Abstract
Moderate doses of stimulant drugs are known to enhance memory encoding and consolidation, but their effects on memory retrieval have not been explored in depth. In laboratory animals, stimulants seem to improve retrieval of emotional memories, but comparable studies have not been carried out in humans. In the present study, we examined the effects of dextroamphetamine (AMP) on retrieval of emotional and unemotional stimuli in healthy young adults, using doses that enhanced memory formation when administered before encoding in our previous study. During 3 sessions, healthy volunteers (n = 31) received 2 doses of AMP (10 and 20 mg) and placebo in counter-balanced order under double-blind conditions. During each session, they first viewed emotional and unemotional pictures and words in a drug-free state, and then 2 days later their memory was tested, 1 hour after AMP or placebo administration. Dextroamphetamine did not affect the number of emotional or unemotional stimuli remembered, but both doses increased recall intrusions and false recognition. Dextroamphetamine (20 mg) also increased the number of positively rated picture descriptions and words generated during free recall. These data provide the first evidence that therapeutic range doses of stimulant drugs can increase memory retrieval errors. The ability of AMP to positively bias recollection of prior events could contribute to its potential for abuse.
Keywords: memory, amphetamine, human, arousal, drug abuse
Stimulant drugs are widely used for their cognitive-enhancing properties, both in the treatment of cognitive deficits (eg, attention-deficit/hyperactivity disorder),1 as well as by healthy individuals to boost cognitive performance.2 Research in both humans and laboratory animals supports the idea that stimulant drugs can improve performance on a variety of cognitive tasks (for reviews, see Smith and Farah,3 McGaugh and Roozendaal,4 and de Jongh et al5). Notably, however, this research has focused primarily on the processing of new information (eg, attention, working memory, and associative learning), rather than retrieval of previously learned information. Thus, relatively little is known about how stimulant drugs affect memory retrieval.
A large literature indicates that moderate doses of stimulant drugs can enhance new memory formation. In humans, the prototypic stimulant drug dextroamphetamine (AMP) improves episodic memory when administered at the time of learning,6–9 and it also enhances other functions related to memory encoding processes, including attention,10 working memory,11,12 associative learning,6 and cognitive flexibility.13 Interestingly, AMP also improves episodic memory when it is administered immediately after encoding,14 indicating that it also enhances memory consolidation. In rodents as well, AMP typically enhances both learning (encoding) and retention (consolidation) of conditioned behaviors.15–20
Far fewer studies have examined the effects of stimulant drugs on memory retrieval, and the available data are mixed. In rodents, AMP enhances retrieval of both aversively motivated21–23 and appetitively motivated24 conditioned behaviors. However, in humans, AMP administered at retrieval testing did not improve memory for previously studied lists of neutral words.25,26 One reason for this apparent discrepancy may be that human studies assessed memory for lists of unemotional words, whereas the rodent studies assessed memory for emotionally arousing procedures. Indeed, in a recent study with humans, we found that AMP administered before encoding enhanced memory for emotionally arousing pictures more than for neutral pictures,27 raising the possibility that AMP may also selectively affect retrieval of emotional memories.
In addition to these memory-enhancing effects, AMP also increases arousal, and nonpharmacological manipulations that increase arousal (eg, stress) typically impair memory retrieval in both humans and nonhuman animals (for reviews, see Roozendaal and McGaugh28 and Wolf29). Moreover, in humans, experimentally increasing arousal state before retrieval testing impairs memory more strongly for previously studied emotional stimuli than unemotional stimuli.30–33 This suggests that AMP may preferentially impair emotional memory retrieval, just as it preferentially enhanced emotional memory formation (eg, similar to stress34). Thus, AMP may either enhance or impair retrieval of emotional memories.
The present study investigated the acute effects of AMP on emotional and unemotional memory retrieval in healthy human volunteers. The design was similar to our previous study in which subjects viewed emotional pictures on 1 day and their recognition memory was tested 2 days later.27 However, in the previous study, they received AMP on the study day whereas in the current study, they received AMP (10 or 20 mg) or placebo only on the retrieval day. All participants received all 3 drug conditions, in counterbalanced order under double-blind conditions. In addition to the picture recognition measure used in our previous study, this study also included more difficult memory tasks (ie, free recall and word stimuli). Finally, we also looked at confidence ratings as an indicator of the subjective strength of correct and incorrect memory responses.
MATERIALS AND METHODS
Design
In this within-subject study, healthy volunteers received placebo, and 10- and 20-mg AMP under double-blind conditions, in counterbalanced order. For each drug condition, they attended 2 laboratory visits—a 1-hour visit (encoding phase) where they viewed emotional (positively and negatively valenced) and unemotional (neutral valence) pictures and words under drug-free conditions, and a 4-hour visit (retrieval phase) 2 days later, where they ingested capsules containing placebo or AMP before completing memory tests. This study was approved by the University of Chicago Institutional Review Board.
Procedure
Subjects
Healthy volunteers aged 18 to 35 years (n = 31; 16 women) were recruited via posters, advertisements, and word-of-mouth referrals. Prospective participants underwent an in-person psychiatric interview and physician-supervised physical examination including an electrocardiogram, and they completed a health questionnaire with detailed information on current and lifetime drug use. Exclusion criteria included current Axis I Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition disorder including substance dependence (American Psychiatric Association 1994) except tobacco dependence. Volunteers were excluded if they had a history of psychosis or mania, less than a high school education, lack of fluency in English, a body mass index outside 19 to 26 kg/m2, high blood pressure (> 140/90), an abnormal electrocardiogram, reported daily use of any medication other than birth control, or were pregnant, lactating, or planning to become pregnant in the next 3 months. Women not taking hormonal contraceptives (n = 10 of 16) were tested during their follicular phase only because hormonal fluctuations of the menstrual cycle can influence responses to the drug.35
Session Protocols
Qualifying participants attended a 1-hour orientation session to become acquainted with the study procedures, provide informed consent, and practice study tasks and questionnaires. They were informed that the study was investigating the effects of drugs on mood and memory, and, to minimize drug-related expectancies, they were told that they might receive a placebo, stimulant, sedative/tranquilizer, or a marijuana-like drug. Participants were instructed to consume their normal amounts of caffeine and nicotine before sessions, but to abstain from using alcohol and over-the-counter, prescription, and illicit drugs for 24 hours before the sessions. They were informed that they would be tested for drug use before each session to verify abstinence. Participants were also advised to get their normal amounts of sleep, and not to eat solid food for 2 hours before the retrieval phase visits to allow for proper drug absorption.
Participants were tested individually in comfortably furnished rooms with a television and VCR, magazines, and a computer for administering questionnaires and tasks. They were allowed to watch television, neutral movies, or read when no measures were being obtained, but they were not allowed to sleep, work, or study, and they had no access to cell phones or Internet. Upon arrival for each visit, compliance was tested for breath alcohol level (Alco-sensor III; Intoximeters, St Louis, MO), and urine drug (ToxCup; Branan Medical Co, Irvine, CA) and pregnancy (hCG assay, Aimstrip; Craig Medical, Vista, CA).
During the encoding phase visits, participants first completed mood questionnaires and physiological measures were obtained (see “Memory Tasks”). After this, they viewed and rated a set of pictures and then a set of words (unique to each experimental session) before leaving the laboratory. During the retrieval phase visits, participants first completed precapsule mood ratings and physiological measures, and then ingested capsules (0 minute) containing placebo or active drug with 100-mL water. Physiological measures were obtained every 30 minutes for the remainder of the visit. Memory testing began 1 hour after capsules were administered (+60 minutes), and lasted roughly 2 hours. Mood and drug effect rating scales were administered multiple times across each session. The sequence of the memory measures was picture recall (+60 minutes), word recall (+75 minutes), picture recognition (+90 minutes), and word recognition (+120 minutes). Participants had breaks of 3 to 5 minutes every half hour during memory testing. At the end of the retrieval phase visits, participants completed an end-of-session questionnaire (ESQ), and were allowed to leave providing residual subjective and physiological drug effects had subsided. All participants were fully debriefed at study completion.
Memory Tasks
Materials
The picture stimuli consisted of 360 pictures drawn from the International Affective Picture System (IAPS36). The pictures were divided into 6 sets of 60, comprised of photographs depicting positive (pleasant), neutral, and negative (unpleasant) scenes (20 each category), according to normative ratings. The positive and negative pictures were matched on extremity of valence and arousal. Normed valence ratings of IAPS pictures range from 1 (very negative) to 9 (very positive), and arousal ratings range from 1 (not at all arousing) to 9 (very arousing). Positive pictures selected for this study had a mean valence of 7.05 (range, 6–8.34), and mean arousal of 5.58 (range, 4.51–7.35); neutral pictures had a mean valence of 5.07 (range, 4.03–5.99), and a mean arousal of 3.35 (range, 1.72–4.36); negative pictures had a mean valence of 2.73 (range, 1.46–3.92), and a mean arousal of 5.6 (range, 4.53–7.29).36 The word stimuli consisted of 180 personality trait words taken from Anderson.37 The words were divided into 6 sets of 30, each consisting of positive, neutral, and negative personality trait words (10 each category), and the 6 word sets were matched on word length and meaningfulness. Valence categories were assigned based on normed ratings of trait “likableness.”37 Positive trait words selected for this study had a mean likableness of 5.0 (range, 4.6–5.7), mean meaningfulness of 3.7 (range, 3.5–3.9), and an average length of 8.6 letters (range, 4–15); neutral trait words had a mean likableness of 2.7 (range, 2.2–3.5), mean meaningfulness of 3.6 (range, 3.5–3.9), and an average length of 9.0 letters (range, 3–15); negative trait words had a mean likableness of 0.9 (range, 0.3–1.5), mean meaningfulness of 3.7 (range, 3.5–3.9), and an average length of 8.6 letters (range, 4–15).37
Encoding Phase
During each encoding phase visit, participants first viewed and rated 1 picture set and then 1 word set under drug-free conditions. The stimuli were displayed 1 at a time on a computer screen for 3000 milliseconds each, and were pseudorandomized such that no more than 2 items from the same valence category were presented consecutively; for the word task, 2 words were not included in the same study set if they had synonymous meanings. Set order was counterbalanced across participants. Participants initiated the presentation of each item using the keyboard. To ensure that the stimuli would be meaningfully processed, participants were required to rate each item for perceived valence (pictures and words) and arousal (pictures) or meaningfulness (words). For the picture task, valence was defined as how positive and how negative participants felt in response to the picture, and arousal was defined as how stimulated, excited, or awake they felt in response.38 For the word task, valence was defined as how positive and how negative an attribute the trait is, and meaningfulness was defined as how much meaning the word had to them. Valence and arousal ratings were self-paced. Valence ratings were measured using the evaluative space grid,39 a 2-dimensional grid allowing independent ratings of positivity and negativity from 0 (not at all) to 4 (extreme). Arousal and meaningfulness ratings were measured using Likert scales from 1 (not at all) to 9 (very).
Retrieval Phase
At the retrieval phase visits 2 days after the encoding visit, memory for the pictures and words was assessed by both free recall and recognition. Picture recall was always assessed first, followed by word recall, picture recognition, and then word recognition. No feedback was provided during memory testing.
For the free recall measure, participants were given 15 minutes to write down brief descriptions of as many pictures as they remembered, and 10 minutes to write down as many words as they remembered from their visit 2 days earlier. They were told to provide sufficient detail for the pictures so that raters could discriminate them. Participants also rated each item according to current perceived valence (“positive,” “neutral,” or “negative”) and how confident they were that they had viewed the item at the previous encoding phase visit (“very confident,” “somewhat confident,” or “not confident”). Recalled items were scored as either correct (studied) or intrusions (not studied) by 4 independent raters and a fifth rater was used in the event of a split decision; interrater reliability was 97%. (Three percent of picture descriptions could not be reliably scored by raters as “correct/incorrect” because they were either too vague or details were incorrect. Thus, to explore the source of the AMP’s effects on picture recall accuracy, the data were also rescored using a more liberal criterion. In this liberal scoring criterion, items were scored as correct if either [1] their descriptions contained few details, but could have corresponded to a picture viewed during the respective encoding visit, or [2] minor details of studied pictures were inaccurately remembered. On average, between only 1 and 2 picture descriptions fell in to this questionable category on each session, and because adopting a more liberal scoring criterion did not substantially affect the findings obtained on this or other measures, only the results from the strict scoring criterion are presented.) Main memory outcome measures included the correct recall rate (proportion of studied items recalled), number of intrusions, and total number of attempts (correct recalls and intrusions combined).
For the recognition tests, participants viewed all of the stimuli studied during the respective encoding phase visit (targets), interspersed with an equal number of matched, nonstudied stimuli (lures). These sets were counterbalanced across conditions so that each set appeared in the studied and nonstudied conditions in an equal number of times across participants. Words with synonymous meanings were not included within the same set. Participants were instructed to identify pictures and words they had seen 2 days earlier during the encoding phase visit (yes/no), rate their confidence of each memory judgment on a Likert scale from 1 (not confident) to 9 (very confident), and to rate each item according to their current perception (ie, valence and arousal/meaningfulness). Main memory outcome measures included hit rate (proportion of studied items correctly identified as studied), false alarm rate (proportion of nonstudied items incorrectly identified as studied), and accuracy (calculated as hit rate minus false alarm rate). This method of subtracting false alarms from hits is widely used to control for changes in base rate responding.40
Subjective Mood and Drug Response Measures
Subjective mood and drug effects were assessed using the Profile of Mood States (POMS41), the Addiction Research Center Inventory (ARCI42) including marijuana scale,43 a modified version of the Drug Effects Questionnaire (DEQ44), and an ESQ. The POMS and ARCI were administered at baseline, and 60 and 150 minutes postcapsule, the DEQ was administered at 60, 90, 120, and 150 minutes postcapsule, and the ESQ was administered at 210 minutes postcapsule.
Drug Effects Questionnaire
On the DEQ, participants indicate how much they currently feel a drug effect, like the drug’s current effects, dislike the drug’s current effects, feel high, and would be interested to take the same dose of the same drug again in the future. Participants rated their responses on 100 mm sliding scales from “not at all / neutral” to “very much.”
Profile of Mood States
The POMS is a 72-item adjective checklist on which individuals report their current mood on a 5-point scale from 0 (not at all) to 4 (extremely). Eight clusters (scales) of items are separated empirically by factor analysis (Friendliness, Anxiety, Elation, Anger, Fatigue, Depression, Confusion, and Vigor). Two summary scales are derived from the other scales: Arousal = (Anxiety + Vigor) − (Fatigue + Confusion); Positive Mood = Elation − Depression.
Addiction Research Center Inventory
The ARCI is a 53-item true-false questionnaire with 6 empirically derived scales including the Amphetamine scale which measures amphetamine-related effects; the Morphine-Benzedrine Group scale which measures drug-induced euphoria; the Lysergic Acid Diethylamide scale which measures dysphoria and somatic symptoms; the Pentobarbital-Chlorpromazine Group scalewhich measures sedation; the Benzedrine Group scalewhich measures intellectual efficiency and energy; and the Marijuana scale which assesses cannabis-related effects.
End-of-Session Questionnaire
The ESQ consists of questions designed to measure participants’ perception of drug effects and performance during the visit, including (1) desire to take the drug again “yes/no,” (2) best guess of drug received (eg, stimulant, sedative, marijuana-like drug, or placebo), (3) overall drug preference (sliding scale ranging from negative 5 [strongly dislike] to 5 [strongly like]), and (4) overall motivation to perform memory tasks to the best of the individual’s ability (sliding scale ranging from 0 [not at all motivated] to 10 [extremely motivated]).
Physiologic Effects Measures
Blood pressure and heart rate were measured shortly after arrival on all visits, and every 30 minutes postcapsule during the retrieval phase visits, using a portable digital blood pressure monitor (A&D Medical/Life Source, San Jose, CA).
Drugs
Dextroamphetamine (Barr Laboratories, Pomona, NY) was placed in opaque size 00 capsules in doses of 10 or 20 mg, with dextrose filler. Placebo capsules contained only dextrose. Capsules were administered in counterbalanced order under doubleblind conditions. These doses enhance memory encoding of thesame picture stimuli used in this study,27 and also words6 using an analogous procedure.
Statistical Analyses
Memory data were analyzed using both (i) 2-way repeated-measures analysis of variance (ANOVA) with drug dose (0, 10, and 20 mg AMP) and valence as the within-subjects factors; and (ii) individual 1-way repeated-measures ANOVA to examine the drug effects on items of each valence category. Linear main effects of drug dose are reported unless otherwise specified. Where violations of sphericity were apparent in ANOVA, Greenhouse-Geisser corrected results are presented. α was set at P = 0.05 for all analyses, and trend-level main effects of drug dose (P < 0.1) were followed up by post hoc paired t tests, to examine dose dependency. The data from 3 participants for the word recognition task were lost due to a computer malfunction leaving an n = 28 for that measure. An analogous approach was used for subjective stimulus evaluation measures. Dextroamphetamine’s effects on subjective mood and drug effect ratings and physiologic state were analyzed using mean change-from-precapsule-baseline values, calculated across the memory testing period (with the exception of the DEQ, which was not administered at baseline, and the ESQ, which was administered only at the session’s end).
RESULTS
Participant Characteristics
A total of 31 healthy young adults (16 women) completed the study. Their average age was 22.6 (SD, 3.4) years, all had at least a high school education (mean [SD], 14.7 [1.7] years of education), and most were white. Most reported low to moderate recent use of alcohol (8.3 [6.5] alcoholic beverages per week) and caffeine (7.7 [4.7] caffeinated beverages per week). Six participants were daily smokers (12.8 [1.6] cigarettes/d) and 4 were occasional cigarette smokers. Several reported some other recreational drug use, but most had very little experience with prescription or illicit stimulants (see Table 1, Supplementary Digital Content 1, http://links.lww.com/JCP/A214).
Effects of AMP on Memory Retrieval by Normative Valence
Picture Recall
Placebo
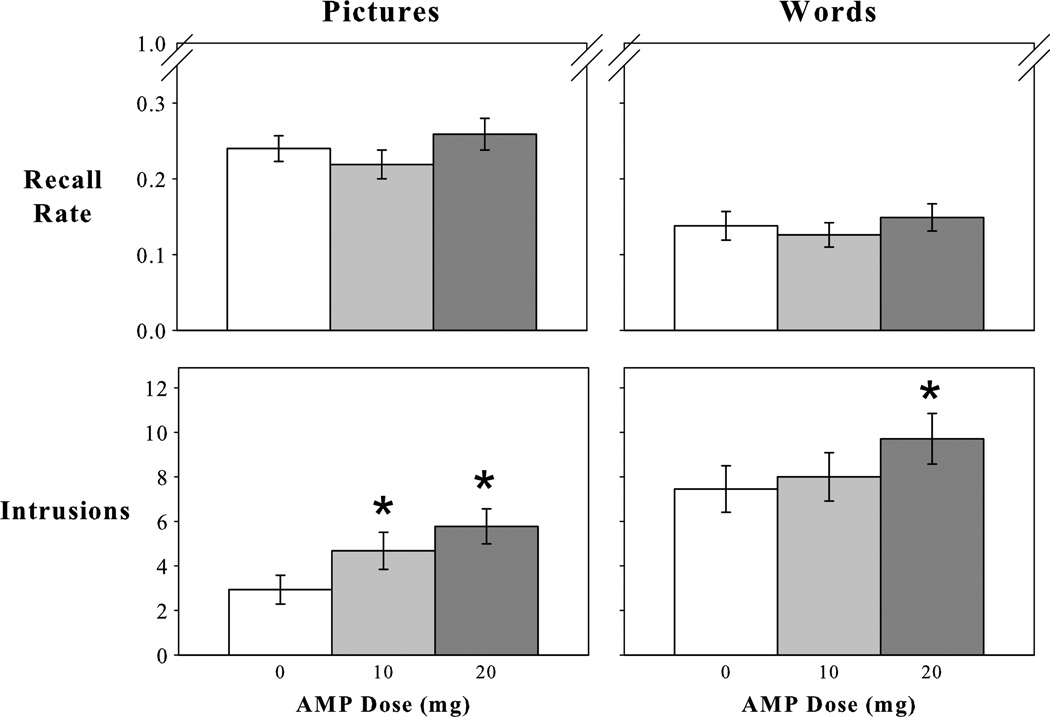
In the placebo condition, on average, participants recalled 25% of the studied pictures and generated 2.9 memory intrusions (Fig. 1). Intrusions included descriptions of pictures from an earlier testing session (39%), descriptions that were unrelated to pictures contained in the studied set (extraneous intrusions; 35%), or descriptions that were only marginally related to viewed pictures (26%). Neutral studied pictures were recalled less often, and with lower confidence, than both positive and negative studied pictures (main effect of picture valence: omnibus F2,60 = 12.3, P < 0.001, MSE = 0.01, ηρ2 = 0.29; t30 > 4, P < 0.001; Table 1). Positive and negative studied pictures were recalled with similar frequency and confidence (t30 < 1, P > 0.37).
Figure 1.
Effect of pretest amphetamine (AMP) on free recall. AMP did not affect the proportion of studied pictures or words recalled (ie, recall rate), but it increased erroneous recall of nonstudied pictures and words (ie, intrusions). Values represent means (SEM). *P < 0.05 compared to placebo.
TABLE 1.
Effects of Pretest Amphetamine (AMP) on Free Recall of Studied Pictures and Words by Normative Valence Category
| Pictures | Words | |||||
|---|---|---|---|---|---|---|
| Dose, mg | Positive | Neutral | Negative | Positive | Neutral | Negative |
| 0 | 0.28 (0.02) | 0.17 (0.02) | 0.29 (0.02) | 0.18 (0.03) | 0.13 (0.03) | 0.1 (0.02) |
| 10 | 0.24 (0.03) | 0.15 (0.02) | 0.28 (0.02) | 0.17 (0.03) | 0.11 (0.02) | 0.12 (0.02) |
| 20 | 0.28 (0.02) | 0.16 (0.02) | 0.28 (0.03) | 0.23 (0.03) | 0.08 (0.02) | 0.14 (0.02) |
Values are mean (SEM) raw recall rates (ie, proportion of studied items recalled at test).
Dextroamphetamine
Dextroamphetamine did not affect the total number of studied pictures recalled, or participants’ confidence in their correct recall, regardless of normative picture valence (P > 0.05). In sharp contrast, both doses of AMP robustly increased memory intrusions (F1,30 = 17.1, P < 0.001, MSE = 5.60, ηρ2 = 0.36). This effect was obtained for both high confidence intrusions (F1,30 = 5, P = 0.034, MSE = 1.57, ηρ2 = 0.14; significant at 20 mg AMP only) and moderate confidence intrusions (F1,30 = 4.6, P = 0.041, MSE = 3.62, ηρ2 = 0.13; significant at both doses), and there was a trend for low confidence intrusions (F1,30 = 3.6, P = 0.067, MSE = 2.37, ηρ2 = 0.11). Dextroamphetamine did not alter the proportion of intrusion types.
Picture Recognition
Placebo
In the placebo condition, participants discriminated studied from nonstudied pictures with very high accuracy (target hit rate, 96%; lure false alarm rate, 4%), and accuracy was similar for all valences (P > 0.06; Table 2). Participants were more confident in their correct recognition of negative studied pictures (mean, 8.6) than positive (mean, 8.4) or neutral (mean, 8.3) pictures (main effect of valence: F2,60 = 4.6, P = 0.013, MSE = 0.12, ηρ2 = 0.13; t30 ≥ 2.5, P ≤ 0.02), but confidence ratings did not differ between positive and neutral hits (P = 0.45). Given the low false alarm rate, false alarm confidence data are not reported.
TABLE 2.
Effects of Pretest Amphetamine (AMP) on Recognition of Studied Pictures and Words by Normative Valence Category
| Recognition Measure |
Dose, mg | Pictures | Words | ||||
|---|---|---|---|---|---|---|---|
| Positive | Neutral | Negative | Positive | Neutral | Negative | ||
| Hit rate | 0 | 0.96 (0.01) | 0.96 (0.01) | 0.96 (0.01) | 0.9 (0.02) | 0.83 (0.03) | 0.87 (0.02) |
| 10 | 0.95 (0.01) | 0.96 (0.02) | 0.97 (0.01) | 0.89 (0.02) | 0.85 (0.02) | 0.84 (0.02) | |
| 20 | 0.96 (0.01) | 0.94 (0.01) | 0.97 (0.01) | 0.88 (0.02) | 0.83 (0.03) | 0.84 (0.03) | |
| False alarm rate | 0 | 0.05 (0.01) | 0.03 (0.01) | 0.03 (0.01) | 0.47 (0.04) | 0.24 (0.03) | 0.28 (0.03) |
| 10 | 0.05 (0.01) | 0.04 (0.01) | 0.03 (0.01) | 0.5 (0.05) | 0.29 (0.04) | 0.35 (0.04) | |
| 20 | 0.04 (0.01) | 0.05 (0.01) | 0.03 (0.01) | 0.53 (0.03) | 0.29 (0.03) | 0.39 (0.04) | |
Values are mean (SEM) raw hit rates (ie, proportion of studied items correctly recognized as studied at test) and false alarm rates (ie, proportion of nonstudied items incorrectly recognized as studied at test).
Dextroamphetamine
Dextroamphetamine did not alter picture recognition accuracy, or hit or false alarm rates, overall, or for items of any valence category in particular (P > 0.06).
Word Recall
Placebo
In the placebo condition, on average, participants recalled 14% of the studied words and generated 7.7 memory intrusions (Fig. 1). Although the main effect of word valence on correct recall missed significance (omnibus P = 0.06), positive studied words were recalled more often than negative studied words (t30 = 2.7, P = 0.012; Table 1). Studied words from all normative valence categories were recalled with equivalent confidence (P > 0.09).
Dextroamphetamine
Dextroamphetamine did not affect the total number of studied words recalled, or participants’ confidence in their correct recall, regardless of normative word valence (P > 0.05). Importantly, as was the case with picture recall, AMP increased memory intrusions with word recall (F1,30 = 9.2, P = 0.005, MSE = 14.15, ηρ2 = 0.24). This effect was evident at all confidence levels, but only reached significance for the lowest confidence bin (F1,30 = 7.2, P = 0.012, MSE = 3.61, ηρ2 = 0.19; significant at 20 mg AMP only).
Word Recognition
Placebo
In the placebo condition, studied and nonstudied words were discriminated with high accuracy (target hit rate, 86%; lure false alarm rate, 33%), and accuracy was lower for positive words than both negative and neutral words (F2,54 = 9.1, P < 0.001, MSE = 0.03, ηρ2 = 0.25; t27 > 3.6, P < 0.001; negative vs neutral: P = 0.88). This accuracy effect was primarily driven by enhanced false recognition of positive lures, which were incorrectly endorsed as studied nearly twice as often as either neutral or negative lures (F2,54 = 22.3, P < 0.001, MSE = 0.02, ηρ2 = 0.45; both t27 > 5.2 and P < 0.001; negative vs neutral: P = 0.34; Table 2). The main effect of word valence on hit rate missed significance (P = 0.08), but pairwise comparisons indicated that hit rate was higher for positive than neutral studied words (t27 = 2.6, P = 0.014; other P > 0.27). Hit and false alarm confidence ratings did not vary substantially depending on word valence (P > 0.11).
Dextroamphetamine
Dextroamphetamine did not affect recognition of studied words overall (P = 0.38; hit rates, 86% and 85% for the 10 and 20 mg AMP conditions, respectively), or those of any valence in particular (P > 0.06). However, both doses increased false recognition of nonstudied words (lure false alarm rates: 10 mg, 38%; 20 mg, 40%; F1,27 = 6.7, P = 0.015,MSE = 0.01, ηρ2 = 0.2; both t27 > 2.3 and P < 0.03, compared to placebo), resulting in a significant reduction in discrimination accuracy (F1,27 = 8.8, P = 0.006, MSE = 0.01, ηρ2 = 0.25; both t27 > 2.2 and P ≤ 0.03, compared to placebo). There were no Dose × Valence interactions at any memory measure (P > 0.31), and AMP had no effects on confidence ratings (P > 0.08).
Effects of AMP on Recall by Subjective Valence
We also explored the possible effect of AMP on emotional bias in recall using measures of subjective, rather than normative, valence. We examined the number of items generated during free recall that subjects had rated as positive, neutral, or negative, regardless of whether their recalls were correct or incorrect (Fig. 2).
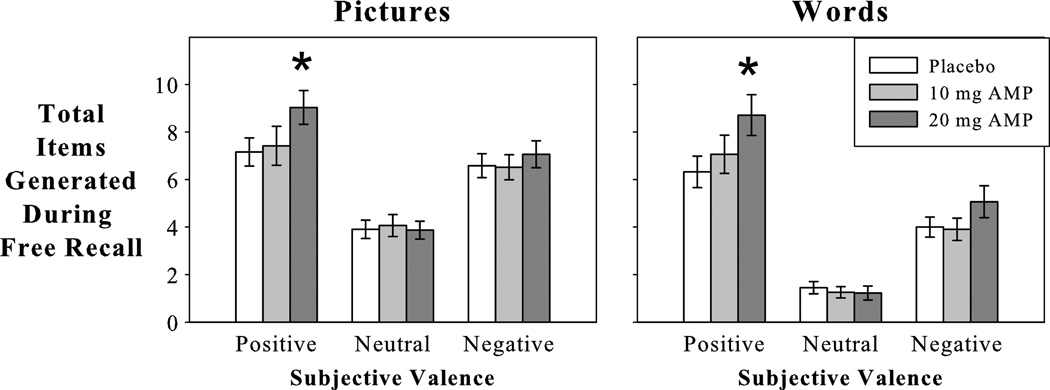
Figure 2.
Effect of pretest amphetamine (AMP) on total recall attempts (studied items and intrusions combined) according to subjective valence ratings. AMP increased the number of positively rated picture descriptions and words. Values represent means (SEM). *P < 0.05 compared to placebo.
Placebo
In the placebo condition, participants generated significantly more “positive” and “negative” than “neutral” picture descriptions (omnibus F2,60 = 16.2, P < 0.001, MSE = 5.77, ηρ2 = 0.35; both t30 > 4.5 and P < 0.001; “positive” vs “negative” P = 0.22). Likewise during word free recall, participants generated significantly more “positive” than “negative” words (omnibus F2,60 = 29.5, P < 0.001, MSE = 6.24, ηρ2 = 0.5; t30 = 3.8, P = 0.001), and “neutral” words were generated less frequently than either “positive” or “negative” words (t30 > 4.7, P < 0.001).
Dextroamphetamine
Dextroamphetamine primarily increased the number of “positive” picture descriptions and trait words generated during free recall. After AMP, participants generated significantly more “positive” picture descriptions (Dose × Valence: F1,30 = 4.4, P = 0.044, MSE = 3.44, ηρ2 = 0.13; positive valence dose: F1,30 = 7.4, P = 0.011, MSE = 7.36, ηρ2 = 0.2; 10 mg: P = 0.71; 20 mg: t30 = 2.7, P = 0.011, compared to placebo) and words (Dose × Valence: F1,30 = 11.6, P = 0.002, MSE = 3.39, ηρ2 = 0.28; positive valence dose: F1,30 = 8.4, P = 0.007, MSE = 10.52, ηρ2 = 0.22; 10 mg: P = 0.37; 20 mg: t30 = 2.9, P = 0.007, compared to placebo). The number of picture descriptions and words rated as either “neutral” or “negative” did not vary with increasing doses of AMP (P > 0.08).
Subjective Evaluation of Stimulus Content During Recognition Testing
Placebo
In the placebo condition, participants’ subjective valence ratings of the picture and word stimuli were highly consistent with normative data. Also as expected, participants rated both normatively positive (mean, 4) and negative (mean, 4.6) pictures as being significantly more arousing than normatively neutral pictures (mean, 2.2; F1.5,44 = 71.7, P < 0.001, MSE = 0.97, ηρ2 = 0.71; both t30 > 9.2 and P < 0.001), although negative pictures were rated as significantly more arousing than positive pictures (t30 = 2.9, P = 0.007). Negative trait words were rated by participants as more “meaningful” (mean, 5.4) than neutral trait words (mean, 5) in the placebo condition (t27 = 2.2, P = 0.035), although meaningfulness ratings did not differ between positive (mean, 5.1) and either neutral or negative trait words (P > 0.32), and the main effect of valence was not significant (P = 0.25).
Dextroamphetamine
Participants rated positive trait words as more “positive” (Dose × Valence: F1,27 = 4.2, P = 0.049, MSE = 0.04, ηρ2 = 0.14; significant at 20 mg for positive words only) and “meaningful” (Dose × Valence: F1,27 = 6.1, P = 0.02, MSE = 0.17, ηρ2 = 0.18; significant at both doses for positive words only) in the AMP conditions relative to placebo during the recognition test. Other than this general effect on positive items, AMP had minimal effects on participants’ subjective ratings of the picture and word stimuli during recognition testing overall.
Effects of AMP on Subjective Mood, Drug Response, and Physiologic Measures
Dextroamphetamine produced the expected subjective and physiologic effects consistent with its profile as a stimulant drug of abuse (see Table 2, Supplementary Digital Content 2, http://links.lww.com/JCP/A215). Participants preferred the effects of AMP over placebo, especially the higher dose, and both doses of AMP increased measures of positive mood. Both doses also increased blood pressure and heart rate, and the higher dose increased subjective arousal. Dextroamphetamine (20 mg) increased the motivation to complete the memory tests. Dextroamphetamine (10 mg) was identified as a stimulant drug 23% of sessions, and AMP (20 mg) was correctly identified on 55% of sessions.
DISCUSSION
We found that AMP impaired memory retrieval in healthy young adults at the same doses that enhanced memory formation in previous studies.6,27 Dextroamphetamine did not alter recall or recognition of previously studied items, but it markedly increased the number of recall intrusions and falsely recognized test items. This pattern was observed for both picture and word stimuli, and did not depend on stimulus emotionality. Moreover, the increase in false recognition was accompanied by an increase in subjects’ confidence in their ratings, particularly for the pictures, suggesting that the drug produced an illusion of recollection rather than a simple bias in endorsing nonstudied items (cf. Roediger and McDermott45).
To our knowledge, this is the first evidence that moderate doses of a stimulant drug increase memory retrieval errors. Two earlier studies in humans found similar doses of AMP (ie, 0.2 mg/kg in healthy young adults,26 and 0.5 mg/kg in hyperactive children25) at retrieval did not affect recall of previously studied neutral words. However, those studies did not report memory intrusions. We found that AMP can impair retrieval accuracy by increasing intrusions. This finding that AMP increases memory errors seems to contrast with rodent studies indicating AMP enhances retrieval of previously learned conditioned motivated behaviors.22,24 This discrepancy could be due to a species difference, or it could be related to differences in dose, route of administration, or context. Differences in the experimental procedures could also have been an important factor as the rodent studies did not include a measure of memory intrusions, and further used behaviors maintained by food reward24 or shock,22 which are probably much more salient than the emotional images and words used in our study. Our finding that AMP can impair cognition at moderate doses has clear clinical relevance as AMP is commonly prescribed for attentiondeficit/ hyperactivity disorder, to combat fatigue and in patients with narcolepsy.
These retrieval-impairing effects of AMP are broadly consistent with the effects of other manipulations that heighten arousal state. As noted previously, there are reports of stress impairing memory retrieval in both humans and rodents (for a review, see Wolf29); however, this literature primarily concerns studies in which stress was administered before encoding rather than at the time of retrieval (see Smeets et al46). In a recent study, Diekelmann et al47 found that cortisol administered at the time of retrieval reduced false recall of words that were related to studied information, but correct recall was also reduced, suggesting that the cortisol impaired memory in general. Thus, although both cortisol and stimulant drugs seem to have impairing effects on memory retrieval, they may have differential effects on retrieval of studied information,46 and false recollection.
Interestingly, AMP produced a positivity bias in participants’ recall. That is, during free recall, subjects generated more subjectively positive picture descriptions and personality trait words. This is consistent with the positive mood-altering effects of AMP, and fits with the theory that emotional memory can be mood-congruent—that is, that people remember information better if its valence matches their affective mood state at the time of retrieval (for reviews, see Eich48 and Bower and Forgas49). Drug users sometimes report that they use drugs to divert their focus from negative life experiences.50,51 Thus, drugs may induce positive biases in memory that could then increase the likelihood of using the drug in the future.
In summary, the present study yielded 2 important findings. First, AMP increased memory errors in healthy young adults, mainly by increasing the rate of intrusions. This is clinically important information for people who use stimulant drugs to enhance cognition, or in preparation for tests. This effect might be especially pronounced if the drug is combined with a stressful setting. It remains to be determined whether similar impairments are detected with other, related drugs, or in patient populations. Second, AMP tended to positively bias memory recall, an effect that may contribute toAMP’s abuse potential. Taken together, these findings highlight the importance of characterizing the effects of stimulants and other drugs on memory function.
Supplementary Material
Acknowledgments
This research was supported by NIDA DA02812 and DA031796, and Dr Ballard was supported by T32 DA007255 and F31 DA030863.
During the past 3 years, Dr de Wit has received funding from Unilever for an unrelated research study.
Footnotes
Supplemental digital contents are available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.psychopharmacology.com).
AUTHOR DISCLOSURE INFORMATION
The authors have no other disclosures or conflicts of interest to report.
REFERENCES
- 1.Kratochvil CJ. ADHD pharmacotherapy: rates of stimulant use and cardiovascular risk. Am J Psychiatry. 2012;169:112–114. doi: 10.1176/appi.ajp.2011.11111703. [DOI] [PubMed] [Google Scholar]
- 2.Franke AG, Lieb K, Hildt E. What users think about the differences between caffeine and illicit/prescription stimulants for cognitive enhancement. PLoS One. 2012;7:e40047. doi: 10.1371/journal.pone.0040047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith ME, Farah MJ. Are prescription stimulants “smart pills”? The epidemiology and cognitive neuroscience of prescription stimulant use by normal healthy individuals. Psychol Bull. 2011;137:717–741. doi: 10.1037/a0023825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGaugh JL, Roozendaal B. Drug enhancement of memory consolidation: historical perspective and neurobiological implications. Psychopharmacology (Berl) 2009;202:3–14. doi: 10.1007/s00213-008-1285-6. [DOI] [PubMed] [Google Scholar]
- 5.de Jongh R, Bolt I, Schermer M, et al. Botox for the brain: enhancement of cognition, mood and pro-social behavior and blunting of unwanted memories. Neurosci Biobehav Rev. 2008;32:760–776. doi: 10.1016/j.neubiorev.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Ballard ME, Gallo DA, de Wit H. Psychoactive drugs and false memory: comparison of dextroamphetamine and delta-9-tetrahydrocannabinol on false recognition. Psychopharmacology (Berl) 2012;219:15–24. doi: 10.1007/s00213-011-2374-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mintzer MZ, Griffiths RR. Triazolam-amphetamine interaction: dissociation of effects on memory versus arousal. J Psychopharmacol. 2003;17:17–29. doi: 10.1177/0269881103017001689. [DOI] [PubMed] [Google Scholar]
- 8.Zeeuws I, Soetens E. Verbal memory performance improved via an acute administration of d-amphetamine. Hum Psychopharmacol. 2007;22:279–287. doi: 10.1002/hup.848. [DOI] [PubMed] [Google Scholar]
- 9.Breitenstein C, Wailke S, Bushuven S, et al. d-amphetamine boosts language learning independent of its cardiovascular and motor arousing effects. Neuropsychopharmacology. 2004;29:1704–1714. doi: 10.1038/sj.npp.1300464. [DOI] [PubMed] [Google Scholar]
- 10.Hamidovic A, Dlugos A, Palmer AA, et al. Catechol-O-methyltransferase val158met genotype modulates sustained attention in both the drug-free state and in response to amphetamine. Psychiatr Genet. 2010;20:85–92. doi: 10.1097/YPG.0b013e32833a1f3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tipper CM, Cairo TA, Woodward TS, et al. Processing efficiency of a verbal working memory system is modulated by amphetamine: an fMRI investigation. Psychopharmacology (Berl) 2005;180:634–643. doi: 10.1007/s00213-005-0025-4. [DOI] [PubMed] [Google Scholar]
- 12.Mattay VS, Callicott JH, Bertolino A, et al. Effects of dextroamphetamine on cognitive performance and cortical activation. Neuroimage. 2000;12:268–275. doi: 10.1006/nimg.2000.0610. [DOI] [PubMed] [Google Scholar]
- 13.Kempton S, Vance A, Maruff P, et al. Executive function and attention deficit hyperactivity disorder: stimulant medication and better executive function performance in children. Psychol Med. 1999;29:527–538. doi: 10.1017/s0033291799008338. [DOI] [PubMed] [Google Scholar]
- 14.Soetens E, D’Hooge R, Hueting JE. Amphetamine enhances human-memory consolidation. Neurosci Lett. 1993;161:9–12. doi: 10.1016/0304-3940(93)90127-7. [DOI] [PubMed] [Google Scholar]
- 15.Janak PH, Martinez JL., Jr Cocaine and amphetamine facilitate retention of jump-up responding in rats. Pharmacol Biochem Behav. 1992;41:837–840. doi: 10.1016/0091-3057(92)90235-8. [DOI] [PubMed] [Google Scholar]
- 16.Lee EH, Ma YL. Amphetamine enhances memory retention and facilitates norepinephrine release from the hippocampus in rats. Brain Res Bull. 1995;37:411–416. doi: 10.1016/0361-9230(95)00039-9. [DOI] [PubMed] [Google Scholar]
- 17.Martinez JL, Jr, Jensen RA, Messing RB, et al. Central and peripheral actions of amphetamine on memory storage. Brain Res. 1980;182:157–166. doi: 10.1016/0006-8993(80)90838-0. [DOI] [PubMed] [Google Scholar]
- 18.Wood SC, Anagnostaras SG. Memory and psychostimulants: modulation of Pavlovian fear conditioning by amphetamine in C57BL/6 mice. Psychopharmacology (Berl) 2009;202:197–206. doi: 10.1007/s00213-008-1185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies JA, Jackson B, Redfern PH. The effect of amantadine, l-dopa, (plus)-amphetamine and apomorphine on the acquisition of the conditioned avoidance response. Neuropharmacology. 1974;13:199–204. doi: 10.1016/0028-3908(74)90107-5. [DOI] [PubMed] [Google Scholar]
- 20.Fulginiti S, Cancela LM. Effect of naloxone and amphetamine on acquisition and memory consolidation of active avoidance responses in rats. Psychopharmacology (Berl) 1983;79:45–48. doi: 10.1007/BF00433015. [DOI] [PubMed] [Google Scholar]
- 21.Quartermain D, Altman HJ. Facilitation of retrieval by d-amphetamine following anisomycin-induced amnesia. Physiol Psychol. 1982;110:283–292. [Google Scholar]
- 22.Quartermain D, Judge ME, Jung H. Amphetamine enhances retrieval following diverse sources of forgetting. Physiol Behav. 1988;43:239–241. doi: 10.1016/0031-9384(88)90245-4. [DOI] [PubMed] [Google Scholar]
- 23.Quartermain D, Jung H. Persistence of retrieval enhancement by amphetamine following scopolamine-induced amnesia. Pharmacol Biochem Behav. 1989;33:51–54. doi: 10.1016/0091-3057(89)90428-0. [DOI] [PubMed] [Google Scholar]
- 24.Sara SJ, Deweer B. Memory retrieval enhanced by amphetamine after a long retention interval. Behav Neural Biol. 1982;36:146–160. doi: 10.1016/s0163-1047(82)90145-5. [DOI] [PubMed] [Google Scholar]
- 25.Hurst PM, Radlow R, Chubb NC, et al. Effects of d-amphetamine on acquisition, persistence, and recall. Am J Psychol. 1969;82:307–319. [PubMed] [Google Scholar]
- 26.Weingartner H, Langer D, Grice J, et al. Acquisition and retrieval of information in amphetamine-treated hyperactive children. Psychiatry Res. 1982;6:21–29. doi: 10.1016/0165-1781(82)90034-8. [DOI] [PubMed] [Google Scholar]
- 27.Ballard ME, Gallo DA, de Wit H. Pre-encoding administration of amphetamine or THC preferentially modulates emotional memory in humans. Psychopharmacology (Berl) 2013;226:515–529. doi: 10.1007/s00213-012-2924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roozendaal B, McGaugh JL. Memory modulation. Behav Neurosci. 2011;125:797–824. doi: 10.1037/a0026187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf OT. Stress and memory in humans: twelve years of progress? Brain Res. 2009;1293:142–154. doi: 10.1016/j.brainres.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Kuhlmann S, Piel M, Wolf OT. Impaired memory retrieval after psychosocial stress in healthy young men. J Neurosci. 2005;25:2977–2982. doi: 10.1523/JNEUROSCI.5139-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao LY, Shi J, Zhang XL, et al. Psychosocial stress enhances non-drug-related positive memory retrieval in male abstinent heroin addicts. Neurosci Lett. 2010;485:16–20. doi: 10.1016/j.neulet.2010.08.046. [DOI] [PubMed] [Google Scholar]
- 32.Smeets T. Acute stress impairs memory retrieval independent of time of day. Psychoneuroendocrinology. 2011;36:495–501. doi: 10.1016/j.psyneuen.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Tollenaar MS, Elzinga BM, Spinhoven P, et al. The effects of cortisol increase on long-term memory retrieval during and after acute psychosocial stress. Acta Psychol (Amst) 2008;127:542–552. doi: 10.1016/j.actpsy.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Payne JD, Jackson ED, Hoscheidt S, et al. Stress administered prior to encoding impairs neutral but enhances emotional long-term episodic memories. Learn Mem. 2007;14:861–868. doi: 10.1101/lm.743507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White TL, Justice AJ, de Wit H. Differential subjective effects of d-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002;73:729–741. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- 36.Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. Gainesville, FL: NIMH Center for the Study of Emotion and Attention, University of Florida; 1999. [Google Scholar]
- 37.Anderson NH. Likableness ratings of 555 personality-trait words. J Pers Soc Psychol. 1968;9:272–279. doi: 10.1037/h0025907. [DOI] [PubMed] [Google Scholar]
- 38.Lang PJ, Greenwald MK, Bradley MM, et al. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- 39.Larsen JT, Norris CJ, McGraw AP, et al. The evaluative space grid: a single-item measure of positivity and negativity. Cogn Emot. 2009;23:28. [Google Scholar]
- 40.Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- 41.McNair DM, Lorr M, Droppleman LF. A Manual for the Profile of Mood States. San Diego, CA: EaIT; 1971. [Google Scholar]
- 42.Martin WR, Sloan JW, Sapira JD, et al. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- 43.Chait LD, Fischman MW, Schuster CR. ‘Hangover’ effects the morning after marijuana smoking. Drug Alcohol Depend. 1985;15:229–238. doi: 10.1016/0376-8716(85)90002-x. [DOI] [PubMed] [Google Scholar]
- 44.Fischman MW, Foltin RW. Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. Br J Addict. 1991;86:1563–1570. doi: 10.1111/j.1360-0443.1991.tb01749.x. [DOI] [PubMed] [Google Scholar]
- 45.Roediger HL, III, McDermott KB. Creating false memories: remembering words not presented in lists. J Exp Psychol Learn Mem Cogn. 1995;21:803–814. [Google Scholar]
- 46.Smeets T, Otgaar H, Candel I, et al. True or false? Memory is differentially affected by stress-induced cortisol elevations and sympathetic activity at consolidation and retrieval. Psychoneuroendocrinology. 2008;33:1378–1386. doi: 10.1016/j.psyneuen.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 47.Diekelmann S, Wilhelm I, Wagner U, et al. Elevated cortisol at retrieval suppresses false memories in parallel with correct memories. J Cogn Neurosci. 2011;23:772–781. doi: 10.1162/jocn.2010.21493. [DOI] [PubMed] [Google Scholar]
- 48.Eich E. Mood-dependent Memory. International Encyclopedia of the Social & Behavioral Sciences. Oxford: Pergamon; 2001. pp. 10014–10017. [Google Scholar]
- 49.Bower GH, Forgas JP. Affect, memory, and social cognition. In: Eich E, Kihlstrom JF, Bower GH, et al., editors. Cognition and Emotion. New York, NY: Oxford University Press; 2000. pp. 87–168. [Google Scholar]
- 50.Jurich AP, Polson CJ, Jurich JA, et al. Family factors in the lives of drug users and abusers. Adolescence. 1985;20:143–159. [PubMed] [Google Scholar]
- 51.Pandina RJ, Johnson VL. Why people, use, abuse, and become dependent on drugs: progress toward a heuristic model. In: Glantz MD, Hartel CR, editors. Drug Abuse: Origins & Interventions. Washington, DC: American Psychological Association; 1999. pp. 119–147. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.