Abstract
Proper microtubule nucleation during cell division requires augmin, a microtubule-associated hetero-octameric protein complex. In current models, augmin recruits γ-tubulin, via its hDgt6 subunit’s C-terminus, to nucleate microtubules within spindles. However, augmin’s biochemical complexity has restricted analysis of its structural organization and function. Here, we reconstitute human augmin and show it is a Y-shaped complex that can adopt multiple conformations. Further, we find that a dimeric sub-complex retains in vitro microtubule-binding properties of octameric complexes, but not proper metaphase spindle localization. Addition of octameric augmin complexes to Xenopus egg extracts promotes microtubule aster formation, an activity enhanced by Ran-GTP. This activity requires microtubule binding, but not the characterized hDgt6 γ-tubulin-recruitment domain. Tetrameric sub-complexes induce asters, but activity and microtubule bundling within asters are reduced compared to octameric complexes. Together, our findings shed light on augmin’s structural organization, microtubule binding properties and define subunits required for its function in organizing microtubule-based structures.
INTRODUCTION
Error-free cell division depends on the regulated nucleation of microtubules, polar polymers of α/β-tubulin1–3. There have been important advances in our understanding of how microtubule nucleation at centrosomes is mediated by the recruitment of γ-tubulin and associated proteins (called the γ-tubulin ring complex, or γ-TuRC)4–6. It has also been established that centrosomes are not required for the assembly of meiotic and mitotic spindles7–9. As a result, much attention has been focused on examining two centrosome-independent microtubule formation pathways. The first is the chromosome-dependent microtubule formation pathway involving Ran GTPase and the Aurora B kinase complex10,11. The second pathway involves augmin, a recently discovered eight protein complex needed to recruit γ-tubulin to microtubules within the spindle12–14.
Several lines of evidence indicate that augmin is needed for proper centrosome-independent microtubule formation in dividing cells. First, the levels of spindle microtubules are reduced when augmin is knocked-down or mutant subunits are present12,13,15. Second, studies in Xenopus egg extracts depleted of augmin reveal that centrosome-independent meiotic spindle assembly around chromatin-coated beads occurs at reduced rates16. Third, microtubule nucleation along the sides of other microtubules has been directly imaged and requires augmin17. Fourth, electron tomography-based analysis reveals that minus-ends of microtubules distributed within the metaphase spindle are reduced in the absence of augmin. Further, these studies suggest that a rod-shaped (29±14 nm) structure, which could be augmin, crosslinks the minus-end of newly formed microtubules to the lattice of pre-existing filaments18. Together, these data have led to a model in which augmin binds the sides of microtubules, recruits γ-tubulin, and promotes the nucleation of a new filament13. A recent study in Drosophila embryos indicates that augmin also contributes to centrosome-dependent astral microtubule assembly19.
In metazoans augmin is comprised of eight subunits: Ccdc5 (HAUS1), Cep27 (HAUS2), hDgt3 (HAUS3), C14orf94 (HAUS4), hDgt5 (HAUS5), hDgt6 (HAUS6), UCHL5IP (HAUS7), and Hice1 (HAUS8)12–14(Fig. 1a). Recombinant Hice1 has been shown to bind microtubules in vitro20. It has been proposed that hDgt6 recruits γ-TuRC to spindle microtubules as overexpression of a truncated construct leads to reduction in γ-tubulin signal in mitotic spindles13. Furthermore, immuno-precipitation experiments suggest interactions between hDgt6 and NEDD1, a component of γ-TuRC13. However, the functions of these different subunits and the overall organization of the augmin complex remain poorly understood.
Figure 1.
Hice1·hDgt6Δ (433–955) are components of distinct augmin sub-complexes. (a) Schematics for the augmin’s 8 subunits: Hice1 (HAUS8), hDgt6 (HAUS6), UCHL5IP (HAUS7), Cep27 (HAUS2), C14orf94 (HAUS4), Ccdc5 (HAUS1), hDgt3 (HAUS3) and hDgt5 (HAUS5). Microtubule binding region (MTBR) (a.a. 1–141) of Hice1 and N-terminal domain (a.a. 1–432) of hDgt6 are highlighted (black). (b) Size exclusion chromatography (Superdex 75) elution profile for Hice1-MTBR. (c) Microtubule co-sedimentation assays to analyze Hice1-MTBR binding. Microtubule binding constants were determined by fitting to a hyperbola (Kd: 9.33+1.44 μM, N = 3 independent experiments; Error bars show S.D.). SDS-PAGE gel image, stained with Coomassie blue, is shown. BSA (final 0.25 mg/ml), used to suppress non-specific interactions, and tubulin are indicated. (d) Circular dichroism spectrum of Hice1-MTBR (black) and PRC1-MTBD (gray, dashed) (25°C) are shown. (e–h) Size exclusion chromatography (Superose 6) elution profiles for Hice1·hDgt6Δ (433–955) dimer (e), tetramer-I (Hice1·hDgt6 (1–432)·UCHL5IP·Cep27) (f), tetramer-II (Hice1·hDgt6 (1–432)·His-C14orf94·Ccdc5) (g), and hexamer (Hice1·hDgt6 (1–432)·His-UCHL5IP·Cep27·His-C14orf94·Ccdc5) (h). For all chromatography analyses peak fractions (volumes indicted) were analyzed by SDS-PAGE (stained with Coomassie blue). Void volume (Vo) is also indicated and absorbance (A.U.) at 280 nm is shown. SDS-PAGE resolved Hice1 to be slightly larger than hDgt6 (1–432), even though the calculated molecular weight of Hice1 is lower than that for hDgt6 (1–432). The tagged-C14orf94 and -UCHL5IP subunits in the hexamer could not be completely resolved by SDS-PAGE.
Here, we report the biochemical reconstitution of the augmin complex with recombinant proteins. Analyses of sub-complexes and electron microscopy reveal the subunit organization and overall architecture of this hetero-octameric complex. Comparisons between direct microtubule binding and metaphase spindle localization of the augmin complexes reveal how different subunits contribute to these properties. We also analyze the activity of the recombinant augmin holo-complex and sub-complexes using a microtubule aster assembly assay. Together, these studies shed light on how centrosome-independent microtubule formation can be mediated by augmin.
RESULTS
Hice1·hDgt6 are core components of distinct augmin sub-complexes
To biochemically characterize and reconstitute this multi-protein complex we first tried co-expressing all eight subunits in bacteria using polycistronic systems21. As these attempts were unsuccessful, we focused on characterizing individual subunits and generating sub-complexes. We first examined Hice1, the subunit that has been shown in vitro to bind microtubules via a region at its N-terminus (hereafter, Hice1-MTBR, for Hice1-microtubule-binding region; a.a. 1–141)20. We expressed and purified Hice1-MTBR and find it is monodisperse by size-exclusion chromatography (Fig. 1b). Co-sedimentation assays revealed that Hice1-MTBR binds microtubules (Kd: ~9 μM, Fig. 1c). Circular dichroism (CD) spectrometry indicated that the Hice1-MTBR is mostly a random coil in solution (Fig. 1d). As a control, we examined the microtubule-binding domain of PRC1 under similar conditions and found it was mainly helical in solution (Fig. 1d), consistent with structural data22. These data suggest that the Hice1 microtubule-binding region is unlikely to have well-defined secondary structure in solution.
We were unable to purify full-length recombinant Hice1 and therefore examined co-expression with hDgt6 N-terminal domain (a.a. 1–432) (hereafter, Hice1·hDgt6Δ (433–955), as an interaction between these has been shown by yeast two-hybrid results13. We found a soluble and stable hetero-dimer formed by Hice1 and hDgt6Δ (433–955) (Fig. 1e). Light scattering analysis indicated that these proteins form a hetero-dimeric complex with molecular weight consistent with the calculated mass (91 kDa, Supplementary Fig. 1a,d).
We next examined if other augmin subunits could be co-purified with the Hice1·hDgt6Δ (433–955) hetero-dimer. After trying different combinations, we obtained two different stable tetrameric complexes. One tetrameric complex (hereafter, tetramer-I) was comprised of Hice1, hDgt6 (1–432), UCHL5IP, and Cep27 (Fig. 1f). The other tetramer (hereafter, tetramer-II) was comprised of Hice1, hDgt6 (1–432), C14orf94 and Ccdc5 (Fig. 1g). Light scattering analysis indicated that these complexes adopt an extended conformation, and the molecular weights of tetramer-I and -II were 149 and 159 kDa, respectively (Supplementary Fig. 1b–d), consistent with the subunits associating with equal stoichiometry.
Next, to test whether the proteins represented in tetramer-I and -II assemble into a larger complex, we co-expressed all six proteins in bacteria. We were able to purify a hetero-hexameric complex, with either His-tags on both C14orf94 and UCHL5IP (Fig. 1h), or with only His-tagged C14orf94 (Supplementary Fig. 1e). Tagged C14orf94 and UCHL5IP could not be resolved by SDS-PAGE, and their presence was independently confirmed (Supplementary Fig. 1f). While we were unable to reconstitute octameric augmin complexes in bacteria, these analyses revealed a connectivity model for the different subunits in augmin (see below).
Biochemical reconstitution of hetero-octameric human augmin complexes
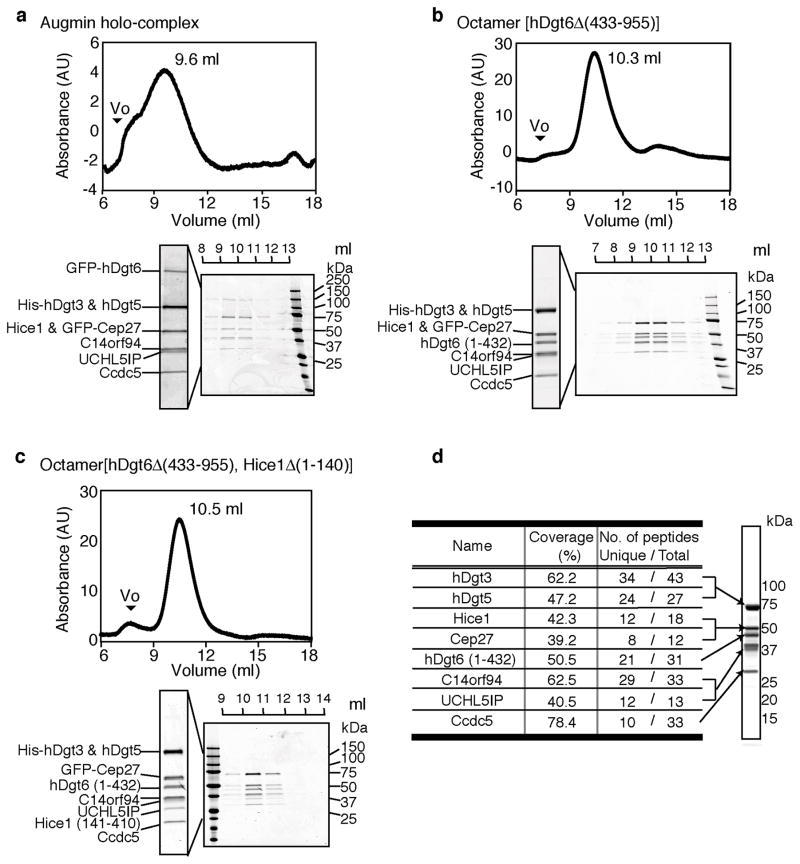
To reconstitute the augmin holo-complex, we used the MultiBac system for poylcistronic gene expression in insect cells23. Remarkably, the entire hetero-octameric complex (hereafter, holo-complex) could be isolated, albeit with low yields. We confirmed the presence of the subunits by western blot analyses (Supplementary Fig. 2) and using SDS-PAGE-based comparisons with another augmin octameric complex characterized by mass spectrometry (see below). The size-exclusion chromatography elution profile and the intensities of bands resolved by SDS-PAGE suggest that subunits in the holo-complex are present at equal stoichiometry (Fig. 2a).
Figure 2.
Reconstitution of the hetero-octameric human augmin complex with recombinant proteins. (a-c) Size exclusion chromatography (Superose 6) elution profiles for GFP-labeled holo-complex (Hice1·GFP-hDgt6·UCHL5IP·GFP-Cep27·C14orf94 ·Ccdc5·His-hDgt3·hDgt5) (a), octamer[hDgt6Δ (433–955)] (Hice1·hDgt6 (1–432) ·UCHL5IP·GFP-Cep27·C14orf94· Ccdc5·His-hDgt3·hDgt5) (b) and octamer[hDgt6Δ (433–955), Hice1Δ (1–140)] (Hice1 (141–410) ·hDgt6 (1–432) ·UCHL5IP·GFP-Cep27·C14orf94· Ccdc5·His-hDgt3·hDgt5) (c) with peak fractions (volumes indicated) analyzed by SDS-PAGE (stained with Coomassie blue). The void volume (Vo) is indicated and absorbance (A.U.) at 280 nm is shown. (d) Analysis of the subunit composition of the augmin octamer[hDgt6Δ (433–955)] by mass spectrometry. Identities, percent sequence coverage, and number of peptides (unique and total) detected are shown.
As we could obtain only small amounts of the holo-complex (~20 μg from one liter of insect cell culture), we generated an octameric complex with a truncated untagged hDgt6 (1–432). This octameric complex (hereafter, octamer[hDgt6Δ (433–955)]) eluted as a single peak during size exclusion chromatography (Fig. 2b). The presence of the different subunits in the octamer[hDgt6Δ (433–955)] was validated by mass spectrometry (Fig. 2d). We also purified a related octameric complex lacking the Hice1 N-terminus (hereafter, octamer[hDgt6Δ (433–955), Hice1Δ (1–140)]), thereby removing a known microtubule-binding domain (Fig. 2c). Together, these data indicate that augmin can be reconstituted by co-expressing eight subunits as recombinant proteins, and hDgt6’s C-terminal residues and Hice1’s N-terminal residues are dispensable for forming an octameric complex.
Characterizing the microtubule interaction of different purified augmin complexes in vitro
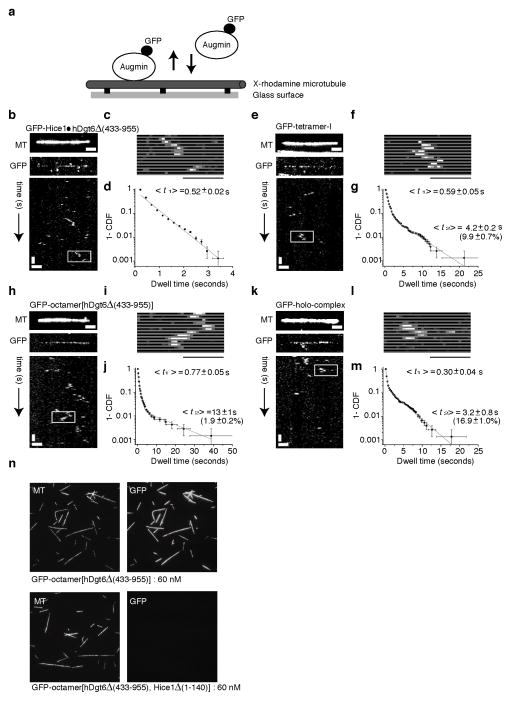
We next used total internal reflection fluorescence (TIRF) microscopy to analyze the interaction of GFP-tagged augmin complexes with microtubules. For these analyses we generated GFP-labeled dimer and tetramer-I complexes in addition to the GFP-labeled octameric complexes. Fluorescence intensity measurements of single molecules indicated that Hice1·hDgt6Δ (433–955) dimer, tetramer-I, and octamer[hDgt6Δ (433–955)] particles have a single GFP tag and the holo-complex has two GFP tags, as expected (Supplementary Fig. 3a–f).
All GFP-tagged augmin complexes bound to microtubules immobilized on coverslips (Fig. 3a,b,c,e,f,h,i,k,l and Supplementary Fig. 3l,m). Analyses of time-lapse sequences using kymographs showed that the different augmin complexes diffused in 1-D along microtubules without detectable directional bias (Supplementary Fig. 3g–k). In addition, all these complexes had similar microtubule association times (t½ less than 1 sec, with a subset (~2–16%) exhibiting longer (~3–13s) lifetimes) (Fig. 3d,g,j,m and Supplementary Fig. 3n). While high intensity spots, most likely due to aggregation under the assay conditions, could be observed for the GFP-tagged holo-complex (Supplementary Fig. 3e), several microtubule-bound particles with the expected intensities were observed and analyzed. At equal concentrations, GFP-tagged octamer[hDgt6Δ (433–955)] bound microtubules, while octamer[hDgt6Δ (433–955), Hice1 Δ (1–140)] did not (Fig. 3n). Together, these analyses reveal that the interaction of augmin with microtubules depends on the Hice1-MTBR, results in 1-D diffusion along the filaments and is not biased to filament ends.
Figure 3.
TIRF microscopy-based analysis of augmin-microtubule interactions. (a) A schematic of the assay. Microtubules immobilized on the glass surface and GFP-tagged complexes were imaged using TIRF microscopy. (b–m) Image of GMPCPP-stabilized microtubules (X-rhodamine- and biotin-labeled) (top), GFP-tagged complexes (maximum intensity projections, 300 images) and corresponding kymographs (below) are shown for GFP-tagged Hice1·hDgt6Δ (433–955) dimer (b), tetramer-I (GFP-Cep27) (e), octamer[hDgt6Δ (433–955)] (h), and holo-complex (k). Scale bars: horizontal, 2 μm; vertical, 2 seconds. (c,f,i,l) The region highlighted (box) in each kymograph is also shown in greater detail as a montage. Scale bar: 2 μm. (d,g,j,m) Binding events of individual GFP-tagged augmin complexes on microtubules were tracked to compute the cumulative distribution function (CDF) (right panels). Mean dwell times <t> and relative amplitudes (in parentheses) were obtained by fitting to bi-exponential functions (gray curve) for each case, while Hice1·hDgt6Δ (433–955) was fit to a mono-exponential. Three or more independent experiments were analyzed in each condition. (n) GMPCPP-stabilized microtubules (X-rhodamine- and biotin-labeled), immobilized on a glass surface, were incubated separately with equal concentrations (60 nM) of octamer[hDgt6Δ (433–955)] and octamer[hDgt6Δ (433–955), Hice1Δ (1–140)]. Under identical imaging conditions, octamer[hDgt6Δ (433–955)] decorated microtubules (upper-left panel) but octamer[hDgt6Δ (433–955), Hice1Δ (1–140)] did not target to microtubules (lower-left panel).
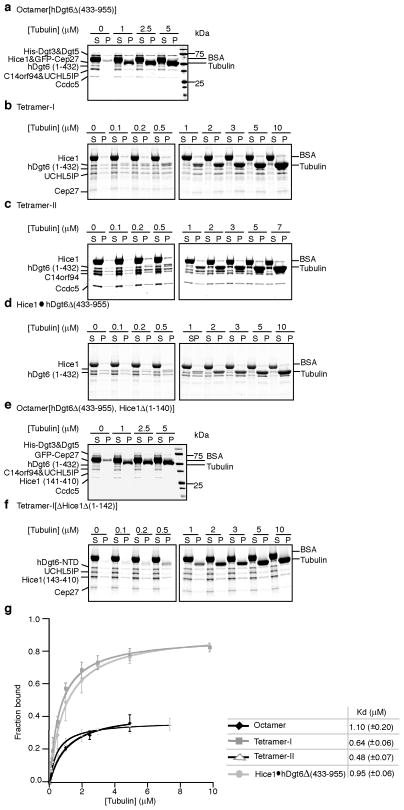
We next measured the microtubule binding affinities of the Hice1·hDgt6Δ (433–955) dimer, tetramer-I, tetramer-II, and octamer[hDgt6Δ (433–955)] using co-sedimentation assays. We found that these multi-protein complexes bound microtubules with more than 10-fold higher affinity (Kd’s: 0.5 to 1.1 μM) than Hice1-MTBR alone (Fig. 4). Octamer[hDgt6Δ (433–955), Hice1Δ (1–140)] and tetramer-I[Hice1Δ (1–142)] did not bind microtubules under these conditions at concentrations up to 10 μM (Fig. 4e,f). Additionally, we visualized microtubule binding of the dimeric and tetrameric complexes by negative stain electron microscopy (EM) (Supplementary Fig. 4a,b). Together, these data indicate that the Hice1-MTBR alone is not sufficient to achieve microtubule-binding affinities comparable to the multi-protein complexes, but this region is needed for strong augmin-microtubule interactions.
Figure 4.
Analyses microtubule binding by augmin complexes. SDS-PAGE analysis of microtubule co-sedimentation assays for octamer[hDgt6Δ (433–955)] (a), tetramer-I (b), tetramer-II (c), Hice1·hDgt6Δ (433–955) (d), octamer[hDgt6Δ (433–955), Hice1Δ (1–140)] (e), and tetramer-I[Hice1Δ (1–142)] (f). BSA (final 0.25 mg/ml), used to suppress non-specific interactions, and tubulin are indicated. (g) Analysis of microtubule binding by octamer[hDgt6Δ (433–955)] (◆), tetramer-I (
 ), tetramer-II (Δ), and Hice1·hDgt6Δ (433–955) (
), tetramer-II (Δ), and Hice1·hDgt6Δ (433–955) (
 ). For the octamer, band intensities of His-Dgt3&Dgt5, C14orf94, UCHL5IP, and Ccdc5 from the SDS-PAGE gels were used to determine average fraction protein bound. For tetramer-I, band intensities of hDgt6 (1–432), UCHL5IP, and Cep27 were used to determine average fraction protein bound. For tetramer-II, band intensities of C14orf94, and Ccdc5 were used to determine average fraction bound. For the dimer, only the hDgt6 (1–432) band intensity was used to determine fraction protein bound. Microtubule binding constants were determined by fitting to a hyperbola. Due to limited amounts of octamer [hDgt6Δ (433–955)] available, co-sedimentation experiments were repeated twice. For the tetramer-I, -II and dimer, three independent experiments were analyzed. Error bars show S.D.
). For the octamer, band intensities of His-Dgt3&Dgt5, C14orf94, UCHL5IP, and Ccdc5 from the SDS-PAGE gels were used to determine average fraction protein bound. For tetramer-I, band intensities of hDgt6 (1–432), UCHL5IP, and Cep27 were used to determine average fraction protein bound. For tetramer-II, band intensities of C14orf94, and Ccdc5 were used to determine average fraction bound. For the dimer, only the hDgt6 (1–432) band intensity was used to determine fraction protein bound. Microtubule binding constants were determined by fitting to a hyperbola. Due to limited amounts of octamer [hDgt6Δ (433–955)] available, co-sedimentation experiments were repeated twice. For the tetramer-I, -II and dimer, three independent experiments were analyzed. Error bars show S.D.
Single particle electron microscopy analysis of augmin complexes
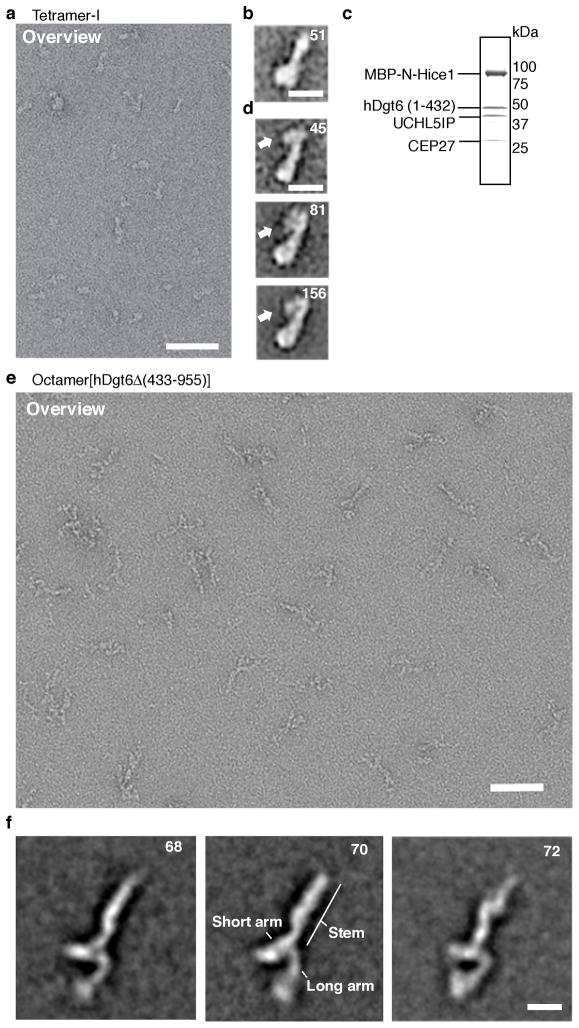
To examine the overall organization of the augmin complex, we used negative stain single particle electron microscopy. Images of tetramer-I (Hice1·hDgt6 (1–432) · UCHL5IP·Cep27) and tetramer-II (Hice1·hDgt6 (1–432) · His-C14orf94·Ccdc5) complexes appeared to be similar (Fig. 5a and Supplementary Fig. 4e,f), and therefore we carried out standard single particle analysis of tetramer-I only. We generated 2D-class averages of tetramer-I and found it to have a “drumstick”-like structure with a length of 16–20 nm (Fig. 5b). To localize the position of the microtubule-binding Hice1 subunit, we generated two tetramer-I complexes containing Hice1 tagged with a maltose-binding protein (MBP) at either the N- or C-terminus. Co-expression, followed by multi-step chromatography, led to homogenous tetrameric protein complexes (Fig. 5c and Supplementary Fig. 4c). 2D averages of these N- and C-terminally labeled tetramer-I complexes revealed additional density at similar positions at the narrow end of the “drumstick” structure (Fig. 5d and Supplementary Fig. 4d). Together, these data indicate that Hice1 localizes at one end of tetramer-I formed by Hice1, hDgt6 (1–432), UCHL5IP and Cep27.
Figure 5.
2-D class averages of augmin complexes examined by electron microscopy. (a) Negative stain electron micrograph of tetramer-I (Hice1·hDgt6 (1–432)·UCHL5IP·Cep27) particles. A field of particles adsorbed onto a glow-discharged carbon grid was stained with 2% (w/v) uranyl acetate and processed for imaging. Scale bar, 50 nm. (b) A representative two-dimensional class average image of tetramer-I particles (N=51). Scale bar, 10 nm (c) SDS-PAGE analysis (stained with Coomassie blue) of purified tetramer-I with MBP-tagged Hice1 N-terminus. (d) Three representative class averages of the MBP-tagged tetramer-I (MBP-Hice1·hDgt6 (1–432)·UCHL5IP· Cep27) particles (N=45, 81 and 156) are shown. The position of the MBP-tag is indicated (arrow). Scale bar, 10 nm (e) Negative stain electron micrograph of octamer[hDgt6Δ (433–955)] particles. A field of octamer[hDgt6Δ (433–955)] particles adsorbed onto a glow-discharged carbon grid was stained with 2% (w/v) uranyl acetate and processed for imaging. Scale bar, 50 nm. (f) Representative class averages of the octamer[hDgt6Δ (433–955)] particles (N=68, 70 and 72). Prominent structure features are indicated. Scale bar, 10 nm.
We next examined the structure of the purified recombinant hetero-octameric complex lacking the C-terminal portion of hDgt6 (octamer[hDgt6Δ (433–955)]). In this complex, Cep27 is tagged with GFP. Analysis of ~8000 augmin particle images revealed classes exhibiting general structural features (representative class averages shown in Fig. 5e,f). The complex is 38–44 nm long with a splayed Y-shape at one end. The angle between the long and short arms of the Y is variable, underscoring the flexibility of the splayed end of the complex seen in the raw images. Attempts to localize tetramer-I in the octamer averages did not yield an unambiguous result. Overall, our findings indicate that the augmin octamer adopts a ~40 nm extended Y-shaped complex, the splayed end of which can access a large number of conformations.
Analyzing augmin targeting to metaphase spindles
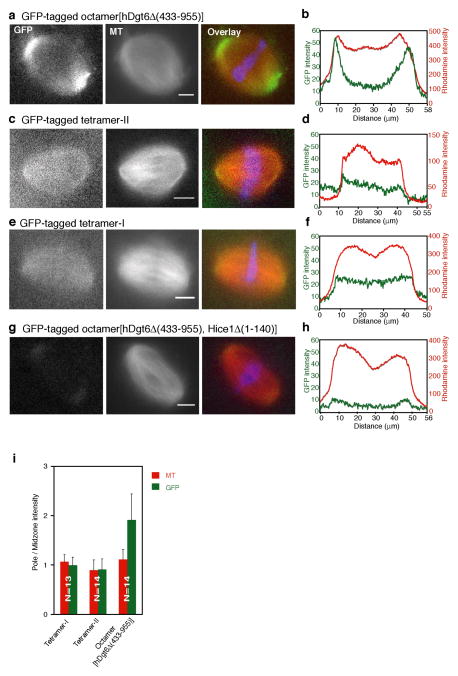
To examine the contributions of the different subunits to augmin’s localization in metaphase spindles we used Xenopus egg extracts24. Using an antibody against Xenopus Ccdc5 (Supplementary Fig. 5a) we find that this subunit localizes along spindle microtubules and accumulates at spindle poles (Supplementary Fig. 5b), consistent with the reported augmin localization in Drosophila meiotic spindles15. Next, we analyzed the localization of recombinant GFP-tagged octamer[hDgt6Δ (433–955)], tetramer-II and tetramer-I (15 nM). While all these complexes targeted to spindle microtubules (Fig. 6a–f and Supplementary Fig. 5c), octamer[hDgt6Δ (433–955)] had a 2-fold higher signal at spindle poles (Fig. 6i). The Hice1·hDgt6Δ (433–955) dimer (15 nM) localized to the spindle, but also targeted to chromosomes, possibly due to non-specific interactions (Supplementary Fig. 5d,e). These results suggest endogenous augmin and octamer[hDgt6Δ (433–955)], but not the other sub-complexes, have a similar protein localization in Xenopus metaphase spindles. As the recombinant holo-complex could only be obtained at concentrations that were ~10-fold lower than other complexes, we were unable to achieve sufficient levels in extracts for detection of its spindle association without perturbing organization due to extract dilution (Supplementary Fig. 5i).
Figure 6.
Analysis of augmin’s localization in metaphase spindles using Xenopus egg extracts. (a–h) Recombinant GFP-tagged octamer[hDgt6Δ (433–955)] (a), tetramer-II (c), tetramer-I (e), and octamer[hDgt6Δ (433–955), Hice1Δ (1–140)] (g) (15 nM) (left panels); tubulin (X-rhodamine-labeled, middle panels); and overlays (right panels; tubulin, red; GFP, green; and DNA, blue), are shown. (b,d,f,h) Corresponding linescans, along the long axis of the spindles, for the GFP (green) and X-rhodamine (red) signals. (i) Analysis of augmin (green) and microtubule (red) levels in spindles. Ratios of average fluorescence signal at spindle poles versus signal in the middle of the spindle for tetramer-I, tetramer-II and octamer[hDgt6Δ (433–955)] are shown. Tetramer-I: 0.99 ± 0.18 SD [N=13 spindles], microtubule, 1.06 ± 0.14 SD [N=13 spindles]; Tetramer-II: 0.91 ± 0.22 SD [N=14 spindles], microtubule, 0.9 ± 0.2 SD [N=14 spindles];octamer[hDgt6Δ (433–955)], 1.91 ± 0.53 SD [N=14 spindles], microtubule, 1.11 ± 0.21 SD [N=14 spindles]. S.D. was determined from data pooled from 3 or more independent experiments.
We next examined octamer[hDgt6Δ (433–955), Hice1Δ (1–140)] (15 nM) localization in spindles. The GFP intensity in the middle of the spindle is similar to that of recombinant GFP alone (Fig. 6h and Supplementary Fig. 5f,g). Interestingly, a weak GFP-signal could be consistently detected at spindle poles (Fig. 6g,h and Supplementary Fig. 5h), suggesting the presence of low affinity microtubule-independent interactions between pole-associated proteins and augmin. Together, these results suggest that augmin sub-complexes with similar microtubule-binding properties in vitro target to metaphase spindles. However, accumulation at spindle poles requires subunits in addition to those present in tetramer-I or -II alone.
Recombinant augmin promotes microtubule aster formation in Xenopus egg extracts
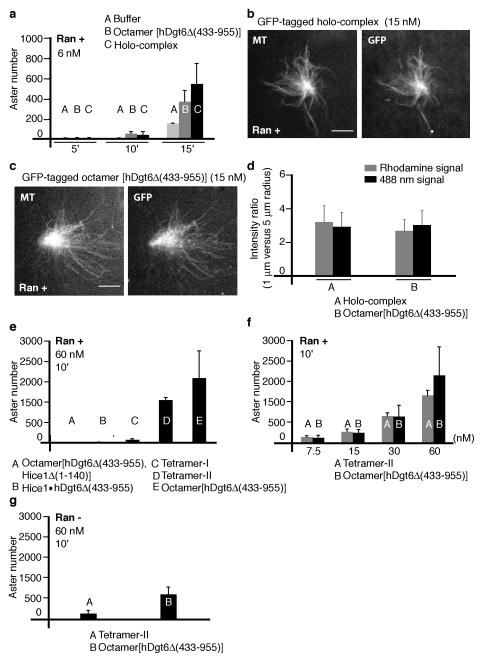
To test the function of the recombinant augmin complexes, we used a microtubule aster formation assay in Xenopus egg extracts16,17. It has recently been shown that augmin depletion delays microtubule aster formation in the presence of Ran(Q69L)16, a mutant form of Ran locked in the GTP-bound state. We find that addition of augmin holo-complex (6 nM) leads to substantially more asters, formed within minutes, in the presence of Ran(Q69L) (15 μM), compared to controls (buffer) (Fig. 7a and Supplementary Fig. 6b, 7a). Aster formation was also promoted by octamer[hDgt6Δ (433–955)] (6 nM), (Fig. 7a and Supplementary Fig. 6a, 7a), albeit with reduced efficiency (15 minutes, ~32% lower number) compared to the holo-complex (Fig. 7a).
Figure 7.
Analysis of augmin-induced microtubule aster formation. Augmin complexes and Ran(Q69L)(15 μM) were added to extracts, incubated for 10 min (or as noted), fixed and processed to determine the number of asters formed. (a) Aster assembly in the presence of buffer (A, light gray), GFP-tagged octamer[hDgt6Δ (433–955)] (6 nM) (B, gray), and holo-complex (6 nM) (C, black). (Mean and S.D. were calculated from 3 independent experiments with separate extract preparations. For each experiment, data were pooled from two measurements) (b,c) Asters formed in the presence of Ran(Q69L) and GFP-tagged holo-complex (b) or octamer[hDgt6Δ (433–955)] at 15 nM (c). Tubulin (X-rhodamine-labeled), left panels; and xCcdc5 antibody staining or GFP fluorescence, right panels, are shown. Scale bar, 10 μm. (d) Analysis of microtubule (rhodamine signal) and augmin (488nm signal) levels in asters. Ratios of average fluorescence at 1μm versus 5 μm radius for holo-complex (A) and octamer[hDgt6Δ (433–955)] (B) induced asters are shown. (Mean and S.D. were calculated from: Holo-complex, N=23 asters; octamer, N=22 asters; Analyzed asters were from 3 separate extract preparations.) (e) Aster formation in the presence of GFP-tagged octamer[hDgt6Δ (433–955), Hice1Δ (1–140)] (A), Hice1·hDgt6Δ (433–955) dimer (B), tetramer-I (C) and tetramer-II (D). GFP-tagged octamer[hDgt6Δ (433–955)] (E) at 60 nM. (Mean and S.D. were calculated from either 3 (dimer, tetramer-I and tetramer-II) or 4 (octamer[hDgt6Δ (433–955), Hice1Δ (1–140)] and octamer[hDgt6Δ (433–955)) independent experiments with separate extract preparations. For each experiment, data were pooled from two measurements) (f) Dose-dependent analysis of aster formation in the presence of tetramer-II (A) and GFP-tagged octamer[hDgt6Δ (433–955)] (B). (Mean and S.D. were calculated from either 3 (tetramer-II) or 5 (octamer[hDgt6 Δ (433–955)]) independent experiments with separate extract preparations. For each experiment, data were pooled from two measurements) (g) Aster assembly in the absence of Ran(Q69L). Aster number for tetramer-II (A, 60 nM) and GFP-tagged octamer[hDgt6Δ (433–955)] (B, 60 nM). (Mean and S.D. were calculated from 3 independent experiments with separate extract preparations. For each experiment, data were pooled from two measurements).
We examined the localization of the GFP-tagged augmin holo-complex and octamer[hDgt6Δ (433–955)] in microtubule asters at 15nM, the highest concentration of the holo-complex we could reliably achieve without loss of aster stability. Both holo-complex and octamer[hDgt6 Δ (433–955)] associated with microtubules in the asters (Fig. 7b–d). We were unable to sufficiently deplete endogenous augmin from the egg extracts using our xCcdc5 antibody (Supplementary Fig. 6c), and therefore could not properly examine if the recruitment of γ-tubulin in the asters generated by octamer[hDgt6Δ (433–955)] was reduced compared to the holo-complex.
We next analyzed aster formation by the recombinant augmin sub-complexes and the octameric complex lacking the Hice1-MTBR. We estimated that the concentration of Ccdc5 in Xenopus extracts is 60 nM (Supplementary Fig. 5a). We were unable to obtain these high concentrations of the holo-complex without diluting the extract and disrupting aster assembly, but could with octamer[hDgt6Δ (433–955)]. Within 10 minutes, this octameric complex (60 nM) induced aster assembly. In contrast, essentially no asters formed in the presence of octamer[hDgt6Δ (433–955), Δ Hice1(1–140)] or Hice1·hDgt6Δ (433–955) dimer (Fig. 7e and Supplementary Fig. 7b, 60 nM). Notably, while some asters were induced by tetramer-I, activity of tetramer-II was comparable to that of octamer[hDgt6Δ (433–955)] in this assay (Fig. 7e (60 nM)). The localization of tetramer-II in asters was similar to that of the holo-complex and octamer[hDgt6Δ (433–955)] (Supplementary Fig. 6d,e (15nM)). Addition of tetramer-II induced aster formation in a dose-dependent manner, with efficiency only slightly lower than that of octamer[hDgt6Δ (433–955)] (Fig. 7f and Supplementary Fig. 7c). Interestingly, octamer[hDgt6Δ (433–955)] and tetramer-II promoted microtubule aster formation even in the absence of Ran(Q69L), but overall efficiency was lower (Fig. 7g and Supplementary Fig. 7d). Together, our data indicate that recombinant augmin promotes microtubule aster formation in Xenopus egg extracts and this Ran GTPase-regulated activity depends on the Hice1-MTBR, but not the hDgt6 C-terminus.
Morphological differences between microtubule asters promoted by octameric and tetrameric augmin complexes
We next compared the morphology of the asters induced by the recombinant tetramer-II and octamer[hDgt6Δ (433–955)] in the presence of Ran(Q69L). We used recombinant complexes at concentrations similar to that of the native protein (60nM). Tetramer-II-induced asters appeared symmetric with a more uniform microtubule density, while octamer[hDgt6Δ (433–955)]-induced asters were more asymmetric and exhibited more regions of dense microtubule ‘bundles’ adjacent to regions void of microtubules (Fig. 8a, b and Supplementary Fig. 6l, m).
Figure 8.
Morphological analysis of asters induced by augmin complexes and schematic for augmin organization and function. Augmin complexes and Ran(Q69L)(15 μM) were added to extracts, fixed and processed. (a, b) Two examples of microtubule asters induced by GFP-tagged tetramer-II (a) and GFP-tagged octamer[hDgt6Δ (433–955)] (b) at 60 nM. Defined angular coordinates are indicated (blue and yellow lines). (c, d) Representative images after polar transformation of asters induced by tetramer-II (c) and octamer[hDgt6Δ (433–955)] (d). The microtubule fluorescence intensity along the radial direction (x-axis, where radius=0 corresponds to the center of the aster), is shown for all angles (y-axis, from 0 to 360 degrees). Labelled radial profiles (blue and yellow lines) correspond to those shown in (a) and (b). (e) Normalized angular intensity values at radius= 8 μm in asters induced by teramer-II and octamer[hDgt6Δ (433–955)]. (f) Coefficient of variation (CV, equal to the standard deviation divided by the mean of the intensity) of tetramer-II and octamer[hDgt6Δ (433–955)] induced asters at 4 and 8μm radii. CV was calculated for half-circle (180 degree) regions containing the highest detected microtubule signal. (Tetramer-II, N=27 asters; octamer, N=37 asters; S.D. was determined from data pooled from 3 independent experiments.) (g) Normalized intensity averaged across all angles plotted as a function of radial distance from the aster center. (h) Normalized angular intensity values at radius=8μm in asters induced by Ran-alone, holo-complex (15 nM) and octamer[hDgt6Δ (433–955)] (15 nM). (i) Coefficient of variation calculated from angular intensity values (Ran(Q69L) alone, N=28 asters; holo-complex, N=31 asters, octamer[hDgt6Δ (433–955)], N=35 asters; S.D. was determined from data pooled across from 3 or more independent experiments.) (j) Normalized intensity averaged across all angles plotted as a function of radial distance from the aster center. (k) Schematic for augmin’s subunit organization and function. Direct interaction between subunits is indicated as a red bar. Hice1 and hDgt6 (1–432) form hetero-dimers. Distinct tetrameric complexes have a common Hice1·hDgt6Δ (433–955) core. The six proteins in these two tetramers form a stable hexamer. Together with hDgt5 and hDgt3, these proteins form an octameric complex. Table provides a summary of the results for microtubule interaction in vitro and microtubule aster formation of augmin complexes.
To quantitatively compare the morphology of the asters induced by these complexes we first performed a coordinate transformation on images (bottom panel, Fig. 8a, b). Here, the fluorescence intensity along the radial direction is plotted for all angles (Fig. 8c, d). The angular intensity values at a specified radius were then determined (Fig. 8e, radius= 8 μm), and the coefficient of variation was calculated (Fig. 8f). For asters induced with tetramer-II, and at radii near the center of the aster, this value was relatively small. In contrast, for octamer[hDgt6Δ (433–955)] induced asters, the coefficient of variation was much larger. These fluctuations in microtubule intensity increased with distance from the aster center (Fig. 8f). Further, determined under a comparable total intensity within the asters, the normalized intensity of all microtubule signal reveals octamer[hDgt6 (433–955)] induced asters have a sharper reduction of microtubule density along the radial direction compared that of tetramer-II induced asters (Fig. 8g and Supplementary Fig. 7e).
We next analyzed asters induced by Ran(Q69L) alone to those generated by Ran(Q69L) and holo-complex or octamer[hDgt6Δ (433–955)], at the highest holo-complex concentration we could reliably achieve in the assay (15 nM, Fig. 7b,c and Supplementary Fig. 6i–k). Our analysis revealed that the coefficient of variation across and the normalized intensity that was analyzed under a comparable total intensity was similar under these conditions (Fig. 8h–j and Supplementary Fig. 6f–h, 7f). Together, these data show that different augmin complexes can generate asters with diverse morphological features, and that the addition of holo-complex and octamer[hDgt6Δ (433–955)] induce structures with morphologies similar to the Ran-induced asters.
DISCUSSION
In summary, the biochemical reconstitution of the hetero-octameric augmin complex has revealed its overall architecture and allowed functional tests. Our electron microscopy analyses indicate that augmin forms an elongated Y-shaped structure. However, due to the resolution of this structure and conformational flexibility of the holo-complex, we are currently unable to position sub-complexes within the holo-complex with high confidence. Based on our analyses thus far, we propose that Hice1·hDgt6Δ (433–955) forms a core scaffold within augmin. The holo-complex C14orf94 and Ccdc5 are likely to be directly connected (Supplementary Fig. 1g), and together interact with Hice1·hDgt6Δ (433–955) independent of other subunits. Currently, we are unable to position hDgt3 and hDgt5 subunits within augmin. Together, our findings provide valuable constraints on how the different subunits interact within the augmin holo-complex (Fig. 8k).
Our findings also help dissect the contributions of the different augmin subunits to its microtubule organizing function. Octamer[hDgt6Δ (433–955)], Hice1·hDgt6Δ (433–955) dimer, and tetramer-I have similar microtubule binding affinities, filament association lifetimes and distributions along stabilized microtubules. As these properties do not differ significantly for the octameric and dimeric complexes, and Hice1·hDgt6Δ (433–955) are present in tetramer-II, we infer that these properties are likely comparable for all these complexes. Further, our data indicate that the Hice1-MTBR mediates direct microtubule binding in augmin complex in vitro and in spindles. While different sub-complexes can bind spindle microtubules, enrichment of these proteins at spindle poles, similar to that of endogenous augmin, requires the additional subunits present in the octameric complex. Our data also indicate that this localization does not depend on the hDgt6 C-terminus that has been shown to interact with γ-tubulin13. Current models predict that augmin should localize at the branch point between the existing ‘mother’ filament and the new ‘daughter’ filament13. However, such localization has not been shown and our own efforts have not been successful thus far. This could be due to limited signal over background in our imaging assays or augmin not preferentially localizing to the filament branch points relative to the sides of the filaments.
We find that microtubule asters with similar size and overall microtubule levels are promoted by tetramer-II and octameric complexes, including one that lacks the hDgt6 C-terminus. The main morphological difference between asters promoted by tetramer-II and octameric complexes is the amount of microtubule bundling. This difference could result from variations in the branching angles between the ‘mother’ and ‘daughter’ filaments, or the efficiency in recruiting γ-tubulin. This aster promoting activity is not observed for other sub-complexes that have similar microtubule binding properties in vitro. C14orf94 and Ccdc5, the two subunits in tetramer-II not present in tetramer-I or the dimeric complexes, are unlikely to substantially modulate the microtubule interaction in vitro. These two subunits may instead mediate regulation of aster assembly by RanGTP (see Fig. 7f,g), or contribute to γ-tubulin recruitment independent of hDgt6. These data, along with our observation that this aster promoting activity requires the Hice1-MTBR, raise the possibility that augmin functions in two ways, by recruiting γ-tubulin and via directly stabilizing microtubules. In vitro assays with dynamic microtubules and the augmin complexes we have reconstituted will help test this model and reveal how this hetero-octameric complex contributes to microtubule formation pathways needed to ensure successful cell division.
Supplementary Material
Acknowledgments
We thank G. Goshima for the gift of UCHL5IP antibody; and L. Pelletier for the antibodies of C14orf94 and Cep27. The SEC-LS/UV/RI instrumentation used for light-scattering analysis was supported by a National Institutes of Health (NIH) award (1S10RR023748-01). TEM studies were conducted at the National Resource for Automated Molecular Microscopy, which is supported by the National Institute of General Medical Sciences (9 P41 GM103310). K.-C.H. was supported by Kimberly Lawrence-Netter Cancer Research Discovery Fund at The Rockefeller University and is currently supported by a Special Fellow Award from The Leukemia & Lymphoma Society. S.F. acknowledges postdoctoral support from an NIH National Research Service Award Fellowship (F32GM099380). R.A.M. acknowledges support from the NIH (GM-052468). T.M.K. acknowledges support from the NIH (GM-65933)
References
- 1.Walczak CE, Heald R. Mechanisms of mitotic spindle assembly and function. Int Rev Cytol. 2008;265:111–158. doi: 10.1016/S0074-7696(07)65003-7. [DOI] [PubMed] [Google Scholar]
- 2.Meunier S, Vernos I. Microtubule assembly during mitosis - from distinct origins to distinct functions? J Cell Sci. 2012;15:2805–2814. doi: 10.1242/jcs.092429. [DOI] [PubMed] [Google Scholar]
- 3.Teixidó-Travesa N, Roig J, Lüders J. The where, when and how of microtubule nucleation - one ring to rule them all. J Cell Sci. 2012;125:4445–4456. doi: 10.1242/jcs.106971. [DOI] [PubMed] [Google Scholar]
- 4.Lüders J, Stearns T. Microtubule-organizing centres: a reevaluation. Nat Rev Mol Cell Biol. 2007;8:161–167. doi: 10.1038/nrm2100. [DOI] [PubMed] [Google Scholar]
- 5.Bettencourt-Dias M, Glover DM. Centrosome biogenesis and function: centrosomics brings new understanding. Nat Rev Mol Cell Biol. 2007;8:451–463. doi: 10.1038/nrm2180. [DOI] [PubMed] [Google Scholar]
- 6.Kollman JM, Merdes A, Mourey L, Agard DA. Microtubule nucleation by γ-tubulin complexes. Nat Rev Mol Cell Biol. 2011;12:709–721. doi: 10.1038/nrm3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wadsworth P, Khodjakov A. E pluribus unum: towards a universal mechanism for spindle assembly. Trends Cell Biol. 2004;14:413–419. doi: 10.1016/j.tcb.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Walczak CE, Cai S, Khodjakov A. Mechanisms of chromosome behaviour during mitosis. Nat Rev Mol Cell Biol. 2010;11:91–102. doi: 10.1038/nrm2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumont J, Desai A. Acentrosomal spindle assembly and chromosome segregation during oocyte meiosis. Trends Cell Biol. 2012;22:241–249. doi: 10.1016/j.tcb.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karsenti E, Vernos I. The mitotic spindle: a self-made machine. Science. 2001;294:543–547. doi: 10.1126/science.1063488. [DOI] [PubMed] [Google Scholar]
- 11.Kelly AE, Funabiki H. Correcting aberrant kinetochore microtubule attachments: an Aurora B-centric view. Curr Opin Cell Biol. 2009;21:51–58. doi: 10.1016/j.ceb.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goshima G, Mayer M, Zhang N, Stuurman N, Vale RD. Augmin: a protein complex required for centrosome-independent microtubule generation within the spindle. J Cell Biol. 2008;181:421–429. doi: 10.1083/jcb.200711053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uehara R, et al. The augmin complex plays a critical role in spindle microtubule generation for mitotic progression and cytokinesis in human cells. Proc Natl Acad Sci USA. 2009;106:6998–7003. doi: 10.1073/pnas.0901587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawo S, et al. HAUS, the 8-subunit human Augmin complex, regulates centrosome and spindle integrity. Curr Biol. 2009;19:816–826. doi: 10.1016/j.cub.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 15.Colombié N, Guszek AA, Meireles AM, Ohkura H. Meiosis-specific stable binding of augmin to acentrosomal spindle poles promotes biased microtubule assembly in oocytes. PLoS Genet. 2013;9:e1003562. doi: 10.1371/journal.pgen.1003562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petry S, Pugieux C, Nédélec FJ, Vale RD. Augmin promotes meiotic spindle formation and bipolarity in Xenopus egg extracts. Proc Natl Acad Sci USA. 2011;108:14473–14478. doi: 10.1073/pnas.1110412108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petry S, Groen AC, Ishihara K, Mitchison TJ, Vale RD. Branching microtubule nucleation in Xenopus egg extracts mediated by augmin and TPX2. Cell. 2013;152:768–777. doi: 10.1016/j.cell.2012.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamasaki T, et al. Augmin-dependent microtubule nucleation at microtubule walls in the spindle. J Cell Biol. 2013;202:25–33. doi: 10.1083/jcb.201304031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayward D, Metz J, Pellacani C, Wakefield JG. Synergy between Multiple Microtubule-Generating Pathways Confers Robustness to Centrosome-Driven Mitotic Spindle Formation. Dev Cell. 2014;28:81–93. doi: 10.1016/j.devcel.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu G, et al. Hice1, a novel microtubule-associated protein required for maintenance of spindle integrity and chromosomal stability in human cells. Mol Cell Biol. 2008;28:3652–3662. doi: 10.1128/MCB.01923-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan S, Kern RC, Selleck W. The pST44 polycistronic expression system for producing protein complexes in Escherichia coli. Protein Expr Purif. 2005;40:385–95. doi: 10.1016/j.pep.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Subramanian R, et al. Insights into antiparallel microtubule crosslinking by PRC1, a conserved nonmotor microtubule binding protein. Cell. 2010;142:433–443. doi: 10.1016/j.cell.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trowitzsch S, Bieniossek C, Nie Y, Garzoni F, Berger I. New baculovirus expression tools for recombinant protein complex production. J Struct Biol. 2010;172:45–54. doi: 10.1016/j.jsb.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Desai A, Murray A, Mitchison TJ, Walczak CE. The use of Xenopus egg extracts to study mitotic spindle assembly and function in vitro. Methods Cell Biol. 1999;61:385–412. doi: 10.1016/s0091-679x(08)61991-3. [DOI] [PubMed] [Google Scholar]
- 25.Suloway C, et al. Automated molecular microscopy: the new Leginon system. J Struct Biol. 2005;151:41–60. doi: 10.1016/j.jsb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Tang G, et al. EMAN2: an extensible image processing suite for electron microscopy. J Struct Biol. 2007;157:38–46. doi: 10.1016/j.jsb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Hohn M, et al. SPARX, a new environment for Cryo-EM image processing. J Struct Biol. 2007;157:47–55. doi: 10.1016/j.jsb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Yang Z, Fang J, Chittuluru J, Asturias FJ, Penczek PA. Iterative stable alignment and clustering of 2D transmission electron microscope images. Structure. 2012;20:237–247. doi: 10.1016/j.str.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith MB, et al. Interactive, computer-assisted tracking of speckle trajectories in fluorescence microscopy: application to actin polymerization and membrane fusion. Biophys J. 2011;101:1794–1804. doi: 10.1016/j.bpj.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Folta-Stogniew E, Williams KR. Determination of molecular masses of proteins in solution: Implementation of an HPLC size exclusion chromatography and laser light scattering service in a core laboratory. J Biomol Tech. 1999;10:51–63. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.