Abstract
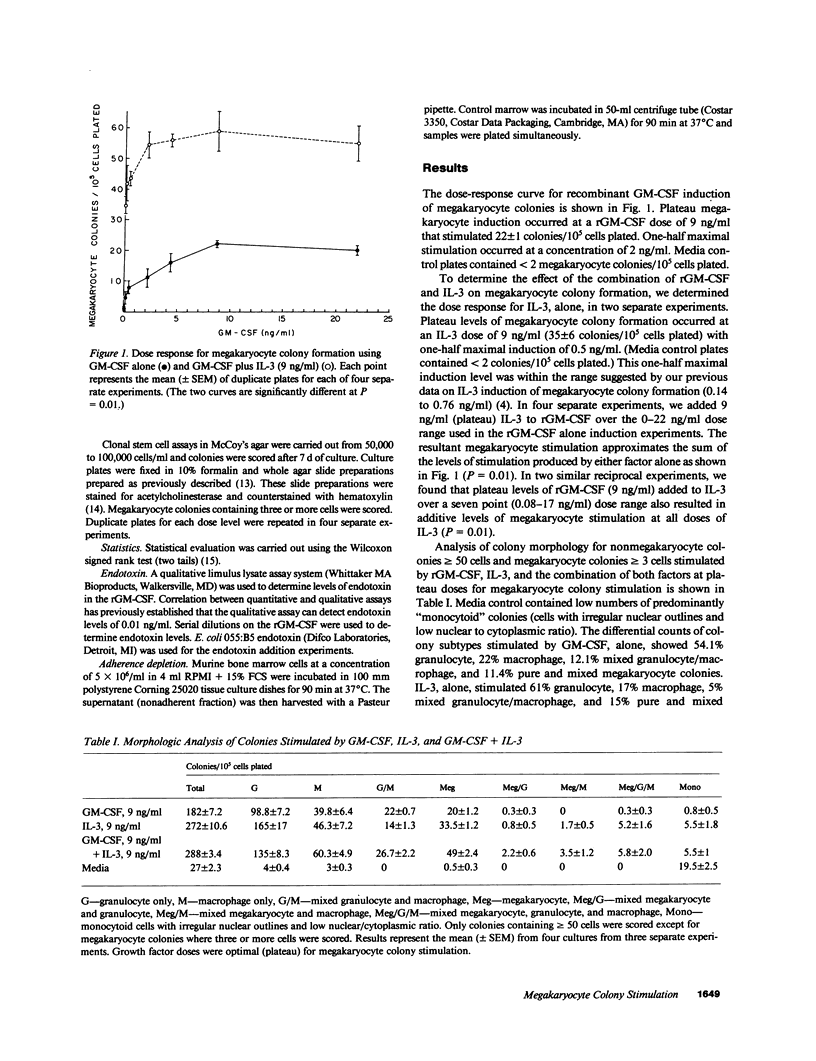
Recombinant murine granulocyte macrophage colony-stimulating factor (rGM-CSF) has been produced in Escherichia coli and purified to homogeneity. GM-CSF has an established role as an in vitro regulator of granulocyte and macrophage colony formation. We have determined that rGM-CSF also has intrinsic activity as a megakaryocyte colony-stimulating factor and that rGM-CSF augments the effect of interleukin 3 (IL-3) on megakaryocyte colony formation. The dose-response curve for megakaryocyte colony induction with rGM-CSF showed plateau megakaryocyte stimulation at 9 ng/ml. When IL-3 (at a plateau dose for megakaryocyte colony induction) was added to rGM-CSF over a 0-22-ng/ml dose range, the resultant megakaryocyte colony stimulation approximated the sum of the levels of stimulation produced by either factor alone. These results establish GM-CSF as a multilineage growth factor with definite megakaryocyte colony-stimulating activity and indicate that both GM-CSF and IL-3 are important in the regulation of megakaryocytopoiesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DeLamarter J. F., Mermod J. J., Liang C. M., Eliason J. F., Thatcher D. R. Recombinant murine GM-CSF from E. coli has biological activity and is neutralized by a specific antiserum. EMBO J. 1985 Oct;4(10):2575–2581. doi: 10.1002/j.1460-2075.1985.tb03973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukes P. P., Egrie J. C., Strickland T. W., Browne J. K., Lin F. K. Megakaryocyte colony stimulating activity of recombinant human and monkey erythropoietin. Prog Clin Biol Res. 1986;215:105–109. [PubMed] [Google Scholar]
- Gualtieri R. J., Shadduck R. K., Baker D. G., Quesenberry P. J. Hematopoietic regulatory factors produced in long-term murine bone marrow cultures and the effect of in vitro irradiation. Blood. 1984 Aug;64(2):516–525. [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Henderson L., Klein F., Palaszynski E. Procedures for the purification of interleukin 3 to homogeneity. J Immunol. 1982 Dec;129(6):2431–2436. [PubMed] [Google Scholar]
- Ishibashi T., Burstein S. A. Interleukin 3 promotes the differentiation of isolated single megakaryocytes. Blood. 1986 May;67(5):1512–1514. [PubMed] [Google Scholar]
- Jackson C. W. Cholinesterase as a possible marker for early cells of the megakaryocytic series. Blood. 1973 Sep;42(3):413–421. [PubMed] [Google Scholar]
- Koike K., Shimizu T., Miyake T., Ihle J. N., Ogawa M. Hemopoietic colony formation by mouse spleen cells in serum-free culture supported by purified erythropoietin and/or interleukin-3. Prog Clin Biol Res. 1986;215:33–49. [PubMed] [Google Scholar]
- Levin J., Levin F. C., Hull D. F., 3rd, Penington D. G. The effects of thrombopoietin on megakaryocyte-cfc, megakaryocytes, and thrombopoiesis: with studies of ploidy and platelet size. Blood. 1982 Oct;60(4):989–998. [PubMed] [Google Scholar]
- Long M. W., Williams N., McDonald T. P. Immature megakaryocytes in the mouse: in vitro relationship to megakaryocyte progenitor cells and mature megakaryocytes. J Cell Physiol. 1982 Sep;112(3):339–344. doi: 10.1002/jcp.1041120305. [DOI] [PubMed] [Google Scholar]
- Lugaro G., Bernasconi G., Casellato M. M. Effect of a seminal inhibin-like factor on in vivo FSH and LH uptake by rat testis. Cell Biol Int Rep. 1984 Oct;8(10):811–811. doi: 10.1016/0309-1651(84)90063-8. [DOI] [PubMed] [Google Scholar]
- McLeod D. L., Shreve M. M., Axelrad A. A. Induction of megakaryocyte colonies with platelet formation in vitro. Nature. 1976 Jun 10;261(5560):492–494. doi: 10.1038/261492a0. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Burgess A. W., Johnson G. R., Nicola N. A., Nice E. C., DeLamarter J., Thatcher D. R., Mermod J. J. In vitro actions on hemopoietic cells of recombinant murine GM-CSF purified after production in Escherichia coli: comparison with purified native GM-CSF. J Cell Physiol. 1986 Sep;128(3):421–431. doi: 10.1002/jcp.1041280311. [DOI] [PubMed] [Google Scholar]
- Oon S. H., Williams N. Biochemical characterization of an in-vitro murine megakaryocyte growth activity: megakaryocyte potentiator. Leuk Res. 1986;10(4):403–411. doi: 10.1016/0145-2126(86)90070-6. [DOI] [PubMed] [Google Scholar]
- Quesenberry P. J., Ihle J. N., McGrath E. The effect of interleukin 3 and GM-CSA-2 on megakaryocyte and myeloid clonal colony formation. Blood. 1985 Jan;65(1):214–217. [PubMed] [Google Scholar]
- Sparrow R. L., Williams N. Megakaryocyte colony stimulating factor: its identity to interleukin-3. Prog Clin Biol Res. 1986;215:123–128. [PubMed] [Google Scholar]
- Vainchenker W., Bouguet J., Guichard J., Breton-Gorius J. Megakaryocyte colony formation from human bone marrow precursors. Blood. 1979 Oct;54(4):940–945. [PubMed] [Google Scholar]
- Williams N., Eger R. R., Jackson H. M., Nelson D. J. Two-factor requirement for murine megakaryocyte colony formation. J Cell Physiol. 1982 Jan;110(1):101–104. doi: 10.1002/jcp.1041100116. [DOI] [PubMed] [Google Scholar]
- Williams N., Jackson H., Iscove N. N., Dukes P. P. The role of erythropoietin, thrombopoietic stimulating factor, and myeloid colony-stimulating factors on murine megakaryocyte colony formation. Exp Hematol. 1984 Oct;12(9):734–740. [PubMed] [Google Scholar]
- Williams N., Jackson H., Ralph P., Nakoinz I. Cell interactions influencing murine marrow megakaryocytes: nature of the potentiator cell in bone marrow. Blood. 1981 Jan;57(1):157–163. [PubMed] [Google Scholar]
- Williams N., Oon S. H., Jackson H., Lim R. Studies on megakaryocyte potentiator: its production and some biochemical characteristics. Prog Clin Biol Res. 1986;215:91–103. [PubMed] [Google Scholar]
- Williams N., Sparrow R., Gill K., Yasmeen D., McNiece I. Murine megakaryocyte colony stimulating factor: its relationship to interleukin 3. Leuk Res. 1985;9(12):1487–1496. doi: 10.1016/0145-2126(85)90041-4. [DOI] [PubMed] [Google Scholar]



