation of drug transporters during infection and inflammation. Mol Interv 7:99–111. [DOI] [PubMed] [Google Scholar]
- Petrovic V, Wang JH, Piquette-Miller M. (2008) Effect of endotoxin on the expression of placental drug transporters and glyburide disposition in pregnant rats. Drug Metab Dispos 36:1944–1950. [DOI] [PubMed] [Google Scholar]
- Pradhan M, Bembinster LA, Baumgarten SC, Frasor J. (2010) Proinflammatory cytokines enhance estrogen-dependent expression of the multidrug transporter gene ABCG2 through estrogen receptor and NFkappaB cooperativity at adjacent response elements. J Biol Chem 285:31100–31106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premyslova M, Chisaka H, Okamura K, Challis JR. (2006) IL-1beta treatment does not co-ordinately up-regulate mPGES-1 and COX-2 mRNA expression, but results in higher degree of cellular and intracellular co-localization of their immunoreactive proteins in human placenta trophoblast cells. Placenta 27:576–586. [DOI] [PubMed] [Google Scholar]
- Roper RL, Phipps RP. (1994) Prostaglandin E2 regulation of the immune response. Adv Prostaglandin Thromboxane Leukot Res 22:101–111. [PubMed] [Google Scholar]
- Roy KR, Reddy GV, Maitreyi L, Agarwal S, Achari C, Vali S, Reddanna P. (2010) Celecoxib inhibits MDR1 expression through COX-2-dependent mechanism in human hepatocellular carcinoma (HepG2) cell line. Cancer Chemother Pharmacol 65:903–911. [DOI] [PubMed] [Google Scholar]
- Schmid A, Thierauch KH, Schleuning WD, Dinter H. (1995) Splice variants of the human EP3 receptor for prostaglandin E2. Eur J Biochem 228:23–30. [DOI] [PubMed] [Google Scholar]
- Serrano MA, Macias RI, Briz O, Monte MJ, Blazquez AG, Williamson C, Kubitz R, Marin JJ. (2007) Expression in human trophoblast and choriocarcinoma cell lines, BeWo, Jeg-3 and JAr of genes involved in the hepatobiliary-like excretory function of the placenta. Placenta 28:107–117. [DOI] [PubMed] [Google Scholar]
- Snijdewint FG, Kaliński P, Wierenga EA, Bos JD, Kapsenberg ML. (1993) Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J Immunol 150:5321–5329. [PubMed] [Google Scholar]
- Surowiak P, Pawełczyk K, Maciejczyk A, Pudełko M, Kołodziej J, Zabel M, Murawa D, Drag M, Gansukh T, Dietel M, et al. (2008) Positive correlation between cyclooxygenase 2 and the expression of ABC transporters in non-small cell lung cancer. Anticancer Res 28:2967–2974. [PubMed] [Google Scholar]
- Trebino CE, Stock JL, Gibbons CP, Naiman BM, Wachtmann TS, Umland JP, Pandher K, Lapointe JM, Saha S, Roach ML, et al. (2003) Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc Natl Acad Sci USA 100:9044–9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Scollard DA, Teng S, Reilly RM, Piquette-Miller M. (2005) Detection of P-glycoprotein activity in endotoxemic rats by 99mTc-sestamibi imaging. J Nucl Med 46:1537–1545. [PubMed] [Google Scholar]
- Yeboah D, Sun M, Kingdom J, Baczyk D, Lye SJ, Matthews SG, Gibb W. (2006) Expression of breast cancer resistance protein (BCRP/ABCG2) in human placenta throughout gestation and at term before and after labor. Can J Physiol Pharmacol 84:1251–1258. [DOI] [PubMed] [Google Scholar]
- Zatelli MC, Luchin A, Tagliati F, Leoni S, Piccin D, Bondanelli M, Rossi R, degli Uberti EC. (2007) Cyclooxygenase-2 inhibitors prevent the development of chemoresistance phenotype in a breast cancer cell line by inhibiting glycoprotein p-170 expression. Endocr Relat Cancer 14:1029–1038. [DOI] [PubMed] [Google Scholar]
- Zhou L, Naraharisetti SB, Wang H, Unadkat JD, Hebert MF, Mao Q. (2008) The breast cancer resistance protein (Bcrp1/Abcg2) limits fetal distribution of glyburide in the pregnant mouse: an Obstetric-Fetal Pharmacology Research Unit Network and University of Washington Specialized Center of Research Study. Mol Pharmacol 73:949–959. [DOI] [PubMed] [Google Scholar]
- Zrieki A, Farinotti R, Buyse M. (2008) Cyclooxygenase inhibitors down regulate P-glycoprotein in human colorectal Caco-2 cell line. Pharm Res 25:1991–2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.